Abstract
Purpose: The objective of this study was to test if combining antecedent systolic blood pressure (SBP) with traditional risk factors and hypertension-mediated organ damage (HMOD) improves risk stratification for subsequent cardiovascular disease.
Materials and methods: 1910 subjects participated in this study. Antecedent SBP was defined as the average of measurements obtained in 1982 and in 1987. Current SBP was obtained in 1993. HMOD were examined in 1993. HMOD was defined as either atherosclerotic plaque(s), increased pulse wave velocity, increased urine albumin creatinine ratio (above the 90th percentile) or left ventricular hypertrophy. Major adverse cardiovascular events (MACE) including myocardial infarction, cerebrovascular disease, heart failure and arrhythmia were obtained from national registries.
Results: Subjects were divided into two age categories: a middle-aged group (aged 41 or 51) and an older group (aged 61 or 71). From 1993 to 2010, 425 events were observed. In multivariable analysis with both current and antecedent SBP adjusted for traditional risk factors, current SBP was associated with each measure of HMOD whilst antecedent SBP was not significantly associated with urine albumin creatinine ratio in the older group, LVMI in the middle-aged group, or the presence of plaque in any of the age groups (all p > 0.15). When current and antecedent SBP were evaluated together, current SBP was not associated with MACE in the middle-aged subgroup [HR = 1.09 (0.96–1.22), p = 0.18] but remained associated with MACE in the older subgroup [HR = 1.21 (1.10–1.34), p < 0.01]. Contrariwise, antecedent SBP was only associated with MACE in the middle-aged subgroup [HR = 1.24 (1.04–1.48), p = 0.02]. Adding antecedent SBP to traditional risk factors did not improve the predictive accuracy of the survival model.
Conclusion: In healthy non-medicated middle-aged subjects, antecedent SBP is associated with cardiovascular outcome independently of current BP, traditional risk factors and HMOD. However, improvement in risk stratification seems to be limited.
Introduction
Elevated blood pressure is a major risk factor for the development of hypertension mediated organ damage (HMOD) and subsequent cardiovascular disease (CVD). In current guidelines for risk stratification, only current blood pressure is taken into account [Citation1,Citation2] disregarding previous measurements and potential accumulated harmful effects. Exposure to high blood pressure during a lifetime increases the risk of CVD. Antecedent blood pressure might predict HMOD and incident CVD independent of current BP [Citation3–6], reflecting that long-term exposure to high blood pressure might be a better predictor of CVD than current blood pressure alone. Markers of HMOD integrate time-dependent damaging effect on the vasculature, predominantly from hypertension but also dyslipidemia, impaired glucose tolerance, obesity and smoking [Citation7,Citation8]. We have previously demonstrated that age strengthens the association between BP and measures of HMOD [Citation9], which might reflect either an age-dependent susceptibility to the harmful effects of increased BP or simply longer exposure duration. Although increasing age and blood pressure are the primary determinants for HMOD, it seems that some types of HMOD can develop and progress in individuals without apparent hypertension [Citation10,Citation11], indicating that vascular damage could be driven by either non-hemodynamic pathways or that these subjects might have a lower BP threshold for developing HMOD, and could therefore result from prolonged but only mildly elevated BP.
In this study, we wanted to evaluate the contribution of antecedent and current blood pressure to HMOD and how this association affects the cardiovascular outcome. We hypothesise that combining previous BP measurements with current risk factors improves prediction of HMOD, thus helping to select subjects for HMOD screening. Furthermore, we hypothesise that combining previous BP measurements with current risk factors including HMOD improves risk stratification for subsequent CVD.
Methods
Study population
In 1982–1984, a random sample of 4807 individuals from the Copenhagen county area were invited to participate in a population survey (3971 participated). Residents still living in the county were reinvited to follow-up visits in 1988 and again in 1993–1994. Participants were aged 30, 40, 50 and 60 at the initial visit, and 41, 51, 61 and 71 at the third visit. Only subjects participating in both the initial visit and the follow-up visit in 1993–1994 were included in the study (n = 2656). Furthermore, participants with missing data for HMOD evaluation, laboratory data or BP measurements were excluded (n = 342). As the aim of this study was to evaluate participants who were untreated and without prior CVD, we excluded individuals with self-reported CVD, diabetes or individuals receiving antihypertensive or lipid-lowering treatment at the third examination in 1993–1994 (n = 404), leaving 1910 participants eligible for this study. Flow chart for the inclusion is provided in the Supplementary Material.
Office blood pressure measurement
BP was measured with the patient sitting after 5 min of rest, with the arm at heart level using a mercury sphygmomanometer. The average of two measurements was reported.
Antecedent systolic BP (SBP) was defined as the average of measurements obtained at the first visit (1982–1984) and at the second visit in 1988. In subjects, who only attended the first and third visits (n = 90), the BP obtained at the first visit was defined as the antecedent BP. Current SBP was the measurement obtained at the third visit (1993–1994). Hypertension was defined as SBP ≥140 mmHg and/or diastolic BP ≥90 mmHg.
Evaluation for hypertension-mediated organ damage
Four measures of HMOD were examined. Participants were defined as having HMOD if they had one or more of the following: presence of atherosclerotic plaque(s), increased pulse wave velocity (PWV), increased urine albumin creatinine ratio (UACR) or left ventricular hypertrophy (LVH).
Atherosclerotic plaque was assessed over both carotid arteries with B-mode ultrasound (Bruel and Kjær 3535; Nærum, Denmark). Plaques were defined as a local thickening of the intima–media layer of more than 50% or a local, sharp increase in echo-density with shadowing.
Pulse wave velocity was calculated as the distance between two piezoelectrical pressure transducers (Hellige GmbH, Freiburg, Germany) placed over the common carotid artery and the femoral artery divided by the pulse wave transit time between the two transducers. Increased PWV was defined as PWV >12 m/s.
Urine albumin concentration was analysed using a turbidimetric method (Hitachi 717 analyser; Roche Diagnostics, Mannheim, Germany) on a single morning urine sample. Urine creatinine was analysed using the Jaffé reaction without deproteinizing and then quantified by a photometric method (Hitachi 717 analyser). Increased urine albumin creatinine ratio (UACR) was defined using cut-off points of 0.73 mg/mmol for men and 1.09 mg/mmol for women, corresponding to the 90th percentile in a healthy population [Citation12].
Left ventricular mass was assessed with echocardiography using M-mode according to standard guidelines [Citation13]. Left ventricular mass index (LVMI) was calculated as left ventricular mass divided by body surface area, calculated by Dubois’ formula. Left ventricular hypertrophy was defined as LVMI >125 g/m2 for men or LVMI >110 g/m2 for women, according to European guidelines [Citation14].
Endpoint classification
Information on cardiovascular events was obtained from national registries after 16 years of follow-up. Data regarding death and migration status, cause of death and hospitalization were obtained from the Civil Registration System, the Danish Cause of Death Register and the Danish National Patient Register, respectively, with last follow-up on 31 December 2010. Data quality from the national registries has high sensitivity, with a proportion of incorrect registrations of 3% evaluated in 2003 [Citation15]. The endpoint for evaluation consisted of major adverse cardiovascular events (MACE), listed in Supplementary Table 1.
Statistical analysis
Continuous variables are summarized by means and standard deviations (SD, approximately normally distributed variables) and medians and interquartile ranges (IQR, non-normally distributed variables); categorical variables are presented by frequencies and corresponding percentages.
Univariable linear and binary logistic regression models were used to evaluate associations between antecedent SBP and HMOD variables. Regression coefficients (linear regression) and odds ratios (OR; logistic regression) with corresponding 95% confidence intervals (CI) are reported. Positively skewed variables, i.e. PWV and UACR were log-transformed to ensure linearity.
To assess BP increases over time, we used linear regression with SBP at each examination point as the outcome and time as the independent variable, stratified for age group and presence of HMOD.
Associations were examined in univariable and multivariable regression models adjusted for Systematic COronary Risk Evaluation (SCORE) risk factors: age, current SBP, sex, smoking status and total cholesterol. Potential effect modification was evaluated by adding interaction terms in the models between antecedent SBP and age group ([41 or 51] versus [61 or 71]), current SBP, sex, smoking status, total cholesterol and the presence of HMOD, respectively.
Receiver-operating characteristic (ROC) curves based on multivariable logistic binary regression models with and without antecedent SBP were compared for each HMOD to test whether antecedent SBP would improve the model to distinguish subjects with HMOD from those without.
Univariable and multivariable Cox proportional hazards regression models were used to evaluate associations between antecedent SBP and incident CVD adjusted for traditional risk factors and the presence of hypertension mediated organ damage. Hazard ratios (HR) with corresponding 95% CIs are presented. Model fit was evaluated by Harrel’s Concordance index (C-index) and Bayesian information criterion (BIC). The significance level was 0.05. All analyses were performed using Stata 15 (StataCorp, College Station, TX, USA).
Results
Baseline data and event rates
The study population consisted of 1910 subjects divided into two age categories; a middle-aged group (aged 41 or 51 years) consisting of 1188 subjects and an older group (aged 61 or 71 years) consisting of 722 subjects. Six percent of subjects aged 41 or 51 and 20% of subjects aged 61 or 71 were hypertensive in 1983–1988. Nineteen percent of subjects aged 41 or 51 had hypertension and 45% of subjects aged 61 or 71 had hypertension in 1993 (). Forty-nine percent of subjects aged 41 or 51 were smokers and 43% of subjects aged 61 or 71 were smokers in 1993, reflecting the Danish population at the time [Citation16].
Table 1. Population characteristics including antecedent blood pressure and markers of hypertension-mediated organ damage.
From 1993 to 2010, during a mean follow-up period of 14.3 years (SD 3.8), 425 events were observed; 173 occurring in the middle-aged age group and 252 in the older age group. Event types and counts are listed in Supplementary Tables 1a and 1b.
Association of current and antecedent blood pressure with markers of hypertension mediated organ damage
In 1993, middle-aged and older subjects with HMOD had higher current and antecedent SBP compared with subjects without HMOD (). Furthermore, the rate of increase in SBP was steeper in patients with HMOD compared to those without in both age groups. When measured in an average increase of SBP per year, middle-aged subjects without HMOD had an increase of 0.32 mmHg compared with subjects with HMOD who had an increase of 0.90 mmHg (p for interaction <0.01; ). Similarly, older subjects without HMOD had an annual increase of 0.67 mmHg in SBP compared with subjects with HMOD who had an annual increase of 1.16 mmHg (p for interaction <0.01; ). In subjects with HMOD, the annual increase of SBP was similar in both age groups: 0.90 versus 1.16 mmHg/year (p for interaction = 0.110). In subjects without HMOD, the annual rate of increase in SBP was steeper in the older group compared to the middle-aged group: 0.67 versus 0.32 mmHg/year (p for interaction = 0.010).
Figure 1. “Progression of systolic blood pressure from 1982 to 1993 stratified for age group and presence of hypertension mediated organ damage”.
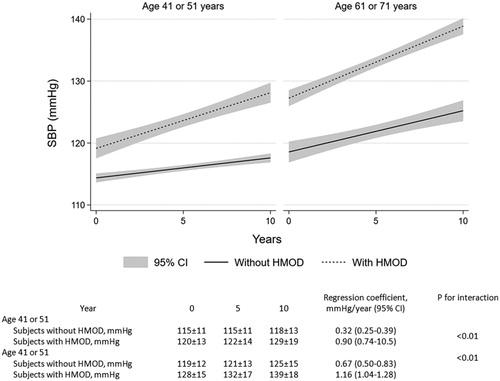
When evaluated in univariable regression models, both antecedent SBP and current SBP were associated with each measure of HMOD, except current SBP and log UACR in the middle-aged group (p = 0.750) ().
Table 2. Association of current and antecedent systolic blood pressure with markers of hypertension mediated organ damage.
In multivariable analysis with both current and antecedent SBP adjusted for sex, cholesterol and smoking (model 2, ), current SBP was associated with each measure of HMOD whilst antecedent SBP was not significantly associated with UACR in the older group, LVMI in the middle-aged group or the presence of plaque in both age groups (all p > 0.15). Antecedent SBP was inversely associated with log UACR in the middle-aged group. This inverse association remained significant in the regression model consisting of both antecedent and current SBP (model 1, ) (p < 0.001) and also in the model adjusted for traditional risk factors (model 2, ) (p = 0.002). However, the association between antecedent SBP and UACR lost significance when evaluated in a model where current SBP was removed (not shown), indicating that the unexpected inverse association between UACR and antecedent SBP could possibly be explained by chance.
The association between antecedent SBP and both LVMI and PWV was accentuated by age in the multivariable model. An increment of 10 mmHg in antecedent SBP corresponded to about a 2.6% increase in PWV in the middle-aged group and a 6.4% increase in PWV in the older group (p for interaction <0.01). Correspondingly, an increment of 10 mmHg in antecedent SBP was associated with an increase of 0.31 g/m2 in LVMI in the middle-aged group and an increase of LVMI of 2.13 g/m2 in the older group (p for interaction = 0.02). Age did not impact the association between current SBP and any measure of HMOD.
Adding antecedent SBP to traditional risk factors did not improve prediction of LVH, presence of carotid plaque, increased PWV or increased UACR as evaluated by an increase in AUC in binary logistic regression (Supplementary Figure 1).
Cardiovascular outcome
Both current and antecedent SBPs were associated with MACE, independently of traditional risk factors in both age groups (Supplementary Table 3). When current and antecedent SBP were evaluated simultaneously in Cox regression models, current SBP was not significantly associated with MACE in the middle-aged group [HR = 1.09 (0.96–1.22), p = 0.18] but remained associated with MACE in the older group [HR = 1.21 (1.10–1.34), p < 0.01]. Contrariwise, antecedent SBP was only associated with MACE in the middle-aged group [HR = 1.24 (1.04–1.48), p = 0.02] with the association attenuated in the older group [HR = 1.04 (0.92–1.18), p = 0.52], when evaluated together with current SBP. Adding the presence of HMOD to the model did not change this relationship, i.e. antecedent SBP was more strongly associated with MACE in the middle-aged group and current SBP more strongly associated with MACE in the older subgroup (). Presence of HMOD was independently associated with MACE after adjusting for traditional risk factors and both current and antecedent SBP with similar HRs in both age groups; middle-aged [HR = 2.11, (1.53–2.91), p < 0.01] and older subgroup [1.96 (1.36–2.28), p < 0.01].
Table 3. Association of current and antecedent systolic blood pressure with incident cardiovascular disease.
The association between antecedent SBP and MACE was negatively modified by age, current SBP, total cholesterol and the presence of HMOD (); however, none of the interaction terms remained significant in the fully adjusted models.
Table 4. Impact of traditional risk factors and presence of hypertension-mediated organ damage on the prognostic value of antecedent systolic blood pressure.
Adding antecedent SBP to traditional risk factors did not improve the predictive power of the Cox regression model; ΔC-index = 0.018, p = 0.09. Including HMOD did, however, improve model performance when compared to a model consisting of both traditional risk factors and antecedent SBP; ΔC-index = 0.015, p = 0.01. No additional model improvement was demonstrated when assessing models including potential interaction terms between antecedent SBP and age, antecedent SBP and current SBP, antecedent SBP and total cholesterol, or antecedent SBP and HMOD (not shown). In the middle-aged subgroup, model fit was slightly improved when substituting current SBP with antecedent SBP, evaluated by the decrease in BIC; however, C-index did not increase significantly. C-index and BIC for each model are provided in Supplementary Table 2.
Discussion
This study has two principal findings: (1) antecedent SBP is associated with measures of HMOD, independently of traditional risk factors; however, combining antecedent SBP with traditional risk factors does not improve discrimination between subjects with and without HMOD; (2) Although antecedent SBP is superior to current SBP in prediction of MACE in middle-aged subjects, clinical implications in terms of improved risk stratification seems limited in healthy non-medicated subjects.
Aging and susceptibility to the harmful effects of blood pressure
In this study, we found that antecedent SBP was a stronger predictor of CVD than current SBP in subjects aged 41 or 51 compared with subjects aged 61 or 71. This is in line with previous studies highlighting the importance of early adulthood to mid-life for the development of HMOD and subsequent CVD [Citation3,Citation17–21]. Adult hypertension may already originate in childhood [Citation22] and SBP trajectories from childhood to early adulthood are associated with HMOD and cardiovascular outcome [Citation17,Citation23–26], reflecting that “watchful waiting” until a patient reaches a certain risk threshold due to aging may be hazardous.
Another explanation for antecedent blood pressure not predicting CVD independently of traditional risk factors in subjects aged 61 or 71 could be due to selection bias, as discussed under limitations. This explanation is supported by the reduced prognostic importance of antecedent SBP in subjects with HMOD, high current SBP or high total cholesterol.
Antecedent blood pressure and risk stratification
Although several studies have shown that antecedent blood pressure is associated with subclinical HMOD and CV outcome independently of current BP [Citation4,Citation6], the evidence for additional value in terms of improved risk stratification beyond traditional risk scoring remains debateable. Identification and employment of BP trajectories in risk stratification has only been evaluated in a few large population-based cohorts without convincing results. In one study, comprised of two prospective extinction cohorts with annual BP measurements over 10 years, the predictive value of models including BP trajectories was comparable to that of models with average BP or single BP measurements [Citation27]. Similar results were seen in the Rancho Bernardo Study, where SBP trajectories provided no added value to average SBP in predicting CVD and all-cause mortality assessed by C-index [Citation28]. Likewise, results from the Lifetime Risk Pooling Project showed no significant improvement in predicting CVD evaluated by C-index when including cumulative SBP. However, the addition of antecedent cumulative SBP did result in a small, albeit significant, net reclassification improvement [Citation29].
This study indicates that although subclinical organ damage is facilitated primarily by hypertension, antecedent blood pressure measurements cannot substitute evaluation for HMOD in terms of risk stratification improvement. This, to our knowledge, has not been demonstrated previously. Furthermore, this study highlights that in terms of blood pressure it is the prior risk status that is responsible for the current health. In middle-aged individuals, the inclusion of prior risk exposure may be valuable in accurately predicting subsequent CVD events.
Limitations
There are some limitations to this study that are important to address. Firstly, we only included non-medicated subjects without prior CVD as reported in 1993. This might introduce a selection bias leading to an underestimation of the association between antecedent blood pressure and both HMOD and cardiovascular outcome. A higher fraction of subjects with previous high blood pressure would more likely receive antihypertensive treatment or already have had a cardiovascular event in 1993, compared with subjects with previous blood pressure measurements in the normal range. However, from a risk stratification perspective, these subjects would already be classified as high risk, regardless of previous BP measurements—leaving knowledge of antecedent BP redundant for the clinician’s evaluation.
Secondly, the design of this study does not allow one to evaluate whether the predictive value of antecedent BP is due to longer exposure to the harmful effects of high BP or it might simply be that more/repeated measurements reduce noise in terms of measurement error and/or variability.
Thirdly, inadequacy in power to detect an improvement in risk stratification when adding antecedent SBP to traditional risk models could arise due to the relatively few number of events, especially when models are compared using the rather conservative Harrell’s concordance index.
Finally, using a broad composite endpoint may constitute a limitation. Although such an endpoint enhances the statistical power of our analysis, inclusion of adverse events such as arrhythmias might dilute associations between exposure variables and more conventional atherosclerotic endpoints, such as myocardial infarction
Conclusion
In healthy non-medicated subjects, antecedent BP is independent of current BP and traditional risk factors associated with markers of HMOD and cardiovascular outcome. However, the clinical applicability of antecedent BP recordings for improved risk stratification seems to be limited.
suptable_20_02.docx
Download MS Word (54.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Krause T, Lovibond K, Caulfield M, on behalf of the Guideline Development Group, et al. Management of hypertension: summary of NICE guidance. BMJ. 2011;343(2):d4891–d4891.
- Hypertension EETFftMoA. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–1938.
- Ghosh AK, Hardy RJ, Francis DP, On behalf of the Medical Research Council National Survey of Health and Development (NHSD) Scientific and Data Collection Team, et al. Midlife blood pressure change and left ventricular mass and remodelling in older age in the 1946 British Birth Cohort Study. Eur Heart J. 2014;35(46):3287–3295.
- Bonifonte A, Ayer T, Veledar E, et al. Antecedent blood pressure as a predictor of cardiovascular disease. J Am Soc Hypertens. 2015;9(9):690–696 e1.
- Vasan RS, Massaro JM, Wilson PW, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2002;105(1):48–53.
- Seshadri S, Wolf PA, Beiser A, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: the Framingham Study. Arch Intern Med. 2001;161(19):2343–2350.
- Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens. 2008;26(6):1049–1057.
- Scuteri A, Orru M, Morrell CH, et al. Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis. 2012;221(1):189–197.
- Olesen TB, Pareek M, Stidsen JV, et al. Impact of age on the association between 24-h ambulatory blood pressure measurements and target organ damage. J Hypertens. 2018;36(9):1895–1901.
- Schmieder RE. The role of non-haemodynamic factors of the genesis of LVH. Nephrol Dial Transplant. 2005;20(12):2610–2612.
- Ursavas A, Karadag M, Gullulu M, et al. Low-grade urinary albumin excretion in normotensive/non-diabetic obstructive sleep apnea patients. Sleep Breath. 2008;12(3):217–222.
- Olsen MH, Hansen TW, Christensen MK, et al. Cardiovascular risk prediction by N-terminal pro brain natriuretic peptide and high sensitivity C-reactive protein is affected by age and sex. J Hypertens. 2008;26(1):26–34.
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367.
- Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–1536.
- Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Clemmensen KK, Lynge E, Clemmensen IH. Nationwide tobacco surveys and sales data in Denmark from 1920 to 2010. Dan Med J. 2012;59(6):A4448.
- Zhang T, Li S, Bazzano L, et al. Trajectories of childhood blood pressure and adult left ventricular hypertrophy: the Bogalusa Heart Study. Hypertension. 2018;72(1):93–101.
- Fan JH, Wang JB, Wang SM, et al. Longitudinal change in blood pressure is associated with cardiovascular disease mortality in a Chinese cohort. Heart. 2018;104(21):1764–1771.
- Ghosh AK, Hughes AD, Francis D, et al. Midlife blood pressure predicts future diastolic dysfunction independently of blood pressure. Heart. 2016;102(17):1380–1387.
- Inker LA, Okparavero A, Tighiouart H, et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis. 2015;66(2):240–248.
- High blood pressure in midlife linked to brain decline. Harv Heart Lett. 2014;25(2):8.
- Shen W, Zhang T, Li S, et al. Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: the Bogalusa Heart Study. Hypertension. 2017;70(1):66–74.
- Hao G, Wang X, Treiber FA, et al. Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk: results from the 23-year longitudinal Georgia Stress and Heart Study. Hypertension. 2017;69(3):435–442.
- Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66(6):1108–1115.
- Hartiala O, Kajander S, Knuuti J, et al. Life-course risk factor levels and coronary artery calcification. The Cardiovascular Risk in Young Finns Study. Int J Cardiol. 2016;225:23–29.
- Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311(5):490–497.
- Tielemans SM, Geleijnse JM, Menotti A, et al. Ten-year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the Minnesota Business and Professional Men Study and the Zutphen Study. J Am Heart Assoc. 2015;4(3):e001378.
- Tielemans S, Geleijnse JM, Laughlin GA, et al. Blood pressure trajectories in relation to cardiovascular mortality: the Rancho Bernardo Study. J Hum Hypertens. 2017;31(8):515–519.
- Pool LR, Ning H, Wilkins J, et al. Use of long-term cumulative blood pressure in cardiovascular risk prediction models. JAMA Cardiol. 2018;3(11):1096–1100.
