Abstract
Purpose: To investigate contemporary results of percutaneous transluminal renal angioplasty (PTRA).
Materials and Methods: A multicentre retrospective study analysing all patients treated with PTRA for primary symptomatic renal artery stenosis (RAS) between 2010 and 2013 at four tertiary centres. Procedures during the preceding four years were counted to evaluate for change in PTRA frequency.
Results: The number of PTRA procedures decreased by approximately 50% from 2006 to 2013. Patients treated in the post-ASTRAL period (n = 224) had a significant reduction in mean systolic pressure (168 to 146 mmHg, p < 0.01), diastolic pressure (84 to 76 mmHg, p < 0.01), number of anti-hypertensive drugs (3.54 to 3.05, p < 0.01), and anti-hypertensive treatment index (21.75 to 16.92, p < 0.01) compared to before PTRA. These improvements were maintained at one year and at the last clinical evaluation after a mean follow-up of 4.31 years. Renal function increased transiently without sustained improvement, or deterioration, during later follow-up. Thirteen patients (5.8%) eventually required dialysis, nine of these had eGFR <20 ml/min/1.73 m2 before PTRA. There was no difference in outcomes between subgroups differentiated by different indications for PTRA.
Conclusion: The frequency of PTRA has decreased, indicating a higher threshold for invasive treatment of RAS in recent years. The reduction in blood pressures, the reduced need for anti-hypertensive medication, and stabilization of renal function over time suggest a clinical benefit for most patients who are now being treated with PTRA.
Introduction
Renal artery stenosis (RAS) is associated with hypertension, renal failure, cardiovascular events, and increased mortality. Percutaneous transluminal renal angioplasty (PTRA) was introduced in 1978 [Citation1]. The method has since been refined, with dedicated low-profile devices and reduced complication rates. This led to a widening of treatment indications, peaking in the late 1990s and early 2000s. In parallel, medical treatment has also improved, with better blood pressure control, renin-angiotensin blockade, anti-platelet therapy, and lipid-lowering agents. This called for prospective randomized trials to investigate whether PTRA offered additional clinical benefit compared to best medical treatment (BMT) alone.
In November 2009, the result of the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial was published in New England Journal of Medicine, showing no additional clinical benefit with PTRA and BMT compared to BMT alone [Citation2]. This led to a shift in treatment strategy for patients with symptomatic RAS. The results of the ASTRAL trial were later confirmed by the larger CORAL trial [Citation3].
Both the ASTRAL trial and the CORAL trial randomized only a low proportion of all eligible patients at the participating centres; in particular, patients with severe symptoms were not included. Guidelines have continued to recommend PTRA for certain patients with RAS, as those with fibromuscular dysplasia, patients with flash pulmonary oedema or congestive heart failure, and patients with single kidney or bilateral RAS and acute renal failure [Citation4,Citation5], despite a lack of evidence for its efficiency compared to modern pharmacological treatment alone.
The aim of the present study was to evaluate the clinical effect of PTRA in patients with primary RAS treated after the shift in strategy that was initiated by the results of the ASTRAL trial and to determine whether contemporary outcomes are better or worse for some indications than for others.
Materials and methods
Patients
Consecutive patients treated with PTRA for RAS at four Swedish University Hospitals in the period 2006‒2013 were identified using the patient registration system of the interventional radiology department at each site. Patients were differentiated according to two equally long time periods, separated by the publication of the ASTRAL trial in late 2009 [Citation2]. Patients treated during the first period (2006‒2009), the pre-ASTRAL group, served as reference for treatment frequency and only the number of PTRA treatments was recorded during this period. Patients treated during the second period (2010‒2013), the post-ASTRAL group, was the study cohort of this report, for which detailed pre-, per-, and postoperative information was retrieved from the medical records. Only patients with primary and symptomatic RAS were included. Patients with RAS secondary to previous invasive aortic treatment and also patients with prophylactic PTRA of asymptomatic RAS before resection of the contralateral kidney were excluded. Patients who received retreatment of recurrent primary symptomatic RAS during both time periods were included. Baseline demographics at the time of the PTRA procedure in the post-ASTRAL group (2010‒2013) are shown in .
Table 1. Baseline demographics of study cohort, 2010‒2013.
Methods
Pre-, per-, and postoperative data on patients in the post-ASTRAL group were collected on site at each participating centre. Information on indications for PTRA, preoperative co-morbidities, systolic and diastolic blood pressures, serum creatinine, postoperative complications, and anti-hypertensive medications were retrieved from the medical records of the hospital and from external healthcare units when needed. Specific procedural information including preoperative diagnostic imaging, trans-stenotic pressure measurements, laterality of treated renal artery, size of stent/balloon and information on perioperative complications was found in the radiology information system (RIS) of each hospital. Clinical outcomes were recorded directly after PTRA (post-PTRA), after one year, and at the latest clinical evaluation. Informed consent was obtained from all surviving patients before requesting journal excerpts from external healthcare units. The study was approved by the Regional Ethical Review Board in Gothenburg (Dnr. 874-149).
Renal function was evaluated with the estimated glomerular filtration rate (eGFR), calculated with the four-variable equation described in the Modification of Diet in Renal Disease (MDRD) study [Citation6]. Pharmacological treatment was evaluated both from the number of prescribed anti-hypertensive drugs and from a calculated pharmacological treatment index (TI). A dose score was determined for each anti-hypertensive drug. The score ranged from 0 to 10; 0 indicated no medication and 10 indicated the upper dose recommended by the Swedish environmental classification of pharmaceuticals (www.fass.se). TI was calculated for each patient at each time point by adding the scores of all anti-hypertensive drugs prescribed, as previously described by Delin et al. [Citation7,Citation8].
Statistics
Values are presented as mean with 95% confidence interval (CI). Systolic and diastolic blood pressures, estimated glomerular filtration rate (eGFR), TI, and number of drugs at the follow-up time points (post PTRA, after one year, and at the last control) were compared with corresponding values before PTRA, using linear regression analyses. Each follow-up time was coded using binary dummy variables allowing for complete flexibility in the development over time.
Results
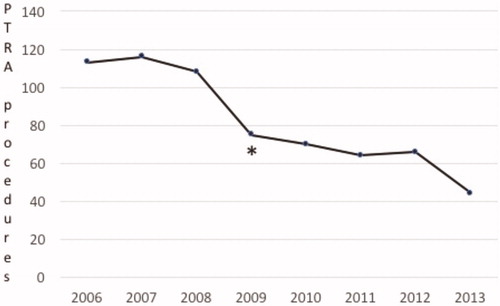
The number of PTRA procedures per year decreased between 2006 and 2013 (). During the period 2006‒2009, in total 412 PTRA procedures were performed at the participating centres, whereas between 2010 and 2013, the post-ASTRAL study period, 244 procedures were performed. Between the first three years of the study period (2006‒2008) and the last three years (2011‒2013), the number of PTRA procedures had decreased by approximately 50%.
Figure 1. Numbers of PTRA procedures performed in the period 2006–2013. The asterisk denotes the time of publication of the ASTRAL trial.
Baseline demographics of the study cohort, patients treated between 2010 and 2013, are summarised in . The 244 PTRA procedures were done in 224 patients. Twenty patients with two separate interventions were either re-treated due to a restenosis in the same renal artery or underwent new treatment of RAS in the contralateral kidney. All patients were on anti-hypertensive medication. The most common indication for PTRA was therapy-resistant hypertension requiring three or more anti-hypertensive drugs (177 patients, 79%), often in combination with declining renal function (103 patients, 46%) or impaired renal function associated with prescription of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) (23 patients, 10,2%). In 26 patients (11%), only one kidney was functioning. Cardiovascular co-morbidities were common: 44 patients (20%) had ischaemic heart disease and 48 patients (21%) had heart failure. Eleven patients (5%) had cerebrovascular disease. Two patients (0.9%) had temporary renal replacement therapy at the time of the PTRA.
Periprocedural data are shown in . Computer tomography angiography (CTA) and magnetic resonance angiography (MRA) were the most common preoperative diagnostic modalities. Periprocedural intravascular pressure measurements were done in 169 of 224 patients (75%), but only in 84 (37%) were the measurements done both pre- and post-dilatation. Some 228 of 260 treated renal arteries (88%) were stented, whereas 32 (12%) were balloon-dilated without stent placement.
Table 2. Procedure-related data in 224 patients subjected to PTRA.
In four patients with bilateral RAS, revascularization was possible in only one renal artery. All other revascularizations (256 of 260, 98%) were successful. There were four major complications (1.8%). Two patients had cholesterol embolism with rapid renal impairment, and one suffered from thrombo-embolism to the lower extremities and to a kidney. Puncture site occlusion of an atherosclerotic common femoral artery in one patient was treated with thromboendarterectomy. Minor complications occurred in 24 patients (10.71%). They were mainly small puncture site haematomas, 14 in the groyne and 2 after puncture of the brachial artery. Three patients with haematomas adjacent to the revascularized kidney were treated conservatively and their haematomas resolved spontaneously. Two patients had minor allergic reactions to the contrast medium, and two suffered from transient pain associated with the procedure.
Clinical outcomes of PTRA in patients treated during the post-ASTRAL period are given in . The mean follow-up time was 4.31 years (1.43‒6.95 [95%CI]) and 64 patients (29%) died during follow-up. Complete data were not found in 22 patients (9.8%) at the one-year control and in 29 patients (13%) at the last control. In summary, both systolic and diastolic blood pressures were significantly reduced compared to pre-PTRA levels, and this reduction was maintained at one year and at the last follow-up. Both the number of anti-hypertensive drugs and the TI were reduced after PTRA, and this reduction was maintained at one year and at the last follow-up. There was a transient increase in eGFR directly after PTRA, but after one year and at the last follow-up eGFR was not significantly different from that before PTRA. Thirteen patients (5.8%) reached end-stage kidney disease with the need for dialysis during follow-up. Nine of these had eGFR <20 before PTRA.
Table 3. Changes in blood pressure, renal function, and anti-hypertensive treatment over time in 224 patients subjected to PTRA.
In order to investigate if improvements after PTRA depended on indications other than atherosclerotic RAS, regression analyses were also performed without the 25 patients treated for RAS due to fibromuscular dysplasia and Takayasús arteritis. This did not affect the magnitude of improvements after treatment.
In subgroup analyses based on indication for the PTRA treatment, there were no significant differences in clinical outcomes between different indications (data not shown).
Discussion
This multi-centre study investigated the clinical response to PTRA in 224 consecutive patients who were treated after publication of the ASTRAL trial in 2009 [Citation2]. Significant improvement was found in blood pressure control: a reduction in systolic and diastolic pressures and a reduced need for anti-hypertensive medication. These improvements remained during a mean follow-up time of 4.3 years. Renal function (eGFR) improved transiently after PTRA, but at one year and beyond, eGFR was not significantly different from pre-PTRA levels. There were few serious complications related to the procedures, but the risk of dialysis remains after PTRA in patients with very low renal function.
The number of PTRA treatments was halved during the observation period of eight years. This reduction started already one year before publication of the ASTRAL trial, in 2008. This may have been due to oral communication of trial results prior to the formal publication, or to a developing general uncertainty at the time concerning the additional value of PTRA in some patients. Indeed, the other large RCT, the US-based CORAL trial, addressing the same clinical question and published in January 2014, had already started to recruit patients in 2005 [Citation3]. Changes in the general population [Citation9], or advances in pharmacological blood pressure management may have led to this, with more appropriate dosing, and the use of more effective drug combinations which allowed for individually tailored medication with higher patient compliance [Citation10,Citation11].
The decrease in systolic blood pressure, a mean reduction of >20 mmHg, was maintained over the follow-up period. The decrease in diastolic blood pressure also persisted over time but was smaller in magnitude (9 mmHg). These results correlate well with those presented by other authors [Citation12–17].
There was a significant reduction in numbers and doses of anti-hypertensive drugs, findings shown also in the DRASTIC and EMMA studies [Citation18,Citation19], and in a recent study by Courand et al. [Citation17]. These results contrast with the findings of the CORAL study [Citation3], where the number of medications increased equally over time for both PTRA-treated patients and patients on BMT only. This difference may have been due to the fact that CORAL trial patients had less severe hypertension (with a mean systolic pressure of 150 mmHg as opposed to 168 mmHg in our cohort) and they required fewer anti-hypertensive drugs (mean 2.1 drugs at baseline, as compared to 3.5 in our patients).
There was no persistent improvement in renal function after PTRA, but on the other hand there was no significant deterioration either. After a small increase directly after PTRA, eGFR returned to pre-PTRA levels at one year and at the last clinical control. Given that deteriorating renal function was a common indication for PTRA in our cohort, this stabilization over time may also be regarded as a positive result, similar to what has been described by others [Citation20]. But in the subset of patients with very low renal function, eGFR below 20, the risk of need for dialysis remains and these patients, with severe vascular and renal disease, should be cautiously evaluated before deciding on PTRA treatment.
Major complications were rare, occurring in only 1.8% of patients. This is less than in the ASTRAL trial (4.7%) and similar to the proportion in the ASPIRE 2 study (2.4%) [Citation21]. We believe that this reflects the fact that patient selection and revascularizations were done by highly trained specialist physicians and operators, with great experience in treating this group of patients.
The limitations of the current study include the retrospective design without a control population and the relatively limited number of patients. With more patients, it might have been possible to determine variance in outcomes between subgroups of patients, differentiated by different indications. Unfortunately, few population-based registries capture these interventions. As PTRA procedures are becoming less common, the need for registries is even more apparent. Of course, prospective randomized controlled trials are the ultimate tool for evaluation of treatment efficacy, but as the balance of opinion regarding invasive or non-invasive treatment of RAS has shifted towards patients with more severe disease, the challenge of doing such trials has increased even further.
In conclusion, invasive treatment of RAS has decreased, indicating a higher threshold for PTRA compared to before the publication of the ASTRAL trial. The positive effect on blood pressure control and stabilization of renal function over time with contemporary indications and techniques indicates that patients with therapy-resistant hypertension requiring three or more anti-hypertensive drugs, haemodynamically significant RAS and enough remaining renal function do benefit from endovascular revascularization.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gruntzig A, Kuhlmann U, Vetter W, et al. Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal-artery stenosis. Lancet. 1978;311(8068):801–802.
- Wheatley K, Ives N, Gray R, et al.; ASTRAL Investigators. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361(20):1953–1962.
- Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370(1):13–22.
- Aboyans V, Ricco JB, Bartelink ML, et al.; ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS. Eur Heart J. 2018;39(9):763–816.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(13):1425–1443.
- Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. JASN. 2007;18(10):2749–2757.
- Delin K. Renal hypertension. A clinical study of the use of renin measurements for preoperative diagnosis [dissertation]. Gothenburg: University of Gothenburg1982.
- Jensen G, Annerstedt M, Klingenstierna H, et al. Survival and quality of life after renal angioplasty: a five-year follow-up study. Scand J Urol Nephrol. 2009;43(3):236–241.
- Ribacke M, Tibblin G, Rosengren A, et al. Is hypertension changing? Blood pressure development in cohorts of 50-year-old men between 1963 and 1993. Blood Press. 1996;5(3):134–138.
- Dahlöf B, Dahlöf Sever P, Poulter N, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906.
- Holmquist C, Hasselström J, Bengtsson Boström K, et al. Improved treatment and control of hypertension in Swedish primary care: results from the Swedish primary care cardiovascular database. J Hypertens. 2017;35(10):2102–2108.
- Jaff MR, Bates M, Sullivan T, et al.; on behalf of the HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Cathet Cardiovasc Intervent. 2012;80(3):343–350.
- Lederman RJ, Mendelsohn FO, Santos R, et al. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J. 2001;142(2):314–323.
- Zeller T, Frank U, Muller C, et al. Stent-supported angioplasty of severe atherosclerotic renal artery stenosis preserves renal function and improves blood pressure control: long-term results from a prospective registry of 456 lesions. J Endov Ther. 2004;11(2):95–106.
- Prajapati JS, Jain SR, Joshi H, et al. Response of blood pressure after percutaneous transluminal renal artery angioplasty and stenting. WJC. 2013;5(7):247–253.
- Laird JR, Rundback J, Zierler RE, et al. Safety and efficacy of renal artery stenting following suboptimal renal angioplasty for de novo and restenotic ostial lesions: results from a nonrandomized, prospective multicenter registry. J Vasc Interv Radiol. 2010;21(5):627–637.
- Courand P-Y, Dinic M, Lorthioir A, et al. Resistant hypertension and atherosclerotic renal artery stenosis. effects of angioplasty on ambulatory blood pressure. Hypertension. 2019;74(6):1516–1523.
- Plouin PF, Chatellier G, Darne B, et al. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension. 1998;31(3):823–829.
- van Jaarsveld BC, Krijnen P. Prospective studies of diagnosis and intervention: the Dutch experience. Semin. Nephrol. 2000;20(5):463–473.
- Mousa AY, AbuRahma AF, Bozzay J, et al. Update on intervention versus medical therapy for atherosclerotic renal artery stenosis. J Vasc Surg. 2015;61(6):1613–1623.
- Rocha-Singh K, Jaff M, Rosenfield K. Evaluation of the safety and effectiveness of renal Artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol. 2005;46(5):776–783.
