Abstract
Purpose: Masked hypertension (MHT) is characterised as an office normotension in the presence of out-of-office hypertension, and can be further categorised as isolated daytime (dMHT), night-time (nMHT) or day-night MHT (dnMHT) according to the time when hypertension is present. MHT is associated with adverse cardiovascular outcome. However, no previous studies contrasted these MHT subtypes in their associations with target organ damage (TOD).
Materials and methods: Consecutive untreated patients referred for ambulatory blood pressure (BP) monitoring to our Hypertension Clinic were recruited. Office and ambulatory BPs were measured using the Omron 7051 and SpaceLabs 90217 monitors, respectively. The BP thresholds of daytime and night-time hypertension were of ≥135/85 mmHg and ≥120/70 mmHg, respectively. We performed various TOD measurements, including carotid-femoral pulse wave velocity (cfPWV), carotid intima-media thickness (cIMT), left ventricular mass index (LVMI) and E/E’, estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (UACR).
Results: The 1808 participants (mean age, 51 years; women, 52%) included 672 (37.2%) MHT subjects, among whom 123 (18.3%) had dMHT, 78 (11.6%) nMHT, and 471 (70.1%) dnMHT. In all participants as well as patients with office normotension (n = 1222), ambulatory daytime and night-time BPs were similarly associated with all TOD measurements (p ≥ 0.20) after multivariate adjustment. Compared to normotensive subjects (p < 0.05), patients with dMHT had faster cfPWV (7.81 vs. 7.58 m/s) and thicker cIMT (637.6 vs. 610.4 µm), patients with nMHT had thicker cIMT (641.8 vs. 610.4 µm) and increased UACR (0.79 vs. 0.59 mg/mmol), and patients with dnMHT had all worse TOD measures mentioned-above plus elevated eGFR (120.7 vs. 116.8 ml/min/1.73m2).
Conclusion: MHT was associated with TOD irrespective of subtype, although TOD varied slightly across these subtypes. The study highlights the importance of controlling both daytime and night-time BP in hypertensive patients.
Introduction
The combined use of office and out-of-office blood pressure (BP) monitoring can help identify various subtypes of hypertension and improve the accuracy of hypertension diagnosis [Citation1]. Masked hypertension (MHT), firstly introduced by Thomas Pickering in 2002, was characterised as a normal office BP in the presence of an elevated out-of-office BP [Citation2]. Abundant research has demonstrated that masked hypertension, regardless of whether treated or untreated with antihypertensive drugs, was associated with target organ damage and increased risk of cardiovascular mortality and morbidity compared to consistent normotension on both office and out-of-office BP measurements [Citation3,Citation4].
Based on 24-h ambulatory BP monitoring, MHT can be further categorised as isolated daytime (dMHT), night-time (nMHT) or day-night MHT (dnMHT) according to the time when hypertension is present during a day. Up to now, no previous studies have contrasted these subtypes of MHT in their associations with subclinical target organ damage. Furthermore, inconsistent results were reported when comparing the associations of daytime and night-time BPs with target organ damage [Citation5–7] and cardiovascular outcome [Citation8–10]. Therefore, the purpose of the present study was to address whether dMHT, nMHT and dnMHT, and daytime versus night-time BPs would be associated with indices of target organ damage in a different strength. To avoid any possible confounding effect of antihypertensive drug treatment, we did this research by taking the advantage of the data from our current untreated outpatient cohort.
Methods
Study population
As previously described [Citation11,Citation12], we recruited consecutive patients referred for ambulatory BP monitoring to the Hypertension Outpatient Clinic, Ruijin Hospital, Shanghai, China, if the patients were never on antihypertensive drug treatment or had discontinued their BP lowering drugs for ≥2 weeks. We adhered to the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All patients gave informed written consent after the investigators explained the purpose, contents, risks and benefits of the research.
Of the patients referred from December 2008 to June 2015, 1859 were eligible for inclusion in the present analysis because they had both office and 24-h ambulatory BPs and at least one of the target organ damage indices measured. From the present analysis, we excluded 24 participants who had an invalid ambulatory BP recording (less than 20 h duration or less than 20 or 7 readings during awakening and asleep period, respectively), 7 patients who initiated antihypertensive drug treatment during the study examinations, and 20 patients with missing values in biochemical measurements. Thus, the number of participants analysed in this study totalled 1808.
BP measurement
Participants were asked to avoid drinking caffeine and alcohol, eating, heavy physical activity, or smoking for at least 2 h before the clinic visit. Using the Omron HEM-7051 oscillometric device (Omron HealthCare, Kyoto, Japan) [Citation13] with appropriate-sized cuffs, office BP was measured after the participants had rested in the sitting position for ≥5 min with their back and arms supported. Three consecutive BP readings at 1-min intervals were obtained at each clinic visit. The average of the nine readings at the three clinic visits within about two weeks was used for analyses. Office hypertension was an average BP of ≥140 mmHg systolic or ≥90 mmHg diastolic [Citation1].
Validated oscillometric SpaceLabs 90217 monitors (SpaceLabs, Redmond, WA, USA) [Citation14] were programmed to obtain ambulatory BP readings at 20-min intervals during 06:00–22:00 and 30-min intervals during 22:00–06:00. Participants were instructed to act and work as usual and to keep their measurement arm still and relaxed during measurements. For the present analysis, daytime was defined as 08:00–18:00 and night-time as 23:00–05:00 [Citation15]. The fixed time intervals eliminate the transition period in the morning and evening when BP changes rapidly [Citation16]. Ambulatory hypertension was a 24-h BP of ≥130 mmHg systolic or ≥80 mmHg diastolic [Citation1]. The thresholds for daytime BP were 135 mmHg systolic and 85 mmHg diastolic and for night-time 120 mmHg and 70 mmHg, respectively [Citation1].
Using above-mentioned thresholds, white-coat hypertension was defined as a raised office BP in the presence of a normal 24-h BP, MHT as an elevated 24-h ambulatory BP with normal office BP, and sustained hypertension as a consistently elevated BP on both office and ambulatory measurements. MHT was further subdivided into dMHT, nMHT, and dnMHT according to the presence of daytime and night-time hypertension.
Measurements of target organ damage
During the office visit, trained observers performed the arterial measurements in a quiet room. Using a high fidelity SPC-301 micromanometer (Millar Instruments, Houston, TX, USA) interfaced with a laptop computer running the SphygmoCor software version 7.1 (AtCor Medical, West Ryde, New South Wales, Australia), cfPWV was measured by sequential ECG-gated recordings of the arterial pressure waveform at the carotid and femoral arteries. Distances from the suprasternal notch to the carotid sampling site (distance A) and from the suprasternal notch to the femoral sampling site (distance B) were measured. Pulse wave travel distance was distance B minus distance A. Pulse transit time was the average of 10 consecutive beats. cfPWV was the distance in metres divided by the transit time in seconds [Citation17].
Carotid ultrasonography was performed at left and right common carotid arteries using an echo-tracking ultrasonic system (ArtLab, Esaote, Italy). The acquired data were analysed offline. The layers of intima and media were automatically identified via the radiofrequency signal analysis [Citation18]. The segment of the common carotid artery 1-cm proximal to the bifurcation was chosen. Average carotid intima-media thickness (IMT) of the left and right sides was used for analysis.
Two experienced doctors performed echocardiograms according to the recommendations of the American Society of Echocardiography [Citation19] using a Phillips IE33 device (Phillips, Eindhoven, The Netherlands) interfaced with a 2.5-MHz phased array probe. The measurements included interventricular septal thickness, posterior wall thickness, and end-diastole left ventricular diameter. Left ventricular mass was calculated according to the Devereux’s formula [Citation20], and indexed by estimated body surface area as left ventricular mass index (LVMI) [Citation21]. The ratio of mitral velocity to early diastolic velocity of the mitral annulus (E/E’) was derived from the tissue Doppler imaging [Citation22].
Venous blood samples, collected after overnight fasting, were analysed by automated enzymatic methods for serum glucose, cholesterol, creatinine and uric acid. Diabetes mellitus was a plasma glucose level of 7.0 mmol/L or higher or use of anti-diabetic drugs [Citation23]. Estimated glomerular filtration rate (eGFR) was calculated by the CKD-EPI creatinine equations [Citation24]. A first-morning urine sample was collected for measurement of the urinary albumin (in milligram) and creatinine (in millimoles) concentrations.
Questionnaire and other measurements
During the clinical examinations, trained technicians administered a standardised questionnaire to inquire about each participant’s medical history, intake of medications, and smoking and alcohol drinking habits. Participants wore light indoor clothing without shoes for body weight measurement. Body mass index was the body weight in kilograms divided by the body height in metres squared.
Statistical analysis
For database management and statistical analysis, we used SAS software, version 9.4 (SAS Institute, Cary, NC). For comparisons of means and proportions, we applied the analysis of variance method and the χ2 test, respectively. The urinary ACR was log-transformed for analysis. We performed Pearson correlation analysis and determined the partial correlation coefficients of target organ damage indices with daytime and night-time BPs and compared the coefficients using the Hotelling–William test. In the multivariate analyses, we considered sex, age, body mass index, 24-h heart rate, serum fasting glucose, serum total cholesterol, smoking and alcohol drinking as covariates. Statistical significance was an α-level of < 0.05 on 2-sided tests.
Results
Clinical characteristics by MHT subtype
Of the 1808 participants, 48.4% were male, 4.5% had diabetes mellitus, and 16.9% and 19.5% (n = 352) were smokers and alcohol drinkers, respectively (Supplementary Table S1). Age averaged 51.1 ± 10.6 years. The median (5th–95th percentile interval) number of readings averaged to estimate the 24-h, daytime and night-time ambulatory BPs were 60 (47–64), 27 (19–31) and 12 (10–12), respectively (Supplementary Table S2). The mean systolic/diastolic BPs were 132.6/82.2 mmHg for office measurement and 127.7/82.1 mmHg for the 24-h ambulatory measurement.
The 1808 participants consisted of 550 (30.4%) normotension, 672 (37.2%) MHT, 81 (4.5%) white-coat hypertension, and 505 (27.9%) sustained hypertension (). Compared to the normotensive subjects (p < 0.001), patients with MHT were younger (49.5 vs. 54.1 years), had greater body mass index (24.8 vs. 23.9 kg/m2), and were more likely to be male (57.7 vs. 33.8%), smokers (22.4 vs. 9.7%) and alcohol drinkers (25.2 vs. 11.5%, Supplementary Table S1). Of the 672 participants with MHT, 123 (18.3%) were dMHT, 78 (11.6%) nMHT, and 471 (70.1%) dnMHT (). Compared to patients with dMHT, patients with nMHT were older, and had similar office and 24-h BPs, and lower daytime and higher night-time BPs by definition (). Patients with dnMHT had higher office, daytime, night-time and 24-h BPs than those with dMHT and nMHT. There was no statistically significant difference (p ≥ 0.11) in body mass index, serum lipids and glucose level, sex distributions, smokers, alcohol drinkers, or diabetes mellitus among the three subtypes of MHT ().
Figure 1. Distribution of subtypes of hypertension (A) and masked hypertension (B). Values are number of patients (% of the corresponding total). MHT: masked hypertension.
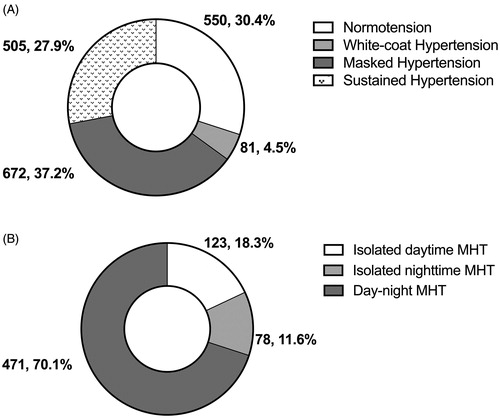
Table 1. Characteristics of the participants by subtype of masked hypertension.
Associations of MHT subtype with target organ damage
In unadjusted analyses, compared to normotensive subjects, patients with nMHT (0.77 vs. 0.62 mg/mmol) and those with dnMHT (0.69 vs. 0.62 mg/mmol) had significantly (p < 0.05) higher urinary ACR. In addition, patients with dnMHT had faster cfPWV (7.77 vs. 7.58 m/s, p = 0.015) than normotensive subjects (). No significant difference in carotid IMT, LVMI, E/E’ and eGFR was observed among the studied groups ().
Table 2. Unadjusted and adjusted means of target organ damage indices by subtype of masked hypertension.
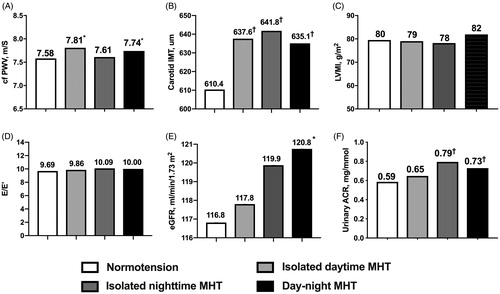
After adjustment for age, sex, body mass index, 24-h pulse rate, current smoking and alcohol drinking, serum total cholesterol and fasting glucose, compared to normotension (p < 0.05), dMHT was associated with increased cfPWV and carotid IMT, nMHT with carotid IMT and urinary ACR, and dnMHT with all TOD measures except LVMI (). After full adjustment for office systolic and diastolic BPs in addition to the covariates mentioned above, the significant associations remained unaltered except that of E/E’ with dnMHT (). cfPWV remained higher (p < 0.05) in dMHT (7.81 vs. 7.58 m/s) and dnMHT (7.74 vs. 7.58 m/s) than normotension, as did urinary ACR in nMHT (0.79 vs. 0.59 mg/mmol) and dnMHT (0.73 vs. 0.59 mg/mmol), eGFR (120.7 vs. 116.8 ml/min/1.73 m2) in dnMHT, and carotid IMT in all three subtypes of MHT (dMHT 637.6, nMHT 641.8, and dnMHT 635.1 vs. 610.4 µm). However, among the three subtypes of MHT, there was no significant difference in all indices of target organ damage after full adjustment (p ≥ 0.08).
Figure 2. Fully adjusted means of target organ damage indices according to the subtype of masked hypertension. The differences in cfPWV (A), carotid IMT (B), LVMI (C), E/E’ (D), eGFR (E) and urinary ACR (F) among patients with normotension, isolated daytime MHT, isolated night-time MHT and day-night MHT were compared using the analysis of covariance method. Means were adjusted for age, sex, body mass index, 24-h heart rate, smoking, alcohol drinking, serum total cholesterol and fasting glucose, and office systolic and diastolic blood pressure. ACR: albumin to creatinine ratio; cfPWV: carotid-femoral pulse wave velocity; IMT: intima-media thickness; LVMI: left ventricular mass index; MHT: masked hypertension. Compared to normotension, ∗p < 0.05, †p < 0.01, ‡p < 0.001.
Associations of daytime and night-time BPs with target organ damage
In all 1808 participants, daytime and night-time BPs, were similarly (p ≥ 0.20) and significantly (p < 0.05) associated with all target organ measures after multivariate adjustment except that daytime and night-time diastolic BPs were not associated with eGFR (Supplementary Table S3). In patients with office normotension (n = 1222, ), the correlations remained significant (p < 0.05) except those of daytime systolic BP with eGFR and daytime diastolic BP with cfPWV and carotid IMT. The significant partial correlation coefficients ranged from 0.07 to 0.24, and were similar between daytime and night-time BPs (p ≥ 0.30).
Table 3. Multivariate-adjusted correlation of target organ damage indices with daytime and night-time blood pressures.
Discussion
Using the data of an outpatient population not on antihypertensive medication, our study demonstrated that among the patients with masked hypertension, the proportions of dMHT, nMHT and dnMHT were about 20%, 10% and 70%, respectively, and all three MHT subtypes were associated with target organ damage, although the damages varied slightly across subtype. More importantly, daytime and night-time BPs, when analysed as continuous variables, significantly and similarly associated with target organ indices in all participants as well as in patients with office normotension.
MHT is a special subtype of hypertension that carries a cardiovascular risk between normotension and sustained hypertension [Citation3,Citation25,Citation26]. MHT is often considered as a forerunner of sustained hypertension. Indeed, MHT has a 2- to 4-fold risk of developing clinic [Citation27] or sustained hypertension [Citation28,Citation29] relative to normotension. The prevalence of MHT is approximately 10–20% in the general population [Citation25–30] and 20–50% in individuals with high-normal office BP, diabetes mellitus, chronic kidney disease, or obesity [Citation31–34]. In the current study, the prevalence of MHT was 37%, a little higher than in previous reports in populations not on antihypertensive medication. Possible reasons might include that our study population was relatively young, about half had high-normal office BP, and office BP was the average of 9 readings taken at three visits.
MHT may consist of individuals with heterogeneous characteristics on ambulatory BP measurement. Manios et al. [Citation35] classified MHT into three subgroups according to the daytime systolic and diastolic BP status, and found that patients with isolated systolic MHT (n = 36, IMT = 0.771 mm) or systolic and diastolic combined MHT (n = 37, IMT = 0.775 mm) had significantly thicker carotid IMT than those with isolated diastolic MHT (n = 28, IMT = 0.664 mm) after adjustment for age and other common risk factors. Among 2628 untreated adults, Presta et al. [Citation36] classified 153 patients with MHT into three groups according to the BP dipping status during night-time, i.e. dippers, non-dippers, and reverse dippers, and found that after an average follow-up of 9.8 years, reverse dippers (n = 21) had a significantly higher risk of stroke than dippers (n = 65) after multivariate correction. However, due to the small sample-size as well as limited number of events, this study did not allow any definite conclusion on the clinical relevance of different forms of MHT in terms of cardiovascular prognosis.
In our study, we classified MHT according to the time when ambulatory hypertension is present. The rationale is that the underlying pathophysiological mechanisms and treatment strategies between daytime and night-time hypertension might be different. As discussed by Kawano et al. [Citation37] and Cuspidi et al. [Citation7], daytime hypertension is often associated with an exaggerated BP reactivity to environmental stimuli and lifestyle-related factors such as habitual smoking [Citation38] and daily stress, whereas night-time hypertension and the non-dipping pattern is often seen in salt-sensitive subjects on a high salt diet or in patients with chronic kidney diseases, sleep apnoea or autonomic failure. Accordingly, smoking cessation and control of daily stress are usually recommended for daytime hypertension, whereas salt restriction and the use of long-acting antihypertensive drugs might be extremely important for night-time hypertension.
Up to now, there is a large body of evidence that ambulatory BP is more closely associated with target organ damage and cardiovascular outcome than office BP [Citation3,Citation10,Citation11,Citation25,Citation26,Citation28]. It is therefore not surprising to see that MHT, characterised as an elevation of ambulatory BPs in this study, was associated with target organ damage irrespective of subtype. It is the absolute ambulatory BP level, rather than arbitrary BP categorisation, driving the significant findings. Indeed, as shown in , patients with dMHT had higher night-time BP, and patients with nMHT had higher daytime BP than normotensive subjects.
Isolated nocturnal hypertension was firstly introduced by us and was shown to be associated with increased arterial stiffness [Citation39] and risk of total mortality and cardiovascular events in a similar extent as isolated daytime hypertension [Citation40]. Among patients enrolled in the Pressioni Arteriose Monitorate E Loro Associazioni study, Cuspidi et al. [Citation7] also found that patients with isolated night-time hypertension or isolated daytime hypertension had similar LVMI (89 ± 18 or 90 ± 20 g/m2), intermediate between normotensive (82 ± 19 g/m2) and day-night hypertensive patients (99 ± 24 g/m2). Taken these findings together with our present observations in masked hypertensive patients, we may conclude that isolated BP elevation during the night-time or daytime cannot be regarded as an innocent phenomenon, even when office BP is normal.
Nevertheless, the results of the comparisons between daytime and night-time BPs, analysed as continuous variables, in their associations with target organ damage or clinical hard outcomes had been inconsistent between studies [Citation5–10]. A meta-analysis in 7458 people enrolled in prospective general population studies showed that both daytime and night-time BPs consistently predicted all fatal and non-fatal cardiovascular events [Citation8]. Whereas a subsequent meta-analysis involving patients with hypertension (n = 23,856) and individuals randomly recruited from populations (n = 9641) showed that night-time BP was a stronger predictor of total mortality and composite cardiovascular events than daytime BP [Citation9]. Inconsistent results were also seen when comparing the associations of daytime and night-time BPs with target organ damage [Citation5–7]. At difference from some [Citation6,Citation9] but not all [Citation5,Citation7] previous studies, our present study showed that both daytime and night-time BPs were similarly associated with target organ indices. In the 854 patients in the Japan Morning Surge-Home Blood Pressure Study [Citation5], the correlation coefficients of urinary ACR with night-time and awake systolic BP were similar on both home BP monitoring (p = 0.70) and ambulatory BP monitoring (p = 0.47). While in 305 nondiabetic hypertensive subjects in a prospective study [Citation6], night-time systolic BP, but not daytime systolic BP, was a significant predictor of the development and persistence of left ventricular hypertrophy after a mean of 42 ± 17 months follow-up. These different findings might be attributable to various characteristics of the populations, i.e. risk factors or diseases clustered with daytime or night-time hypertension.
Our study must be interpreted within the context of its potential limitations and strengths. First, the study subjects did not take any antihypertensive medication during the study, which may rule out any possible confounding effect of drug treatment. On the other hand, our results might not be directly applied to those treated patients with masked uncontrolled hypertension. Second, we stuck to the recommendations [Citation1] and reported the quality measures of ambulatory monitoring in detail. Nonetheless, 24-h ambulatory BP monitoring was performed only once. According to our previous data in a randomised clinical trial, MHT persisted over 4 weeks in over two-thirds of patients taking placebo [Citation41]. Finally, as all the other cross-sectional studies, our findings need to be replicated in future prospective studies.
Conclusions
All subtypes of MHT were associated with target organ damage. Daytime and night-time BPs were equally and independently associated with all target organ measures. Isolated BP elevation during daytime or night-time cannot be deemed as an innocent phenomenon. Our study highlights the importance of controlling both daytime and night-time BP in hypertensive patients.
mhttod_spl20200405.docx
Download MS Word (28.2 KB)Acknowledgements
The authors gratefully acknowledge the technical assistance of Jingjing Li, Junwei Li, Beiwen Lv, Jiaye Qian, Yuzhong Shi, Qian Yu, Jie Zhou, Yi Zhou, Yini Zhou, and Jiajun Zong (Shanghai Institute of Hypertension, Shanghai).
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27:314–340.
- Pickering TG, Davidson K, Gerin W, et al. Masked hypertension. Hypertension. 2002;40:795–796.
- Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675–682.
- Zhang DY, Guo QH, An DW, et al. A comparative meta-analysis of prospective observational studies on masked hypertension and masked uncontrolled hypertension defined by ambulatory and home blood pressure. J Hypertens. 2019;37:1775–1785.
- Ishikawa J, Hoshide S, Eguchi K, et al.; for the Japan Morning Surge-Home Blood Pressure Study Investigators Group. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921–928.
- Andrikou I, Tsioufis C, Thomopoulos C, et al. Nighttime vs. daytime blood pressure as a predictor of changes in left ventricular mass in hypertensive subjects. Hypertens Res. 2013;36:967–971.
- Cuspidi C, Facchetti R, Bombelli M, et al. Is night-time hypertension worse than daytime hypertension? A study on cardiac damage in a general population: the PAMELA study. J Hypertens. 2017;35:506–512.
- Boggia J, Li Y, Thijs L, et al.; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229.;
- Hansen TW, Li Y, Boggia J, et al. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10.
- Yang WY, Melgarejo JD, Thijs L, et al.; for The International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420.
- Wei FF, Li Y, Zhang L, et al. Association of target organ damage with 24-hour systolic and diastolic blood pressure levels and hypertension subtypes in untreated Chinese. Hypertension. 2014;63:222–228.
- Wang Y, Zhang DY, Guo QH, et al. Short-term reproducibility of the 24-h ambulatory monitoring of brachial and central hemodynamics in untreated Chinese. Blood Press. 2019;28:250–257.
- Coleman A, Freeman P, Steel S, et al. Validation of the Omron 705IT (HEM-759-E) oscillometric blood pressure monitoring device according to the British Hypertension Society protocol. Blood Press Monit. 2006;11:27–32.
- Baumgart P, Kamp J. Accuracy of the SpaceLabs Medical 90217 ambulatory blood pressure monitor. Blood Press Monit. 1998;3:303–307.
- Zhang L, Li Y, Wei FF, et al. Strategies for classifying patients based on office, home, and ambulatory blood pressure measurement. Hypertension. 2015;65:1258–1265.
- Fagard R, Brguljan J, Thijs L, et al. Prediction of the actual awake and asleep blood pressures by various methods of 24 h pressure analysis. J Hypertens. 1996;14:557–563.
- Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722.
- Di Geso L, Zardi EM, Afeltra A, et al. Comparison between conventional and automated software-guided ultrasound assessment of bilateral common carotids intima-media thickness in patients with rheumatic diseases. Clin Rheumatol. 2012;31:881–884.
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367.
- Devereux RB, Koren MJ, de Simone G, et al. Methods for detection of left ventricular hypertrophy: application to hypertensive heart disease. Eur Heart J. 1993;14:8–15.
- LaBounty TM, Bach DS, Bossone E, et al. Indexing left ventricular wall thickness to body surface area improves prognostic value. Echocardiography. 2019;36:824–830.
- De Sutter J, De Backer J, Van de Veire N, et al. Effects of age, gender, and left ventricular mass on septal mitral annulus velocity (E’) and the ratio of transmitral early peak velocity to E’ (E/E). Am J Cardiol. 2005;95:1020–1023.
- American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–S27.
- Inker LA, Schmid CH, Tighiouart H, et al.; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29.;
- Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508–515.
- Hansen TW, Kikuya M, Thijs L, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25:1554–1564.
- Abdalla M, Booth JN, 3rd, Seals SR, et al. Masked hypertension and incident clinic hypertension among Blacks in the Jackson Heart Study. Hypertension. 2016;68:220–226.
- Sivén SS, Niiranen TJ, Kantola IM, et al. White-coat and masked hypertension as risk factors for progression to sustained hypertension: the Finn-Home study. J Hypertens. 2016;34:54–60.
- Mancia G, Bombelli M, Facchetti R, et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension. 2009;54:226–232.
- Wang GL, Li Y, Staessen JA, et al. Anthropometric and lifestyle factors associated with white-coat, masked and sustained hypertension in a Chinese population. J Hypertens. 2007;25:2398–2405.
- Franklin SS, Thijs L, Li Y, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964–971.
- Gorostidi M, Sarafidis PA, de la Sierra A, et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: A 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis. 2013;62:285–294.
- Drager LF, Diegues-Silva L, Diniz PM, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23:249–254.
- Shimbo D, Newman JD, Schwartz JE. Masked hypertension and prehypertension: diagnostic overlap and interrelationships with left ventricular mass: the Masked Hypertension Study. Am J Hypertens. 2012;25:664–671.
- Manios E, Michas F, Stamatelopoulos K, et al. Association of isolated systolic, isolated diastolic, and systolic-diastolic masked hypertension with carotid artery intima-media thickness. J Clin Hypertens. 2015;17:22–26.
- Presta V, Figliuzzi I, D'Agostino M, et al. Nocturnal blood pressure patterns and cardiovascular outcomes in patients with masked hypertension. J Clin Hypertens. 2018;20:1238–1246.
- Kawano Y, Horio T, Matayoshi T, et al. Masked hypertension: subtypes and target organ damage. Clin Exp Hypertens. 2008;30:289–296.
- Zhang DY, Huang JF, Kang YY, et al. The prevalence of masked hypertension in relation to cigarette smoking in a Chinese male population. J Hypertens. 2020.
- Li Y, Staessen JA, Lu L, et al. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50:333–339.
- Fan HQ, Li Y, Thijs L, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045.
- Wei FF, Li Y, Zhang L, et al. Persistence of masked hypertension in Chinese patients. Am J Hypertens. 2016;29:326–331.
