Abstract
Purpose: Toxicological screenings for identifying antihypertensive drugs proved to be a useful tool for assessing adherence. However, misinterpretation may occur in case of highly metabolised drugs with low renal excretion, as well as for drugs with a prolonged detectability. The aim of the present study was to compare a recently developed therapeutic drug monitoring (TDM) method based on serum concentrations to an urine drug detection method for assessing adherence in outpatients.
Materials and methods: Corresponding urine and blood samples were obtained at the same time from 26 outpatients without supervised medication. Urine and serum analyses were performed using established high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodologies. Adherence was assumed if drugs were detectable in urine or if serum concentrations were above individually calculated lower dose-related concentrations (DRC) or literature-based therapeutic reference ranges (TRR) used as cut-off, respectively.
Results: The identification of analytes in urine as well as the quantitative serum assay were performed for atenolol (n = 6 patients), bisoprolol (n = 8), nebivolol (n = 6), canrenone (n = 6, metabolite of spironolactone), hydrochlorothiazide (n = 12) and furosemide (n = 2). On the basis of drug detectability in urine, adherence was assumed in 88% of prescriptions. In 81% (DRC) and 50% (TRR) of the serum analyses the cut-off value was exceeded, which confirms patients’ adherence in a lower number. Differences in adherence rates were found in five patients, mainly for β-blockers.
Conclusion: This study suggests that assessment of adherence can be performed more precisely on the basis of serum drug concentrations with individually calculated lower DRC than by using the TRR or qualitative urinalysis.
Introduction
Arterial hypertension is still the leading factor in cardiovascular morbidity and mortality. It is a well treatable disease regarding the wide range of pharmacological treatment options. Non- or poor adherence to therapy makes it difficult to achieve satisfactory blood pressure control, significantly increases the cardiovascular risk [Citation1–3] and may cause treatment resistance [Citation4] to a considerable degree [Citation5]. Non-adherence is estimated in a dimension of 30–50% [Citation5]. Therefore, it is important to exclude non-adherence as a cause of pseudo-resistant hypertension. Drug adherence can either be assessed indirectly (i.e. self-reporting, prescription records, pill count, electronic monitoring systems) or by direct methods (toxicological analyses) [Citation6]. Indirect methods may be affected by manipulations and are therefore limited in their reliability to detect non-adherence [Citation7]. Direct methods are objective but detectability of drugs and conclusions on adherence may be affected by pharmacokinetic aspects.
Reports of adherence rates on the basis of different methodologies show discrepancies. The evaluation of medication refill data by Kronish et al. [Citation8] shows poor adherence for diuretics (51%) and β-blockers (28.4%). This is in contrast with data obtained by toxicological screenings where high adherence rates are consistently reported for these classes (β-blockers > 90.4%, diuretics > 81.9%) [Citation9,Citation10]. While indirect methods cannot confirm actual drug ingestion, the qualitative nature of toxicological analyses may lead to overestimation of adherence in case of substances with long excretion times, e.g. of some diuretics and β-blockers. Therefore, setting up a therapeutic drug monitoring (TDM) system using pharmacokinetic data to establish calibration ranges to classify adherence seems to be a diagnostic advancement [Citation11]. Nevertheless, this approach lacks reliable cut-off values to assess adherence. The same applies to a recently published study, in which patients’ adherence was assessed based on concentration ratios (CR). These CR cut-offs have not been investigated in patients with monitored drug ingestion and no specific threshold has been defined [Citation12].
Recently, a TDM methodology was further refined by using lower dose-related concentrations (DRC) and therapeutic reference ranges (TRR) as cut-offs to assess patients’ adherence [Citation13]. The DRC concept seemed to be reliable in patients with confirmed adherence, whereas using TRR tended to underestimate adherence. The present study was performed to compare this new methodology in a number of outpatients without supervised adherence in comparison to an established qualitative urine drug screening to show relevant methodological limitations.
Materials and methods
Study design
The study was carried out at the hypertension clinic of the Division of Cardiology, Cliniques Universitaires Saint-Luc (UCL, Brussels, Belgium). Patients (11 males, 15 females) aged 36–75 (median 61) years, with a median height of 1.71 (range 1.57–1.92) m, a median body weight of 79 (range 50–111) kg, a median body mass index of 27.7 (range 17.1–39.8) kg/m2, a median office blood pressure of 138/79 (range 100/50–195/133) mmHg and a median eGFR of 66.0 (range 36.0–106.5) mL/min/1.73 m2 were part of the study. They were treated with in median 3 (range 1–7, inter-quartile range 2–4) different antihypertensive drugs without supervising their medication intake. From these patients corresponding spot blood and urine samples were obtained at the same visit for toxicological analyses. After centrifugation (3500 × g for 10 min) the serum was separated. All samples were continuously stored at –20 °C to guarantee stability of the analytes [Citation13]. For data evaluation information on medication regimen (drug, dose, dosing interval), times of last scheduled drug intake and of blood and urine sampling were provided. The study was approved by the competent ethics committee and a written informed consent was obtained from the participants on the day of blood and urine sampling.
Toxicological analyses
The analyses and evaluations were essentially performed as previously described [Citation10,Citation13]. In short, an ethyl acetate extraction (1 mL) of 200 µL aliquots of urine was performed after adding internal standards. The content was mixed for 2 min and centrifuged at 13,000 × g for 10 min. The organic phase was then transferred to a silanized glass tube and evaporated. For analysis, the dry residue was reconstituted with 100 µL 0.1% formic acid/acetonitrile (80:20, v/v).
Serum samples were extracted in two steps, where 1 mL acidic ethyl acetate (0.05% formic acid in ethyl acetate) was first mixed with 200 µL of serum and the supernatant decanted. The aqueous phase was extracted again using 1 mL basic ethyl acetate (1.25% ammonia in ethyl acetate) and the supernatant was evaporated together with the acidic extract.
The analyses of blood and urine extracts of antihypertensive drugs were done by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) [Citation10,Citation13]. For hydrochlorothiazide (HCT) and furosemide, negative electrospray interface (ESI) mode was used, atenolol, bisoprolol, metoprolol, nebivolol, canrenone and torasemide were analysed in positive ESI mode. The analysis was performed on an Agilent (Waldbronn, Germany) LC-MS/MS system consisting of a 1290 Infinity Liquid Chromatograph coupled via JetStream Electrospray Interface (ESI) to a G6460A Triple Quadrupole Mass Spectrometer. Analytes were separated on a Kinetex® 2.6 µm XB-C18 100 Å LC column (30 × 2.1 mm) equipped with a guard column from Phenomenex (Aschaffenburg, Germany) at 55 °C. Detection was performed in the dynamic multiple reaction monitoring mode (dMRM) with at least one transition for internal standards and two for analytes. Data acquisition and evaluation was performed using Agilent MassHunter Software (version B.07.00). Analytes were considered detected with a deviation of retention time of less than 0.05 min and a qualifier to quantifier ratio below 20% deviation compared to reference standards.
Urine
For quality control, drug-free urine was used for preparing negative and positive control samples. The latter was spiked with all the reference substances. Analytical detection of the drug or metabolite in urine was considered an indicator of drug adherence.
Serum
For preparation of quality controls, calibration standards and blank sample drug-free serum was used. The calibration ranged from 0.1–20 ng/mL for nebivolol, 2.5–100 ng/mL for bisoprolol, 1–200 ng/mL for metoprolol, 5–1000 ng/ml for atenolol, canrenone, furosemide and HCT and 10–2000 ng/mL for torasemide.
For evaluation of adherence the measured concentrations were compared with the lower limit of published therapeutic reference ranges (TRR) [Citation14,Citation15] and with individually calculated lower limits of the dose-related concentration (DRC), which are considered as promising cut-off for differentiating adherence and non-adherence as recently described by Ritscher et al. [Citation13]. The DRC was individually calculated on the basis of the patient’s drug doses and the drug’s lower DRC factor (). The lower DRC is based on the trough serum concentration taking into account the dosing interval (τ) and the time until blood sampling (Δt). Furthermore, it includes a diminution by one standard deviation of the apparent total clearance to reflect interindividual variations in excretion. Concentrations above these cut-offs (TRR, DRC) were considered as indicator of adherence to the respective drug.
Table 1. Serum concentrations and qualitative results in urine from corresponding spot samples of outpatients regarding β-blockers and diuretics.
Results
In this study urine and serum samples of 26 patients on β-blockers and/or diuretics were evaluated. Six patients were treated with atenolol, eight with bisoprolol, six with nebivolol, twelve with HCT, two with furosemide and six with spironolactone (analysed and evaluated as its active metabolite canrenone). In total this were 40 drug determinations (prescriptions, drugs × patients).
Urine samples
The results of urine analyses () suggest adherence to atenolol, bisoprolol and HCT in all patients. In one urine sample nebivolol was not found (16.7% of nebivolol treated patients), in another one no furosemide was detected (50%) and in two samples expected canrenone was absent (33.3%). Adherence to β-blocker and diuretic medication was assumed in 23 (88.5%) and non-adherence (one or more prescribed drugs not detected) in three patients (11.5%).
Serum samples
Serum concentrations of positive samples ranged from 64.7–564.2 (median 153.1) ng/mL for atenolol, 2.5–39.8 (median 15.2) ng/mL for bisoprolol, 0.32 to 3.48 (median 0.52) ng/mL for nebivolol, 28.6 to 331.0 (median 75.8) ng/mL for HCT, 43.4 to 221.2 (median 69.6) ng/mL for canrenone (administered as spironolactone) and 22.6 ng/mL for furosemide.
For evaluation [Citation13] one approach is the comparison of serum concentrations with published therapeutic ranges [Citation14,Citation15] Only for nebivolol all values were within the therapeutic reference range (any concentration below 20 ng/mL), but a number of concentrations were below the published ranges (). Below were both furosemide values (<2000 ng/mL), most canrenone concentrations (83.3%, five patients <100 ng/mL), a number of atenolol (66.7%, four patients <200 ng/mL) and bisoprolol patients (62.5%, five patients <10 ng/mL) and some HCT patients (25%, three patients <40 ng/mL). In only one patient the atenolol concentration exceeded the higher limit (>500 ng/mL). Using the lower limit of the therapeutic reference range as cut-off for assessing adherence, patients’ adherence to the measured drugs would be assumed in only 13 (50%) of the 26 patients.
Figure 1. Proportion of patients considered adherent according to the lower dose-related concentration (DRC, serum), the lower limit of therapeutic reference ranges (TRR, serum) and the detectability in the qualitative urinalysis. Data are given for each antihypertensive drug and for the total adherence.
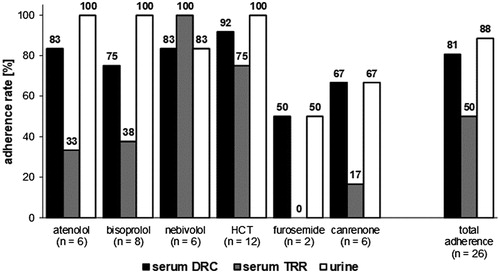
The comparison of the quantitative serum data with the lower limits of the dose related concentrations (c.f. ) as indicator of medication adherence suggests that only one patient (50%) adhered to furosemide, four (66.7%) to spironolactone, six (75%) to bisoprolol, five (83.3%) to atenolol, five (83.3%) to nebivolol and eleven (91.7%) to HCT. In combination, 21 patients (80.8%) would be considered as adherent to their β-blocker and diuretic medication.
Urine vs serum samples
To compare the results of urine and serum samples, the individually calculated lower DRC was used as cut-off criterion which is considered superior to comparison with published therapeutic reference ranges [Citation13–15]. In terms of adherence assessment, the qualitative urine screening result agreed with the quantitative serum assay for furosemide and canrenone. However, this does not apply to the β-blockers and HCT. Overall one patient (8.3%) on HCT, one patient (16.7%) on atenolol, one patient (16.7%) on nebivolol and two patients (25%) on bisoprolol were rated as adherent according to the urine screening whereas the quantitative serum results suggested a non-adherence. In contrast, one patient (16.7%) on nebivolol was classified as non-adherent according to the urinalysis and as adherent regarding the DRC approach ().
Discussion
As explained in the introduction, hypertension is a well treatable disease but affected by a considerable degree of non-adherence [Citation5]. Indirect methods to assess adherence may be affected by manipulations and are therefore not ultimately reliable [Citation7], but also direct methods may have limitations. One of these are pharmacokinetic aspects which were subject to the present study.
Some substances are metabolised to a large extent and are hardly excreted unchanged via the kidneys (dihydropyridine derivatives [Citation16,Citation17], nebivolol [Citation18]). With toxicological analyses of urine this might lead to misclassification as non-adherent. On the other hand substances with prolonged excretion may still be detectable in urine (e.g. HCT [Citation19]) despite poor adherence, leading to an overestimation of adherence. In order to reduce misinterpretation a quantitative serum assay was evaluated as established for psychiatric TDM [Citation20]. This was shown to be reliable in a number of patients with supervised medication adherence [Citation13] and was now applied to blood samples from outpatients who also provided urine samples allowing the comparison of the performance of both methods. The 26 patients of the present study were treated with in median three different drugs. Since the analyses only looked at β-blockers and diuretics, adherence rates referring to individual patients should not be taken as representative for adherence to all drugs of the medication regimen.
Concentrations in serum
The concentrations measured above the respective lower DRC () are in accordance with those found in the previous evaluation study including only patients with confirmed adherence [Citation13] and with ranges reported in other studies [Citation11,Citation21–23].
Assessment of adherence on the basis of serum concentrations
The lower DRC was used as cut-off for differentiation of adherence. This value is calculated individually for different dosing regimens based on the concept of Hiemke et al. [Citation13,Citation20]. Overall, eight of 40 values (20%) were beneath the individual lower limit of the dose-related reference ranges which indicates that the respective drug was not ingested as required (non-adherence) or that the subject exhibited a deviation in typical pharmacokinetics.
As found in the previous study [Citation13] a rather high proportion of the measured concentrations (50%) failed to comply with published reference data on therapeutic concentrations. Of the 40 determined values 19 (47.5%) fell below the lower limit of the concentration range considered therapeutic [Citation14,Citation15], because these ranges may not reflect concentrations of low doses as part of complex antihypertension medication regimens [Citation13]. This relates especially to the low doses of the β-blocker bisoprolol as well as the diuretics furosemide and spironolactone (assayed via its active metabolite canrenone) (). Therefore, the present study suggests that the evaluation of adherence using lower DRCs is the more appropriate approach.
Assessment of adherence on the basis of qualitative urine analysis results
In the present group of outpatients, adherence rates for the two investigated antihypertensive drug classes according to detection in urine samples were high (95% for β-blockers and 80% for diuretics) which is in accordance with results of previous studies using qualitative toxicological analysis to assess adherence [Citation9,Citation10,Citation24]. Overall, in only 3 of 26 patients (11.5%) non-adherence would have been assumed.
Discrepancy between urine and serum
Adherence rates differ between classes of antihypertensive medications and between different methods of adherence assessment. Apart from indirect methods qualitative analysis methods may also be imprecise. This is supported by the low adherence as found for diuretics and β-blockers based on medication refill data [Citation8] while data from studies using urine or plasma analysis suggests high adherence rates [Citation9,Citation24]. In the evaluation of this discrepancy it must be taken into account that current toxicological methods are qualitative in nature and may still be positive even if some time passed since the last drug ingestion due to the high sensitivity of the applied analytical methods. Therefore, the current study was performed to evaluate a more sophisticated and improved method to detect ‘pseudo-resistant hypertension’ caused by non-adherence.
The present data showed that the results of urine and serum analysis did not match in the case of atenolol, bisoprolol, nebivolol and HCT medication. Atenolol may have an extended detectability in urine due to the fact that the drug is predominantly (95%) eliminated via the kidneys and that the typical elimination half-life of 6–9 h may be increased up to 36 h in renally impaired individuals [Citation21]. This would especially apply to patient #1 () with an eGFR of 42.17 mL/min/1.73 m2 (CKD-EPI) having a moderate reduction of renal function (CKD stage G3b). It is not surprising that atenolol is still detectable in the urine sample even despite occasional non-adherence. Detectability in urine over several dosing intervals can also be expected for bisoprolol and HCT. Approximately 90% of bisoprolol are bioavailable after oral administration and approximately 50% of the administered dose is excreted renally unchanged with an elimination half-life of 9 to 12 h [Citation25] and more than 95% of the absorbed HCT (approx. 70%) is eliminated unchanged via the kidneys [Citation26,Citation27] with an elimination half-life of approximately 10 h [Citation23]. Therefore, high renal excretion rates may occur despite acute non-adherence and may lead to misinterpretation of adherence (). The urine analyses lead to the conclusion that all patients on atenolol, bisoprolol and HCT were adherent to the drugs while the serum analyses in four of 26 cases (15.4%) revealed concentrations lower than the respective lower DRC. This suggests that one or more dosing intervals have been skipped, indicating at least partial non-adherence. This illustrates, that urine assays are surely capable of reliably detecting non-adherence over several dosing intervals but that serum analyses may be more sensitive in detecting acute omission of ingestion of prescribed drugs.
Figure 2. Analytical data (extracted ion chromatograms, equally scaled for direct comparison) of nebivolol in serum and urine. Patient #23, #24 and #26 exhibited similar serum concentrations and were considered adherent according to the DRC approach. In comparison, the urinalysis was considered positive for patients #23 and #24 but negative for patient #26.
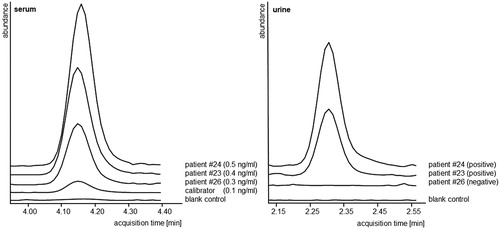
With qualitative urine assays not only an overestimation of adherence is possible, but also an underestimation in cases of analytes which are highly metabolised and hardly excreted via the kidneys. For instance, nebivolol is highly metabolised and less than 0.5% of the unchanged drug is eliminated renally [Citation18] and might therefore not be detected in urine assays. In the present urine samples, nebivolol was not detected in one sample (patient #26, ), which would consequently result in classification of this patient as non-adherent for this drug. In contrast, this patient exhibited a serum concentration above the respective lower DRC for nebivolol (), suggesting typical pharmacokinetics and adherence. Though the applied analytical method is highly sensitive and able to detect nebivolol in patients’ urine samples [Citation10] (c.f. ) the results of patient #26 suggest a limitation. An improvement of the urine method could be the inclusion of metabolites [Citation28] but overall, the present approach of analysis in serum is superior in targeting the specific active drugs and may lead to a better assessment of drug adherence. However, the issue remains that adherence is a constantly changing process [Citation29], which cannot be covered by these direct methods. In particular, acute improvements in drug adherence, like the ‘white coat adherence’ phenomenon, cannot be differentiated and may lead to false assumptions.
The present study was performed to evaluate quantitative serum drug concentrations in comparison to a urine assay with respect to adherence assessment. Taken together, these results suggest that drugs with high bioavailability, long half-lives and renal excretion rates as well as highly metabolised substances, which are hardly excreted via the kidneys, may lead to over- or underestimation of adherence via urine analysis. The approach of assessing adherence on the basis of individually calculated lower DRCs as cut-off for serum concentrations might be superior to qualitative urine analysis regarding those drugs. The application of this approach to a wider range of antihypertensive drugs should be investigated in further studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Shin S, Song H, Oh S-K, et al. Effect of antihypertensive medication adherence on hospitalization for cardiovascular disease and mortality in hypertensive patients. Hypertens Res. 2013;36(11):1000–1005.
- Dragomir A, Côté R, Roy L, et al. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Medical Care. 2010;48:418–425.
- Corrao G, Parodi A, Nicotra F, et al. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29(3):610–618.
- Mennini FS, Marcellusi A, Schulenburg J., et al. Cost of poor adherence to anti-hypertensive therapy in five European countries. Eur J Health Econ. 2015;16(1):65–72.
- Sabaté E. Adherence to long-term therapies: evidence for action [Internet] [cited 2018 Dec 14]. Available from: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1.
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–1090.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497.
- Kronish IM, Woodward M, Sergie Z, et al. Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123(15):1611–1621.
- Ewen S, Meyer MR, Cremers B, et al. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol. 2015;104(12):1097–1105.
- Schmieder RE, Ott C, Schmid A, et al. Adherence to antihypertensive medication in treatment-resistant hypertension undergoing renal denervation. J Am Heart Assoc. 2016;5:e002343.
- Gundersen POM, Helland A, Spigset O, et al. Quantification of 21 antihypertensive drugs in serum using UHPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1089:84–93.
- Punt AM, Stienstra NA, van Kleef MEA, et al. Screening of cardiovascular agents in plasma with LC-MS/MS: a valuable tool for objective drug adherence assessment. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1121:103–110.
- Ritscher S, Opper M, Wunder C, et al. Evaluation of the dose-related concentration approach in therapeutic drug monitoring of diuretics and β-blockers – drug classes with low adherence in antihypertensive therapy. Scientific Reports. 2019;9:15652.
- Schulz M, Iwersen-Bergmann S, Andresen H, et al. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit Care. 2012;16(4):R136–134.
- Repetto MR, Repetto M. Therapeutic, toxic, and lethal concentrations in human fluids of 90 drugs affecting the cardiovascular and hematopoietic systems. Clin Toxicol. 1997;35(4):345–351.
- Barchielli M, Dolfini E, Farina P, et al. Clinical pharmacokinetics of lercanidipine. J Cardiovasc Pharmacol. 1997;29:S1–S15.
- Kleinbloesem CH, van Harten J, van Brummelen P, et al. Liquid chromatographic determination of nifedipine in plasma and of its main metabolite in urine. J Chromatogr B Biomed Sci Appl. 1984;308:209–216.
- Moen MD, Wagstaff AJ. Nebivolol: a review of its use in the management of hypertension and chronic heart failure. Drugs. 2006;66(10):1389–1409; discussion 1410.
- Barbhaiya RH, Craig WA, Perri Corrick-West H, et al. Pharmacokinetics of hydrochlorothiazide in fasted and nonfasted subjects: a comparison of plasma level and urinary excretion methods. J Pharm Sci. 1982;71(2):245–248.
- Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–02):9–62. [cited 2018 Nov 21]
- Kirch W, Görg KG. Clinical pharmacokinetics of atenolol–a review. Eur J Drug Metab Pharmacokinet. 1982;7(2):81–91.
- Haegeli L, Brunner-La Rocca HP, Wenk M, et al. Sublingual administration of furosemide: new application of an old drug. Br J Clin Pharmacol. 2007;0(0):070917231016006–070917231016809.
- Vespasiano CFP, Laurito TL, Iwamoto RD, et al. Bioequivalence study between a fixed-dose single-pill formulation of nebivolol plus hydrochlorothiazide and separate formulations in healthy subjects using high-performance liquid chromatography coupled to tandem mass spectrometry. Biomed Chromatogr. 2017;31(5):e3884–9.
- Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31(4):766–774.
- Lancaster SG, Sorkin EM. Bisoprolol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in hypertension and angina pectoris. Drugs. 1988;36(3):256–285.
- Beermann B, Groschinsky-Grind M. Pharmacokinetics of hydrochlorothiazide in man. Eur J Clin Pharmacol. 1977;12(4):297–303.
- Beermann B, Groschinsky-Grind M, Rosén A. Absorption, metabolism, and excretion of hydrochlorothiazide. Clin Pharmacol Thera. 1976;19(5part1):531–537.
- Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100(11):855–861.
- Wunder C, Persu A, Lengelé J-P, et al. Adherence to antihypertensive drug treatment in patients with apparently treatment-resistant hypertension in the INSPiRED pilot study. Blood Press. 2019;28(3):168–172.
