Abstract
Purpose: Outcome after ischaemic stroke (AIS) depends on multiple factors, including values of blood pressure (BP) and arterial stiffness (AS) in the early phase. It is also known that stroke outcome is affected by BP variability; however, the influence of AS oscillations in the early phase of stroke on its prognosis is unknown. The aim of our study was to assess the relationship between changes of AS markers and stroke outcome.
Materials and methods: Baseline clinical data, BP parameters, and markers of AS (pulse wave velocity [PWV], augmentation index [AIx]) were assessed 1, 6, and >90 days after AIS. The outcomes were defined using modified Rankin scale (mRS) score: early favourable (EFO) and early poor (EPO), as mRS ≤1 and >2 points at discharge, respectively; late favourable (LFO) and late poor (LPO), as mRS ≤1 and >2 points on day >90, respectively.
Results: In the recruited 50 patients (62.2 ± 12.1 years, 68% males), BP and PWV decreased while AIx did not change within 90 days after AIS. Twenty-eight patients (56%) had EFO, 10 (20%) – EPO, 29 (58%) – LFO, and 9 (18%) – LPO. In univariate analysis, rise in AIx in days 1–6 was associated with EFO (odds ratio [OR] = 1.09, 95% confidence interval [CI] = 1.02–1.17, p = 0.01) and LFO (OR = 1.08; 95%CI = 1.01–1.14, p = 0.02), whereas decrease in AIx in days 1–6 was associated with EPO (OR = 1.07, 95%CI = 1.00–1.15, p = 0.05). For EFO and LFO, the relationships remained significant after including confounders (p = 0.03 and p = 0.03, respectively).
Conclusions: Rise in AIx within one week after ischaemic stroke may be of additional importance in determining better early and late favourable functional outcome.
Introduction
Cerebrovascular diseases are the leading contributors of death worldwide with stroke being the second most common cause of mortality [Citation1]. Ischaemic stroke is approximately 10 times more common than haemorrhagic stroke [Citation2]. Predictors of stroke outcome include multiple factors, such as age, disease severity according to the National Institutes of Health Stroke Scale (NIHSS) score, diabetes mellitus, in-hospital infections, premorbid disability, and swallowing ability [Citation3,Citation4]. Importantly, stroke prognosis is influenced by many systemic haemodynamic parameters, of which blood pressure is one of the most important [Citation5,Citation6]. It was shown that the relationship between outcome after ischaemic stroke and blood pressure is U-shaped. Data from 17 398 patients participating in the International Stroke Trial showed that low blood pressure is associated with excess of deaths due to coronary artery disease and high blood pressure is associated with early death and late death or dependency as well as with recurrent stroke [Citation7]. Another study revealed that mortality rate was lowest in patients with systolic blood pressure of 150–169 mm Hg and diastolic blood pressure of 100–110 mm Hg (with a shift towards higher values of approximately 10 mm Hg in hypertensive patients) [Citation8]. Also, some novel haemodynamic parameters may be predictive factors of stroke outcome. An example of such a parameter is aortic stiffness. There are several non-invasive aortic stiffness markers, such as central augmentation index (AIx) and pulse wave velocity (PWV) [Citation9,Citation10]. AIx was found to be associated with short-term outcome after acute ischaemic stroke (positive correlation with length of hospitalisation and negative correlation with Barthel index at 1 week and at discharge) [Citation11] and with in-hospital mortality (higher AIx in patients who were discharged) [Citation12]. There is also a relationship between ischaemic stroke outcome and PWV; our group demonstrated that higher PWV was associated with worse short- and long-term outcome [Citation13,Citation14]. The aforementioned parameters depend on various factors and therefore are not constant and are subject to continuous changes. In humans, blood pressure is variable both in short- and long-term periods [Citation15,Citation16]. Stroke additionally predisposes to blood pressure changes, such as acute hypertensive response [Citation17]. In stroke patients, many factors may contribute to blood pressure oscillations, including psychological stress, pain, vomiting, infection, stroke severity, clot burden, collateral sufficiency, or intracranial hypertension [Citation18]. Most of the studies on blood pressure variability (BPV) in stroke patients have shown that increased BPV is associated with poorer prognosis [Citation19]. AIx, related to vascular function, depends also on factors other than arterial stiffness, such as heart rate, systolic ejection period, and peripheral vasoconstriction (which are not constant), and thus variates [Citation20]. PWV also changes over time and the determinants of PWV include age, sex, genetic factors, salt intake, heart rate, and blood pressure [Citation21]. Until now, no data regarding changes of AIx and PWV in ischaemic stroke population are available. Thus, the aim of our study was to assess changes of aortic stiffness markers in patients after acute ischaemic stroke and their impact on stroke outcome.
Materials and methods
Study design and patient population
In our study, we prospectively enrolled ischaemic stroke patients admitted to the Department of Adult Neurology, Medical University of Gdańsk, Poland, between November 2011 and June 2015. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The study protocol has been priorly approved by the Ethics Committee of the Medical University of Gdańsk. Informed consent was obtained from all patients included in the study.
The inclusion criteria were as follows: 1) diagnosis of ischaemic stroke based on clinical presentation and a neuroimaging study (magnetic resonance imaging), 2) age >18 years, 3) symptom onset <24 h before admission to hospital, 4) sinus rhythm. We excluded patients with atrial fibrillation, haemorrhagic stroke, non-vascular brain lesions, and pre-stroke disability (modified Rankin scale score >1).
Data collection and arterial stiffness assessment
After admission, baseline clinical data, including age, weight, height, and NIHSS score, were recorded. In all patients, we carried out measurements of blood pressure and markers of aortic stiffness by means of applanation tonometry (a gold standard for assessment of the arterial stiffness) three times: within 24 h after stroke onset, 6 days after stroke onset, and >90 days after stroke onset. All measurements were performed in accordance with the guidelines presented by Laurent et al. [Citation9]. Blood pressure (BP) was measured using Omron 705 C oscillometric device. Three BP measurements were conducted, and the mean value of the last two measurements was included in the further analysis. Mean blood pressure (MBP) was defined as (systolic blood pressure [SBP] + 2 × diastolic blood pressure [DBP])/3. After obtaining BP values, we performed applanation tonometry using SphygmoCor® device (AtCor Medical Pty Ltd, Sydney, Australia). In this method, pressure waveform is obtained transcutaneously by placing the applanation probe over the carotid artery and then over the femoral artery. The best waveforms are chosen using visual assessment and software quality control system. The PWV value is received through dividing the distance by the transit time and expressed in m/s. The distance is measured between the points over the carotid and femoral arteries, and the transit time is measured between the feet of the carotid and femoral waveforms (the end of the diastole, characterised by the steep rise of the waveform), obtained using the built-in intersecting tangent algorithm. Also, we obtained radial waveforms placing the probe over the radial artery. Then the central aortic waveform was calculated by the software with the use of the generalised transfer function on the basis of DBP and MBP. The AIx, defined as a percentage increase in aortic pressure caused by a reflected pulse wave, was derived from the central aortic waveform and it was normalised for heart rate of 75 bpm (AIxHR) [Citation9,Citation22].
Assessment of outcome
The modified Rankin scale (mRS) score was assessed at discharge and on day >90 and was used as a stroke outcome. Early favourable outcome was defined as mRS ≤1 point at discharge; early poor outcome, as mRS >2 points at discharge; late favourable outcome, as mRS ≤1 point on day >90; and late poor outcome, as mRS >2 points on day >90.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation or as median (interquartile range), as appropriate; and qualitative data, as number (percentage). Comparisons between two subgroups were performed using Student’s t test or Mann Whitney U test, as appropriate. Changes of parameters of BP and aortic stiffness were calculated using repeated measures analysis of variance (ANOVA) followed by Tukey’s post hoc test. The correlations between variables were assessed using Pearson’s correlation. The association between clinical parameters and stroke outcome was obtained using logistic regression and expressed as odds ratio (OR) and 95% confidence interval (95%CI). The p value <0.05 was considered as statistically significant. The statistical analysis was carried out by means of Dell Statistica software (Dell Inc.), version 13.
Results
Baseline characteristics
We prospectively recruited 50 patients, aged 62.2 ± 12.1 years, 34 males (68%) and 16 females (32%). The median baseline NIHSS score was 5 (3–7); in 20 patients (40%), recombinant tissue plasminogen activator (rtPA) was used; and 5 subjects (10%) had stroke in history. Twenty-eight patients (56%) had early favourable outcome; 10 patients (20%), early poor outcome; 29 patients (58%), late favourable outcome; and 9 patients (18%), late poor outcome. Baseline characteristics of the study population are presented in .
Table 1. Baseline characteristics of the study population.
BP and PWV measurements in the whole population
The first measurement of blood pressure parameters and aortic stiffness was carried out within 24 h from symptom onset; the second measurement, on day 6 (5–8); and the third measurement, on day 140 (106–249).
The values of blood pressure parameters are presented in . All of these parameters declined with time, and values obtained on day >90 were lower than those received on day 1 with p values for SBP, DBP, pulse pressure (PP), and MBP being <0.01, <0.01, 0.02, and <0.01, respectively. Also, SBP, DBP, and MBP values on day ≥90 were lower than on day 6 (p = 0.02, p < 0.01, p < 0.01, respectively).
Table 2. Values of blood pressure, pulse wave velocity, and augmentation index normalised for heart rate on day 1, on day 6, and >90 days after stroke onset.
The PWV values were as follows: 10.4 ± 3.4 m/s on day 1, 10.2 ± 3.3 m/s on day 6, and 9.2 ± 3.3 m/s on day >90 (). PWV on day >90 was lower than PWV on days 1 and 6 (p < 0.01 and p = 0.02, respectively).
BP and PWV measurements in stroke subjects with and without hypertension
In patients with a history of hypertension, SBP and MBP values on day 1 were significantly higher than in subjects without previous hypertension (145.3 ± 16.2 mm Hg vs. 139.0 ± 27.9 mm Hg, p = 0.04 and 105.1 ± 12.5 mm Hg vs. 99.1 ± 20.4 mm Hg, p = 0.04; respectively). SBP and MBP on other days as well as DBP and PP on all assessed days did not differ between these subgroups. Also, PWV and AIxHR values on day 1, day 6, and day >90 did not differ between patients with and without hypertension (Supplementary Table 1). Besides, PWV did not change significantly over time, regardless of hypertension history (Supplementary Figure 1).
Aortic stiffness measurements in female and male stroke subjects
We also carried out statistical analysis after taking into account patients’ sex. Differences in PWV between these groups were not statistically significant (p values for day 1, day 6, and day >90 were 0.22, 0.31, and 0.24, respectively) (Supplementary Table 2). In females, AIxHR was higher than in males on day 6 and day >90 (29.6 ± 11.7 mm Hg vs. 24.2 ± 10.3 mm Hg, p = 0.05; 36.4 ± 9.2 mm Hg vs. 23.1 ± 13.1 mm Hg, p < 0.01; respectively). In females, PWV did not change significantly over time, but in males PWV on day >90 was lower than PWV on day 6 (p = 0.01) (Supplementary Figure 2).
Correlation between arterial stiffness and BP
On day 1, PWV was not correlated with any BP parameter (p values for SBP, DBP, and MBP were 0.07, 0.78, and 0.34, respectively), except PP for which positive correlation was found (r = 0.36, p = 0.01). On day 6, PWV was positively correlated with SBP (p < 0.01), MBP (p = 0.03), and PP (p < 0.01), but not with DBP (p = 0.16). Finally, on day >90, PWV was positively correlated with SBP and PP (both p = 0.01), and not with DBP and MBP (p = 0.64 and p = 0.10, respectively). As far as relationship between AIxHR and BP is concerned, on days 1 and 6 no correlation was observed. However, on day >90 SBP and PP were positively correlated with AIxHR (p < 0.01 and p = 0.01, respectively) (Supplementary Table 3).
Measurements of central blood pressure
Aortic systolic pressure did not change in the assessed period and was 131.6 ± 21.6 mm Hg on day 1, 129.3 ± 25.0 mm on day 6, and 124.8 ± 16.4 mm Hg on day >90 (ANOVA, p = 0.10). Aortic pulse pressure was 48.5 ± 14.3 mm Hg, 49.2 ± 16.9 mm Hg, and 47.4 ± 14.3 mm Hg, respectively (ANOVA, p = 0.72). Similarly, AIxHR did not change significantly during the assessed period and the values were as follows: 25.7 ± 13.0 mm Hg on day 1, 26.0 ± 10.9 mm Hg on day 6, and 27.3 ± 13.4 mm Hg on day >90 (p = 0.64).
Association between AIxHR and stroke outcome
Early poor outcome
We have demonstrated differences in the course of AIxHR between patients with and without early poor outcome. In patients with mRS less than 2 points on day 6, AIxHR rose in days 1–6, unlike in subjects with mRS equal to or higher than 2 points on day 6 in whom aortic augmentation index fell in the first 6 days after ischaemic stroke onset (ANOVA, p = 0.04 between days 1 and 6) ().
Figure 1. Changes of aortic augmentation index normalised for heart rate of 75 bpm in stroke patients with and without late favourable outcome. AIxHR: augmentation index normalised for heart rate of 75 bpm. Numbers following the parameter represent the day of its assessment. Vertical bars represent 95% confidence intervals. Indicated p values are derived from Tukey’s post hoc test.
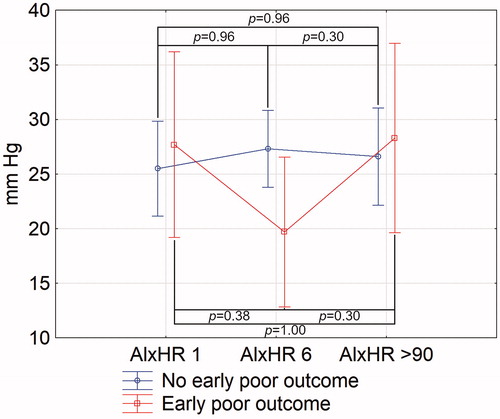
In a univariate logistic regression, decrease in AIxHR was significantly associated with early poor outcome (OR 1.07, 95%CI 1.00–1.15, p = 0.05); however, in a multivariate logistic regression, after adjustment to age, sex, history of hypertension, use of hypertensives before stroke, MBP on day 1, PWV on day 1, baseline NIHSS, and rtPA use, change in AIxHR between days 1 and 6 was not independently associated with early poor outcome (OR = 1.08, 95%CI = 0.98–1.21, p = 0.11; Supplementary Table 4).
Early favourable outcome
AIxHR in stroke patients with early favourable outcome grew between days 1 and 6, and in patients with no early favourable outcome, it declined (ANOVA, p < 0.01 between days 1–6) ().
Figure 2. Changes of aortic augmentation index normalised for heart rate of 75 bpm in stroke patients with and without early poor outcome. AIxHR: augmentation index normalised for heart rate of 75 bpm. Numbers following the parameter represent the day of its assessment. Vertical bars represent 95% confidence intervals. Indicated p values are derived from Tukey’s post hoc test.
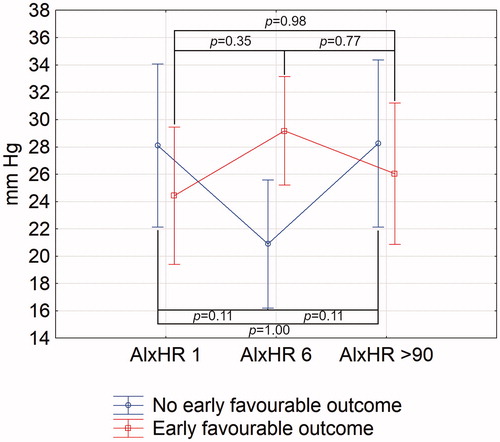
Univariate logistic regression analysis revealed that rise in difference between heart rate-adjusted augmentation index between days 1 and 6 was associated with early favourable outcome (OR = 1.09, 95%CI = 1.02–1.17, p = 0.01). The relationship was also statistically significant in a multivariate logistic regression, independently of age, sex, history of hypertension, use of hypertensives prior to ischaemic stroke, MBP on day 1, PWV on day 1, baseline NIHSS, and rtPA use (OR = 1.34, 95%CI = 1.04–1.74, p = 0.03; Supplementary Table 5). In a multivariate logistic regression model including the above-mentioned factors and types of antihypertensives taken prior to stroke, AIxHR change between days 1 and 6 kept its predictive value (OR = 1.20, 95%CI = 1.02–1.42, p = 0.03) (Supplementary Table 6). By contrast, inclusion of antihypertensives administered during hospitalisation caused loss of significance of AIxHR change (OR = 1.16, 95%CI = 0.997–1.34, p = 0.054). None of the antihypertensive class was of statistically significant importance.
Late favourable outcome
The association between increase in heart rate-corrected aortic augmentation index in days 1 to 6 and late favourable outcome was statistically significant (ANOVA, p = 0.01 between days 1 and 6) ().
Figure 3. Changes of aortic augmentation index normalised for heart rate of 75 bpm in stroke patients with and without early favourable outcome (C). AIxHR: augmentation index normalised for heart rate of 75 bpm. Numbers following the parameter represent the day of its assessment. Vertical bars represent 95% confidence intervals. Indicated p values are derived from Tukey’s post hoc test.
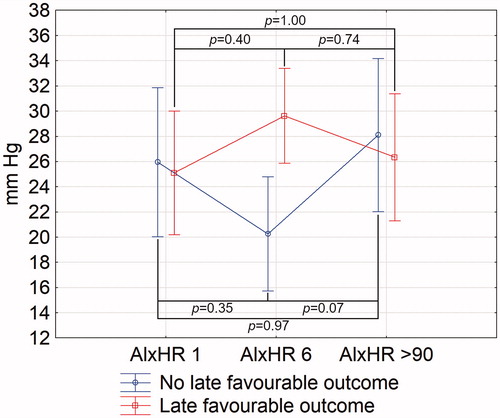
In a univariate logistic regression, change in AIxHR between days 1 and 6 was associated with late favourable outcome (OR = 1.08; 95%CI = 1.01–1.14, p = 0.02). This relationship remained significant after adjustment to sex, age, history of hypertension, use of antihypertensives before stroke, MBP on day 1, PWV on day 1, baseline NIHSS, and rtPA use (OR = 1.14; 95%CI = 1.02–1.28, p = 0.03; Supplementary Table 7). When antihypertensives used before stroke and administered during hospitalisation (separately) were added to the model, AIxHR change were not significantly associated with the outcome (OR = 1.25, 95%CI = 0.998–1.58, p = 0.052; OR = 1.08, 95%CI = 0.97–1.20, p = 0.16, respectively). No type of antihypertensives was related to the stroke outcome.
Late poor outcome
There was no association between the course of AIxHR and late poor outcome (ANOVA, p = 0,24 between days 1 and 6; p = 0.34 between days 1 and >90).
Outcome in stroke subgroups
We also analysed the association between AIxHR and all above-mentioned outcomes in stroke subgroups according to stroke subtype (the Oxfordshire Community Stroke Project classification) and stroke cause (Trial of ORG 10172 in Acute Stroke Treatment classification); however, we did not find any statistical significance.
Discussion
In our study, we prospectively assessed changes of arterial stiffness by means of AIxHR and PWV in patients after acute ischaemic stroke. For the first time, it was revealed that in patients with better early and late functional outcome AIxHR first rose and then fell, while non-favourable outcome was associated with reduction and then increase in AIxHR. Changes in AIxHR in ischaemic stroke patients might contribute to complex assessments of functional outcomes. In multivariate analyses for early favourable and late favourable outcomes, AIxHR change between days 6 and 1 was associated with stroke outcome, independently of NIHSS. However, these relations might be also due to reversed causality or even coincidental. Arterial stiffness may be influenced by endothelial dysfunction or changes of sympathetic drive which impact clinical outcome and, secondarily, arterial stiffness [Citation23]. After taking into account antihypertensive medications taken prior to stroke in a multivariate logistic regression for early favourable outcome, the relationship remained significant. However, the association between change in AIxHR and early favourable outcome after adjustment for in-hospital antihypertensives as well as the relationship between AIxHR change and late favourable outcome after adjustment for antihypertensives administered before stroke was close to but not quite statistically significant (p = 0.054 and p = 0.052, respectively). For late favourable outcome, including antihypertensives taken in hospital also caused loss of statistical significance of AIxHR (p = 0.16). Moreover, in none of the analyses, the classes of antihypertensive agents were associated with the functional outcome. However, the number of participants in this study was far too small to draw any robust conclusions about the significance of mechanisms, timing, and doses of antihypertensives that were given. We did not find an adjusted relationship between change in AIxHR and early and late poor outcomes. Discrepancies in associations between AIxHR and favourable or poorer prognosis might have been simply linked to small samples of patients in this study (only 10 participants in early poor outcome and 9 in late poor outcome group) rather than have essential backgrounds.
Elevated AIxHR is associated with cardio-vascular events and all-cause mortality [Citation10]. However, data on the impact of augmentation index in ischaemic stroke patients are scarce. In previous studies on stroke patients, there was no relationship between AIxHR and functional outcome at discharge [Citation13], higher AIxHR was associated with worse functional outcome in a univariate but not in a multivariate analysis [Citation14], lower AIxHR was associated with better early functional outcome [Citation11], and increased AIx was associated with lower in-hospital mortality [Citation12]. Changes of AIx were evaluated in several clinical studies [Citation20,Citation24,Citation25]. These researches have shown that AIx may be reduced for a prolonged period of time after orthopaedic surgery [Citation24], AIx decreases in patients receiving enalapril or omapatrilat [Citation20], and greater change of AIx during nitrous oxide synthase blockade is associated with better large-artery function [Citation25]. However, none of the previous studies have evaluated changes of AIxHR in ischaemic stroke population. Although rise in AIxHR observed in stroke patients with better outcome (as in our study) may seem strange, it can be explained, at least partially, by the fact that AIxHR depends not only on arterial wall stiffness, but also on other factors, such as the amplitude of the reflected wave, the reflectance point as well as changes in heart rate and ventricular contractility [Citation9]. Noteworthy, the relationship between peripheral BP, central BP, and PWV is very complex and requires further studies to be fully understood.
To our knowledge, this is also the first study assessing the changes of PWV over time in ischaemic stroke patients. To date, changes of PWV were evaluated in healthy populations, in chronic haemodialysis patients, and in depressed patients [Citation26–30]. In one study, it was shown that PWV measured on two consecutive days did not differ significantly [Citation27]. In another study, PWV was assessed before and after haemodialysis, and the study population was classified into three groups: normal PWV before and after haemodialysis (LL), high PWV before and normal PWV after haemodialysis (HL), and high PWV before and after haemodialysis (HH). It was demonstrated that mortality in HL and HH groups was significantly higher than in LL group [Citation29]. A study by Khandoker et al. revealed that depressed patients with suicidal ideation had reduced high frequency and low frequency power of PWV [Citation30].
We have also shown that PWV values are higher during the acute phase than during the late phase of ischaemic stroke. It indicates that during the acute phase PWV is elevated, and then it declines. The explanation of this finding is not known; however, we speculate that fall in PWV from day 1 to day >90 can be caused by several mechanisms. Initially elevated PWV may be evoked by increased sympathetic activity, endothelial dysfunction, or increased blood pressure in the acute phase of stroke (known as acute hypertensive response, AHR) [Citation23]. It is known that ischaemic stroke patients suffer from autonomic dysfunction and that increased sympathetic drive (triggered by cold pressor test, mental stress test [mental arithmetic], and smoking) causes reduction in arterial compliance [Citation31–34]. On the contrary, removal of sympathetic tone was shown to increase arterial distensibility without a significant effect on blood pressure [Citation35]. A decline in endothelial function, observed in stoke patients, is correlated with increased PWV [Citation36,Citation37]. Many patients have no or inadequate pre-stroke antihypertensive treatment. PWV may be reduced by long-term (>6 weeks) treatment with antihypertensives and blood pressure decline [Citation38]. AHR is defined as SBP ≥140 mm Hg and/or DBP ≥90 mm Hg found in two measurements carried out at least 5 min apart within the first 24 h after stroke onset [Citation17]. In our previous study, AHR was independently associated with PWV, though the latter was assessed on day 7 ± 2 [Citation39]. Now, we have demonstrated that on day 1 PWV is not correlated with BP parameters (except for PP); however, on day 6 PWV was positively correlated with most of assessed blood pressure parameters (except for DBP)that is in-line with the conclusion that AHR may be associated with increased aortic stiffness [Citation39]. Non-parallel raise in BP and PWV in the acute phase of stroke may also be explained by some transient or stroke-specific mechanisms [Citation17]. Nevertheless, it should be emphasised that AHR itself was outside the scope of this study.
Another finding from our study is that PWV did not differ between patients with previous hypertension and non-hypertensive subjects. We expected that PWV in hypertensive patients might be higher than in non-hypertensive patients due to the relationship between chronic burden of higher blood pressure and arterial stiffness [Citation40,Citation41], although some studies did not support such a dependence [Citation42,Citation43]. A possible explanation of our results is a small number of patients.
In our two previous studies, it was shown that PWV was independently associated with short-term [Citation13] and long-term [Citation14] outcome after acute ischaemic stroke. In these researches, PWV was assessed approximately 7 days after stroke onset. Our present study suggests that PWV values do not change significantly during the acute phase of ischaemic stroke, thus it indicates that PWV obtained one week after stroke onset can constitute as a marker of arterial stiffness on the first day. This is an encouraging conclusion that may facilitate conducting future studies regarding PWV in the acute ischaemic stroke, since it is more convenient to carry out applanation tonometry several days after ischaemic stroke onset than during the first days of the acute phase, when patients often undergo specific treatment procedures (such as thrombolysis or mechanical thrombectomy), need careful monitoring, and are often uncooperative.
In our study, there were no significant differences in PWV between males and females. This finding is not in accordance with other studies [Citation44,Citation45] which demonstrated higher PWV in males. Of note, female group in our population was relatively small (n = 16), which makes it difficult to draw any reliable conclusions.
Unfortunately, our study has some potential limitations. Firstly, due to logistic difficulties (e.g. severe disability of some patients, subsequent hospitalisations, the need for rehabilitation, consent withdrawal, loss of contact, etc.), the study population is relatively small (50 patients). Thus, the results should be interpreted with caution since small sample size can cause false-positive results and overestimation of the association. The conclusion needs to be confirmed by future studies with larger sample size. Moreover, small study size may have contributed to the fact that the relationship between AIxHR and clinical prognosis in stroke subgroups was not significant. Secondly, due to the aforementioned factors, the time range when the third PWV recording was obtained, is very broad (interquartile range = 106–249 days). However, we aimed to perform PWV analysis late enough from stroke so that hemodynamic stability had already been achieved. Thirdly, our study does not include patients with cardiac arrhythmias (a limitation of applanation tonometry), and the prevalence of atrial fibrillation, the most frequent sustained cardiac arrhythmia, in ischaemic stroke patients is approximately 24% [Citation46]. Fourthly, our stroke population was very heterogeneous and included patients with all stroke subtypes according to OCSP classification. This heterogeneity could have impact on our results since acute stroke, especially right-sided, can impair the autonomic balance [Citation47] which can influence haemodynamic parameters assessed in our study. Fifthly, small number of stroke patients in our study did not allow us to take into account all possible factors that may affect the stroke prognosis; we included only basic variables and many factors were omitted (e.g. lesion size). Furthermore, many of possible confounders were not measured. Obviously, not including all variables that may have impact on ischaemic stroke prognosis in the analyses interferes with their results.
In conclusion, we have shown that AIxHR appears to rise temporarily in stroke patients with better early and late favourable outcome and that PWV is transiently increased in the acute phase of ischaemic stroke.
Supplementary_Materials_FINAL.docx
Download MS Word (21.3 KB)Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- The 10 leading causes of death [Internet]. [cited 2020 Mar 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Andersen KK, Olsen TS, Dehlendorff C, et al. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009;40(6):2068–2072.
- Kenmuir CL, Hammer M, Jovin T, et al. Predictors of outcome in patients presenting with acute ischemic stroke and mild stroke scale scores. J Stroke Cerebrovasc Dis. 2015;24(7):1685–1689.
- Spratt N, Wang Y, Levi C, et al. Clinical study A prospective study of predictors of prolonged hospital stay and disability after stroke. J Clin Neurosci. 2003;10(6):665–669.
- Sprigg N, Gray LJ, Bath PMW, et al. Relationship between outcome and baseline blood pressure and other haemodynamic measures in acute ischaemic stroke: data from the TAIST trial. J Hypertens. 2006;24(7):1413–1417.
- Appleton JP, Sprigg N, Bath PM. Blood pressure management in acute stroke. Stroke Vasc Neurol. 2016;1(2):72–82.
- Okumura K, Ohya Y, Maehara A, et al. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens. 2005;23(6):1217–1223.
- Leonardi-Bee J, Bath PMW, Phillips SJ, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33(5):1315–1320.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605.
- Vlachopoulos C, Aznaouridis K, Rourke MFO, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–1871.
- Soiza RL, Davie MM, Williams D. Use of the augmentation index to predict short-term outcome after acute ischemic stroke. Am J Hypertens. 2010;23(7):737–742.
- Tziomalos K, Bouziana SD, Spanou M, et al. Increased augmentation index is paradoxically associated with lower in-hospital mortality in patients with acute ischemic stroke. Atherosclerosis. 2014;236(1):150–153.
- Gąsecki D, Rojek A, Kwarciany M, et al. Pulse wave velocity is associated with early clinical outcome after ischemic stroke. Atherosclerosis. 2012;225(2):348–352.
- Gąsecki D, Rojek A, Kwarciany M, et al. Aortic stiffness predicts functional outcome in patients after ischemic stroke. Stroke. 2012;43(2):543–544.
- Spronck B, Heusinkveld MHG, Vanmolkot FH, et al. Pressure-dependence of arterial stiffness: potential clinical implications. J. Hypertens. 2015;33(2):330–338.
- Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098.
- Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation. 2008;118(2):176–187.
- Wong AA, Read SJ. Early changes in physiological variables after stroke. Ann Indian Acad Neurol. 2008;11(4):207–220.
- Manning LS, Rothwell PM, Potter JF, et al. Prognostic significance of short-term blood pressure variability in acute stroke: systematic review. Stroke. 2015;46(9):2482–2490.
- Mitchell GF, Lacourcie Y, Arnold JMO, et al. Changes in aortic stiffness and augmentation index after acute converting enzyme or vasopeptidase inhibition. Hypertension. 2005;46(5):1111–1117.
- Obata Y, Mizogami M, Singh S, et al. The effects of hemodynamic changes on pulse wave velocity in cardiothoracic surgical patients. Biomed Res Int. 2016;2016:9640457.
- Mattace-Raso FUS, Hofman A, Verwoert GC, et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values. Eur Heart J. 2010;31(19):2338–2350.
- Auer J, Weber T. Arterial stiffness, central blood pressures, wave reflections and acute hypertensive response in stroke. Atherosclerosis. 2016;251:495–497.
- Fields K, Memtsoudis SG, Mo EE, et al. Changes in the augmentation index and postoperative orthostatic intolerance in orthopedic surgery: a prospective cohort study. Can J Anesth. 2018;65(9):1012–1028.
- Schneider MP, Ott C, Raff U, et al. Change in augmentation index during NOS inhibition, an index of basal NO production, is an independent determinant of large-artery function. Kidney Blood Press Res. 2010;33(5):343–351.
- Liang YL, Teede H, Kotsopoulos D, et al. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci. 1998;95(6):669–679.
- Kallem RR, Meyers KEC, Sawinski DL, et al. Variation and variability in carotid-femoral pulse wave velocity. Art Res. 2013;7(3–4):230–233.
- Tripkovic L, Hart KH, Frost GS, et al. Interindividual and intraindividual variation in pulse wave velocity measurements in a male population. Blood Press Monit. 2014;19(4):233–241.
- Torraca S, Sirico ML, Guastaferro P, et al. Variability of pulse wave velocity and mortality in chronic hemodialysis patients. Hemodial Int. 2011;15:326–333.
- Khandoker AH, Luthra V, Abouallaban Y, et al. Reduced variability in pulse wave velocity in depressed patients with suicidal ideation. Computing in Cardiology. 2015;42:1061–1064.
- Al-Qudah ZA, Yacoub HA, Souayah N. Disorders of the autonomic nervous system after hemispheric cerebrovascular disorders: an update central regulation of the autonomic nervous system the spectrum of autonomic dysfunction secondary to stroke. J Vasc Interv Neurol. 2015;8(4):43–52.
- Boutouyrie P, Lacolley P, Girerd X, et al. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol. 1994;267(4):H1368–76.
- Giannattasio C, Mangoni A, Stella M, et al. Acute effects of smoking on radial artery compliance in humans. J. Hypertens. 1994;12(6):691–696.
- Failla M, Grappiolo A, Carugo S, et al. Effects of cigarette smoking on carotid and radial artery distensibility. J Hypertens. 1997;15:1659–1664.
- Failla M, Grappiolo A, Emanuelli G, et al. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J Hypertens. 1999;17:1117–1123.
- Cherian P, Hankey GJ, Eikelboom JW, et al. Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke. 2003;34(9):2132–2137.
- McEniery CM, Wallace S, Mackenzie IS, et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608.
- Chen X, Huang B, Liu M, et al. Effects of different types of antihypertensive agents on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis. 2015;7(12):2339–2347.
- Kwarciany M, Gąsecki D, Kowalczyk K, et al. Acute hypertensive response in ischemic stroke is associated with increased aortic stiffness. Atherosclerosis. 2016;251:1–5.
- Rourke MFO, Nichols WW, Rourke O. Increase with age and isolated systolic. Hypertension. 2010. 2005;45(4):652–658.
- Franklin SS, Gustin WI, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham heart study. Circulation. 1997;96(1):308–315.
- Wildman RP, Farhat GN, Patel AS, et al. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45(2):187–192.
- Benetos A, Adamopoulos C, Bureau J, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105(10):1202–1207.
- Alecu C, Labat C, Kearney-Schwartz A, et al. Reference values of aortic pulse wave velocity in the elderly. J Hypertens. 2008;26(11):2207–2212.
- Magalhães P, Capingana DP, Silva ABT, et al. Age- and gender-specific reference values of pulse wave velocity for African adults: preliminary results. Age (Dordr). 2013;35(6):2345–2355.
- Otite FO, Khandelwal P, Chaturvedi S, et al. Increasing atrial fibrillation prevalence in acute ischemic stroke and TIA. Neurology. 2016;87(19):2034–2042.
- Colivicchi F, Bassi A, Santini M, et al. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004;35(9):2094–2098.
