Abstract
Purpose
We have summarized key studies regarding the assessment of subclinical macroangiopathic target organ damage (TOD) in type 1 diabetes mellitus (T1DM).
Results
Although chronic complications resulting from hyperglycemia, in particular macroangiopathies, are still the first cause of death in T1DM, there has been growing recognition of the role of hypoglycemia in cardiovascular morbidity and mortality. Subclinical TOD diagnosis ensures early implementation of the complex management aiming at either partial reversal of these complications or at least its downturn. To better identify patients with early TODs, several non-invasive diagnostic techniques are employed, including the ultrasonographic assessment of the intima-media thickness (IMT), computed tomography (CT) for coronary artery calcium (CAC) scores, and pulse wave velocity (PWV) measurement for arterial stiffness evaluation. Various studies reported that T1DM patients present an increased IMT. An increasing IMT fairly correlates with the cardiovascular (CV) events risk even after the adjustment to age, diabetes duration, quality of glucose control as well as the presence of hypertension, and chronic complications. Another, well established marker of the organ damage – CAC score is recommended by ACC/AHA guidelines to assess the overall CV risk in T1DM. Also, the arterial stiffness evaluation with PWV may further improve CV risk prediction, which has been reported in multiple studies including the Framingham Heart Study.
Conclusions
There is shortage of data from prospective studies which could confirm the benefits of early treatment initiation based on the presence of the subclinical organ damage in T1DM. Most evidence comes from T2DM trials, where effective preventive measures were identified i.e.: smoking cessation, reasonable blood glucose control, efficacious hypertension treatment, and dyslipidemia management, as well as renoprotection. There is still a field for further research to see if routine assessment of asymptomatic vascular damage and early implementation of aggressive treatment would reduce mortality excess from CVD in T1DM.
Introduction
In type 1 diabetes mellitus (T1DM), hyperglycemia is by far the most important factor underlying development and progression of functional and structural changes in both small and large blood vessels. Most of the studies assessing blood vessel changes in T1DM focused on microangiopathy. However, the predominant cause of death in T1DM is cardiovascular disease (CVD) [Citation1–5].
The most striking negative impact of T1DM on cardiovascular risk is observed in young and middle-aged patients, especially females. In a cohort study of young adults, the annual mortality rate in patients with T1DM was 8.0% compared with 2.4% in the control group. Moreover, the highest relative mortality risk (almost 12-fold increase) was observed in type 1 diabetic women between 36-45 years of age [Citation2]. In another cohort observation, Laing et al. followed 23,751 T1DM patients for 29 years. Mortality from ischemic heart disease under the age of 40 was related to diabetes in 89% of men and 98% of women [Citation3,Citation4]. The ischemic heart disease mortality rate was increased 4-fold in male patients older than 40 years and 9-fold in those who were younger than 40. In females, the risk increased 7 times in older and almost 40 times in younger patients. Furthermore, in T1DM patients aged 45–55 years, the absolute CVD risk is similar to that in non-diabetic subjects, who are10–15 years older [Citation6]. Thus, T1DM clearly contributes to early vascular aging.
Several studies attempted to identify factors predicting future cardiovascular (CV) events in T1DM patients. The investigators of the EURODIAB Prospective Complications Study Group reported that the albumin excretion rate was a strong predictive factor in both women and men. Furthermore, they found several sex-specific risk factors related to coronary artery disease including HbA1c, waist-to-hip ratio, HDL cholesterol, smoking and autonomic neuropathy in men, and systolic blood pressure, triglycerides and retinopathy in women [Citation7]. The role of albuminuria as a risk marker is supported by a study by Conway et al. who reported that albumin excretion rate, HbA1c and the duration of diabetes are predictors of coronary artery disease mortality, while lower renal function, higher diastolic blood pressure and worse lipid profile predict coronary artery disease morbidity [Citation8]. The study of Waden et al. emphasized the role of HbA1c variability, which was strongly associated with the development of not only microangiopathy, but was also is a predictor of CV events. Importantly, this relationship was independent of the mean HbA1c level and renal status [Citation9].
There is growing interest in methods detecting subclinical macroangiopathy to prevent excessive morbidity and premature deaths due to CVD. Tests used for this purpose should be non-invasive, specific and sensitive allowing proper management of high-risk patients.
In this review, we will summarize the results of the most recent research in the area of subclinical macroangiopathy in T1DM based on the assessment of intima-media thickness (IMT), computed tomography (CT) for coronary artery calcium (CAC) and arterial stiffness. First two tests were recommended for diabetic patients in the 2010 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines [Citation10]. Estimation of arterial stiffness provides additional predictive information about future risk [Citation11,Citation12].
The recommendation for the routine IMT testing in diabetic patients was changed in 2013 ACC/AHA Guidelines due to the results showing the lack of their relationship with CV events and CAC score was showed to be useful if ‘after quantitative risk assessment, a risk-based treatment decision is uncertain’ [Citation13].
2013 European Society of Cardiology Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the European Association for the Study of Diabetes (EASD) considered the above indicators as useful markers of the cardiovascular residual risk and mentioned that the detection of the increased intima-media thickness or arterial stiffness as well as abnormal calcium score should lead to more intensive control of modifiable risk factors [Citation14].
The 2016 ESC Guidelines on CVD prevention indicate that most imaging techniques have not been precisely tested as screening tools for cardiovascular risk assessment and their routine use for CV risk prediction is not recommended. At the same time, these methods might be considered as factors improving estimation of CV risk in patients who are close to decisional thresholds [Citation15].
Assessment of intima-media thickness in T1DM
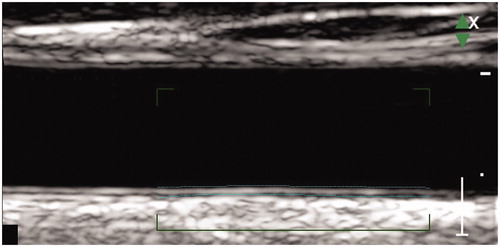
IMT of the common carotid artery (cIMT) is usually measured using an ultrasound device at a posterior wall site in a place located 2–3 cm proximally to the bulb. There are various ways to obtain the measurement. Earlier, B-Mode scanning was first taken, and then the frozen M-Mode image was measured tracking the inner hypoechogenic and the middle echogenic layer (). However, recently specially designed software is used to automatically measure the IMT in up to 300 measure points. The test is easy to perform and its role as a predictive test for CVD is accumulating. Lorenz et al. reported in a systematic review and meta-analysis that cIMT is a strong predictor of future CV events. For a 0.1 mm increase of cIMT, the risk of myocardial infarction increases by 10–15% and the risk of stroke by 13–18%. Single baseline measurement of cIMT gives additional information to CVD risk stratification in the general population especially in an intermediate risk and older adults [Citation16].
Several cross-sectional studies have shown increased IMT in patients with T1DM. The difference, already significant in uncomplicated T1DM [Citation17], is especially evident in patients with chronic complications e.g. nephropathy or retinopathy [Citation18,Citation19]. Importantly, significantly increased cIMT is observed even in children and adolescents with a short T1DM duration [Citation20,Citation21] and adults with newly diagnosed T1DM [Citation22]. This suggests that subclinical cIMT changes develop early in the course of T1DM. Further cIMT increase is greater in T1DM patients than in non-diabetic controls, and is proportional to diabetes duration [Citation23,Citation24].
However, in young type 1 diabetic patients, diabetes seems not to be the main risk for IMT progression [Citation25]. Similarly, in adult population, the association of IMT change with the occurrence of CV events was not proved. The meta-analysis collecting data from 3902 adults with type 2 diabetes mellitus (T2DM) showed no relation between progression of IMT and vascular risk, although it reproduced the link between ‘baseline’ IMT and the risk of CV event [Citation26]. In the REMOVAL study, progression of IMT was not affected by the use of metformin but maximal IMT (a prespecified tertiary outcome) was significantly reduced (−0.013 mm per year) [Citation27].
In the DCCT/EDIC Study, cIMT was strongly related to HbA1c levels. Other predictors of increased cIMT included albumin excretion rate, age, male gender, smoking, and systolic blood pressure. The study has shown that intensive insulin therapy slowed cIMT progression, which was most evident in the first 6 years after DCCT ended, but it was still present after 12 years [Citation28]. This beneficial effect of intensive treatment on subclinical macroangiopathy might provide mechanistic explanation the 27-year follow-up results showing that the intensive group had modesty lower all-cause mortality than the conventional group [Citation29]. There are also data on the possible reduction of IMT as an effect of short-term treatment of hyperglycemia [Citation30].
The relationship between T1DM and carotid damage might be modulated by obesity. Shah et al. evaluated the association between the burden of CVD risk factors over time and follow-up cIMT in young T1DM patients. CVD risk factor burden increased over time, while body mass index (BMI) z score was the only identified risk factor predicting follow-up cIMT at this age [Citation31].
Several other factors could be implicated in T1DM-related cIMT increase. T1DM duration might affect the relationship between cIMT and traditional CV risk factors. In patients with a short duration of T1DM, cIMT was independently associated with triglycerides and nephropathy [Citation22]. In patients with long-term T1DM, cIMT was related not only to BMI, but also to LDL cholesterol [Citation32]. Glucose variability was not shown to correlate with IMT [Citation33].
Patients with microangiopathy, including both nephropathy and retinopathy, have greater cIMT than those without complications [Citation34,Citation35]. Increased cIMT has also been linked to nocturnal hypertension [Citation36,Citation37]. Furthermore, increased cIMT has been associated with impairment of cerebrovascular reactivity [Citation38], skin autofluorescence [Citation39]. Other potential factors affecting IMT include plasma total homocysteine [Citation40].
Finally, the study of Lilje et al. reported association of aortic IMT and femoral IMT with early vascular changes in teenage T1DM patients, while such association was not found for cIMT or brachial IMT [Citation41].
Besides the most common risk predictors for subclinical angiopathy, patient education also plays an important role. Araszkiewicz et al. found that for newly diagnosed T1DM patients a 5-day teaching program in intensive insulin treatment reduces the likelihood of developing macroangiopathic complications defined as IMT thickening. This was independent from other predictors such as HbA1c or diabetic kidney disease [Citation42].
Assessment of computed tomography for coronary calcium in T1DM
Most studies using CT for coronary calcium have been done in T2DM. Coronary artery calcification (CAC) has been found to be predictive for CVD in these patients [Citation43–46]. Below we review studies in patients with T1DM.
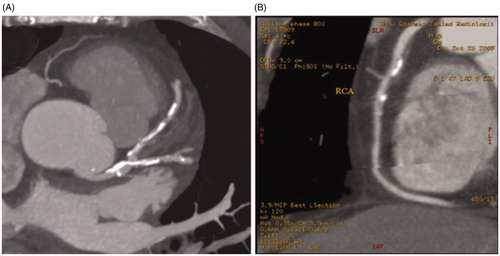
CAC can be detected and quantified using electron beam computed tomography. It is a non-contrast procedure, where contiguous 3-mm CT images are acquired at 100-ms exposure. The scanning area is from the bifurcation of the main pulmonary artery to the diaphragm, in the supine position with the breath held for 35–45 s. Images are electrocardiographically triggered at 80% of RR interval. Areas of calcification are defined by a minimum area of 3 contiguous pixels with an attenuation minimum of 130 Hounsfield Units (HU). The CAC score can be obtained using the standard Agatston method – separately for each region by multiplying the area by the density score (1 for 130–199 HU, 2 for 200–299 HU, 3 for 300–399 HU, 4 for >399 HU). The total CAC score is calculated by adding the scores for all slices separately for the left main, left anterior descending, circumflex and right coronary arteries (). The total radiation dose is about 1–1.5 mSV [Citation47].
Figure 2. CT for coronary artery calcification. (A) Calcium Score for LAD (left anterior descending) – 508; (B) Calcium Score for RCA (right coronary artery) – 154.
Djaberi et al. attempted to answer a question of whether there is any association between cIMT and CAC. They assessed common carotid artery ultrasound and computed tomography of the heart in 150 asymptomatic diabetic patients. Mean cIMT was greater in patients with CAC score > 100 [Citation48].
Raggi et al. tested CAC as a prognostic factor in 903 patients with diabetes (both types 1 and 2). They showed that the increase in CAC score was associated with a greater increase in mortality compared to the non-diabetic group. CAC was related to a 44% increased risk of death for every increase in CAC score group (11–100 to 101–400, 401–1000, >1000) [Citation43]. Interestingly, diabetics and those without diabetes having no coronary artery calcifications had a similar 5-year all-cause survival.
Similarly, a study from Denver confirmed that T1DM patients have accelerated atherosclerosis in coronary arteries. The study assessed the progression of CAC in 109 patients asymptomatic for coronary artery disease, aged 22–50 years with mean diabetes duration of 22 years. CT was performed twice with a mean interval of 2.7 years. 62% of patients at baseline were without CAC, 19% had progression at follow-up. HbA1c >7.5% was a strong risk marker of increased CAC, besides the common risk factors such as age, male sex and duration of T1DM [Citation49]. This mirrors the results from the DCCT/EDIC Study, where most of the cohort underwent CT. The CAC scores were significantly lower in the intensive treatment group. The greatest benefits were among patients in the intensive treatment group with the shortest duration of T1DM. Other risk factors and risk markers for higher CAC scores were: age, male sex, waist-to-hip ratio, cIMT, hypercholesterolemia and hypertension [Citation50].
Glucose variability was also reported to be associated with CAC but the observation was valid only in men [Citation51].
Islet cell transplantation can functionally cure T1DM and appears to stabilize CAC. Lipid status and kidney function were associated with CAC changes [Citation52].
Furthermore, data from Pittsburgh Epidemiology of Diabetes Complications (EDC) showed a correlation between CAC and common CVD risk factors, and also between CAC and clinical coronary artery disease in T1DM. Sensitivity and specificity of CAC for myocardial infarction and coronary artery stenosis in T1DM were 100% and 58% for any CAC score and 95% and 84% for a CAC score 151–168, which was a cut-off point of maximum accuracy. CAC was strongly correlated with age, diabetes duration, systolic blood pressure and autonomic neuropathy [Citation53].
Obesity was shown to be a strong predictor of CAC progression in adults with T1DM independently of dyslipidemia, hypertension or inflammation [Citation54].
There is also a strong interaction between kidney disease and CVD in T1DM patients. Increasing albumin-to-creatinine ratio or decreasing eGFR predicts CAC progression. Furthermore, even in the absence of diabetic kidney disease progression of CAC is faster in patients with T1DM [Citation55].
ACE-Inhibitors were shown to reduce CAC progression in T1DM patients with microalbuminuria [Citation56]. However, results from DCCT/EDIC study did not prove that achieving normoalbuminuria in T1DM patients with previously detected microalbuminuria did not follow with the reduction of cardiovascular events [Citation57].
Reduced heart rate variability predicted progression of CAC in adults with T1DM confirming the link between cardiac autonomic neuropathy and atherosclerosis but the association was present also in non-diabetic patients [Citation58,Citation59].
Taking into account the important role of CAC in the development of CVD, Burge et al. recommend scanning for CAC in all patients with diabetes duration at least 10 years (beginning at the age of 40 years). According to the authors, CAC scan and repeat scan in 4–5 further years (if the initial scan is positive) after instituting aggressive risk factor reduction, can substantially reduce the incidence of atherosclerotic CVD in T1DM [Citation60].
Assessment of arterial stiffness in T1DM
Most of the studies assessing the predictive value of arterial stiffness are based on the applanation tonometry method (). The pulse waveforms are recorded by the micromanometer-tipped pressure transducer probe placed over examined artery (carotid, femoral, radial) (). Analysis of the waveform provides key parameters including central arterial pressures and other indices of arterial stiffness like pulse wave velocity (PWV), aortic augmentation index, aortic augmentation pressure or sub-endocardial viability ratio. Aortic augmentation pressure measures the contribution of the early reflected wave to the central systolic pressure. Aortic augmentation index indicates the size of the increase or decrease in the pulse height as a result of the reflected wave and it is expressed as a percentage. Sub-endocardial viability ratio is a ratio of the diastolic area under the curve to systolic area under the curve of the arterial pulse wave; this makes sub-endocardial viability ratio a possible indicator of myocardial perfusion and indicates the possible infarction risk.
Figure 3. Carotid and femoral pulse waveforms, pulse wave velocity and central clinical parameters obtained by applanation tonometry.
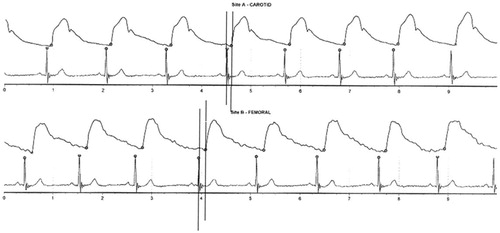
Table 1. Summary of main findings in studies assessing subclinical macroangiopathic target organ damage in type 1 diabetes.
Arterial stiffness measured as aortic PWV is a predictor of CV events, CV and all-cause mortality, which is more pronounced in subjects with a higher baseline CV risk [Citation12]. Moreover, adding evaluation of arterial stiffness to SCORE scale increases sensitivity of risk stratification in general population [Citation61]. The Framingham Heart Study confirmed the role of PWV as a factor associated with increased risk for first CV event as well as an additional factor, which improves risk prediction [Citation62].
Several studies have reported greater arterial stiffness in T1DM patients in comparison to healthy subjects [Citation17,Citation24,Citation63–66]. Peripheral and central vascular changes were shown to be present in adolescents and young adults compared to controls. In multivariate models after adjustment for classical cardiovascular risk factors T1DM was still significantly associated with arterial stiffness [Citation67].
The aforementioned Pittsburgh EDC study evaluated also the arterial function. After 18 years of prospective observation they performed a pulse waveform analysis in 144 T1DM patients. They showed a strong association between autonomic neuropathy (diagnosed on expiration/inspiration ratio) and aortic augmentation pressure, aortic augmentation index and sub-endocardial viability ratio even after adjustment for HbA1c, HDL cholesterol and smoking which were correlated with arterial stiffness in multivariate analysis [Citation63]. Moreover, analysis based on the same cohort revealed hemodynamic parameters, which were associated with CVD in T1DM. Patients underwent, among others, pulse waveform analysis, electron beam CT, and ankle–brachial index (ABI) assessment. This study showed no significant association of aortic augmentation index with any CVD. Aortic augmentation pressure was significantly associated with coronary artery disease after exclusion patients using nitrates. Lower sub-endocardial viability ratio was correlated with a high CAC score (>99). Age and duration of diabetes remained the strongest predictors of higher arterial stiffness [Citation64].
In a cross-correlation study with 676 T1DM patients, arterial stiffness increased with presence and duration of T1DM. Importantly, PWV increased with all the investigated diabetes complications (cardiovascular, renal, retinal, and autonomic abnormalities) independently of other risk factors [Citation68].
In a longitudinal study of 298 young T1DM patients carotid-femoral PWV was assessed with two visits conducted 5 years apart (SEARCH CVD study). The rate of progression in carotid-femoral PWV was independently associated with increases in waist circumference, LDL-cholesterol levels and declining glucose control [Citation69]. Furthermore, higher insulin sensitivity levels were related to lower rate of change in PWV over time indicating that in youth with T1DM lower insulin sensitivity at baseline might be an important risk factor for the development of increased arterial stiffness [Citation70].
A study from Stockholm reported a strong correlation between arterial stiffness and autonomic neuropathy assessed by the analysis heart rate variability [Citation65]. Similarly, in SEARCH CVD study, lower standard deviation of normal R-R interval was associated with higher arterial stiffness and this relation was not attenuated with adjustment for cardiovascular risk factors [Citation71].
Diabetic symmetric polyneuropathy has been associated with increased aortal stiffening but not with cerebral angiopathy in patient with long-term T1DM [Citation72]. Furthermore, the presence of retinopathy in T1DM patients has been linked to increased wave reflection [Citation73]. The association of increased pulse pressure and PWV in both types of diabetes has been confirmed by researchers from Leicester. At the same time, they showed no correlation between aortic augmentation index and diabetes. The same results were found independent of presence or absence of antihypertensive drugs, diabetes type or arterial site (measured from both radial and carotid arteries) [Citation66]. However, the relation between aortic augmentation index and T1DM presence was then related in a population of adolescents and young adults [Citation67].
In asymptomatic patients with diabetes, PWV (but not aortic augmentation index) was also reported to be associated with abnormal myocardial perfusion imaging with adenosine and was proposed as a low-cost tool for the identification of patients with a higher risk of coronary artery disease who might benefit from the further diagnostic process [Citation74].
Thus, T1DM is an independent factor of increased arterial stiffness, which might be the sign of early vascular aging and explain higher rate of CV mortality and morbidity in patients suffering from T1DM.
Assessment of other indicators of subclinical macroangiopathy and cardiovascular risk in T1DM
The necessity of new markers to monitor subclinical manifestations of vascular disease in T1DM led to further research in this field. There is growing evidence indicating that reduced insulin sensitivity might play a role in the pathogenesis of vascular complications in T1DM [Citation75]. Several mechanisms might be implicated including prolonged exposure to supraphysiologic levels of exogenous insulin, weight gain (caused by intensive insulin therapy), insulin’s effects on overall non-essential fatty acid exposure and lipotoxicity, similar genetic and environmental factors that lead to T2DM [Citation75–77]. It is known that greater insulin sensitivity estimation at baseline appears a protective against both micro- and macrovascular complications of T1DM. According to Bjornstad et al., estimation of insulin sensitivity may provide better risk assessment of vascular complications in T1DM [Citation75].
Based on DCCT/EDIC data, other indicators of vascular complications in T1DM include plasma prekallikrein, which levels were significantly positively associated with BMI, HA1c, systolic blood pressure, total cholesterol, LDL cholesterol, and triglycerides (but not with age, sex, T1DM duration or HDL cholesterol). Plasma prekallikrein was also significantly positively associated with progression of both internal and combined IMT, suggesting that it can be regarded as a risk factor of subclinical vascular disease in T1DM [Citation78].
Inflammation plays a key role in the atherosclerotic pathway. Therefore, there is growing interest in the investigation of inflammatory markers to assess CV risk. Prospective studies have shown that elevated levels of circulating lipoprotein-associated phospholipase A2is linked to increased risk of CV events [Citation79]. Lipoprotein-associated phospholipase A2 enters the subintimal space and becomes activated when LDL is oxidized, cleaving oxidized phospholipids and creating pro-inflammatory products (lysophosphatidylcholine and oxidized free fatty acids). The consequence is increased chemotaxis, activation of monocyte-derived macrophages and, as a result, the activation of inflammatory process [Citation80,Citation81]. In T1DM patients, lipoprotein-associated phospholipase A2 was found in both LDL and HDL and was distributed differently in T1DM men without any relationship to CAC score progression [Citation81].
Arpita Basu et al. used nuclear magnetic resonance as the newest techniques that allow us to classify lipoprotein subclasses in greater detail with further evaluation of molar concentrations of size/density-based subclasses. They found that men with T1DM had significant positive associations of some baseline nuclear magnetic resonance-LDL subclasses and common and/or internal cIMT; conventional total-, LDL-cholesterol, non-HDL-cholesterol and common cIMT. Furthermore, common cIMT changes over 12 years were positively associated with large and small LDL particles, and conventional triglycerides. However, such relationship was not observed in women. Thus, nuclear magnetic resonance derived LDL particles (in addition to conventional lipid profiles) might help to identify men with the highest risk for CVD [Citation82].
SUMMARY
CV damage is a frequent, complex and serious complication of T1DM. Subclinical changes underlying CVD are seen already in diabetic children. Young adults with T1DM have a much higher risk for CV events than their healthy equals. On the other hand, we have reports about Golden Medallists – patients who survived ≥50 years with T1DM – with surprisingly low prevalence of chronic complications. H. Keenan et al. reported data in a group of about 400 patients from whom 46.8% did not have any significant microangiopathy [Citation83]. In greater group of corresponding Swedish patients, described by S. Adamsson Eryd et al., only 44% patients had macroangiopathies and 52% had microangiopathies [Citation84]. It seems that those patients have some protective mechanisms from detrimental effects of hyperglycaemia on subclinical organ damage assessed by measurement of cIMT, left ventricular strain and endothelial function [Citation85].
Differences between patients with and without complications show that high HDL cholesterol level, normal BMI, low insulin dose are factors, which promote survival without micro- and macroangiopathies [Citation83,Citation84,Citation86]. Apart from diabetes there are many other risk factors and risk markers of CV complications. The standard risk factors include age, lipid profile, hypertension and smoking. In addition, in the previously mentioned Swedish medallist group, HbA1c was a predictor of CVD independently of duration of T1DM [Citation84].
Diagnosis of the asymptomatic macrovascular complications changes might help to identify individuals in whom more aggressive therapeutic approach might be helpful, however data confirming the clinical advantages of early diagnosis of asymptomatic macrovascular complications in therapy of T1DM are limited.
It should be note that screening of the asymptomatic macrovascular complications in the diabetic patients remains controversial. The American Diabetes Association (ADA) does not recommend routine screening tests for coronary artery disease (CAD) in asymptomatic patients, because high-risk patients should already receive intensive therapy controlling risk factors. However, they do acknowledge the established role of the CAC score as an independent predictor of CV events [Citation87]. At the same time, 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases [Citation88] stress that CT-based CAC score may be considered as a risk modifier in the CV risk assessment of asymptomatic patients with DM at moderate risk. It is emphasised that CAC is not always associated with ischaemia, and to detect a silent myocardial ischaemia, stress testing with myocardial perfusion imaging or stress echocardiography are required. These guidelines recommend ultrasound-based assessment of carotid and/or femoral plaque burden. However, carotid intima-media thickness screening for CV risk assessment is not recommended.
Most research in CV risk assessment and treatment was done in T2DM. Scientific Statement from AHA and ADA [Citation89] on management of CVD risk factors in T1DM stress the most important for preventing macroangiopathies is obviously keeping blood glucose in control. Additionally, for patients with blood pressure > 140/90 mmHg should be started antihypertensive drug, mostly preferred drugs inhibiting renin-angiotensin-aldosterone system. T1DM patients with dyslipidemia and additional CVD risk factor or developed CVD should be treated with statins. Diabetic kidney disease and albuminuria as well-known CVD risk factor should be carefully diagnosed and treated [Citation87,Citation89]. Smoking cessation is mandatory. According to 2018 Practice guidelines for the management of arterial hypertension of the ESH target for systolic blood pressure should be <140mmHg for every patient with diabetes mellitus and <130mmHg for those who tolerate this with benefit of further reduction risk of stroke. There is no beneficial lowering systolic blood pressure <120mmHg [Citation90].
Atherosclerosis is a major contributor to CVD, and it is commonly present in diabetic patients, however, it may have a different course in T1DM vs. T2DM. One of the potential explanations for this phenomenon may be related to observed differences in the lipid profiles in T2DM vs. T1DM which is driven by the various insulin resistance severity. Patients with T2DM tend to have lower HDL cholesterol fraction with higher triglycerides and elevated so-called small dense LDL cholesterol which altogether promote atherogenic process. Patients with T1DM who are treated with insulin are usually characterised by normal and sometimes supernormal lipid profile [Citation91]. This phenomenon is reflected in CV-risk profiling in DM subjects. T2DM patients are usually classified at high or very high CV risk while a considerable fraction of T1DM patients are characterised by moderate CV-risk. In addition, the multislice computed tomography for the evaluation of CAC score and atherosclerotic plaques in patients with T1DM and T2DM provides more insights about the course differences of coronary artery disease. Whereas there are no differences in mean CAC scores and the prevalence of atherosclerosis in T1DM vs. T2DM patients, there is a higher prevalence of multiple vessels stenosis in T2DM which is strongly related to the extent of coronary atherosclerosis after correction for other CV risk factors [Citation92].
At the same time, the results of the DCCT/EDIC Trials showed that atherosclerosis in T1DM is more diffuse and more concentric as compared to T2DM; patients with T1DM have early occurrences of CAD manifested by significant CAC in young adults [Citation93]. In addition, in women with T1DM, menopause increased CAC progression more than in women without diabetes independent of age and other CVD risk factors known to worsen with menopause [Citation94].
Patients with T1DM and T2DM have also differences in the inflammatory response leading to the development of vascular complications. For T2DM, the dominant in the development of atherosclerotic complications are enhanced lipid peroxidation and elevated interleukin-6 levels [Citation95]. At the same time, elevated levels of circulating lipoprotein-associated phospholipase A2 is linked to increased risk of CV events in T1DM [Citation80–81].
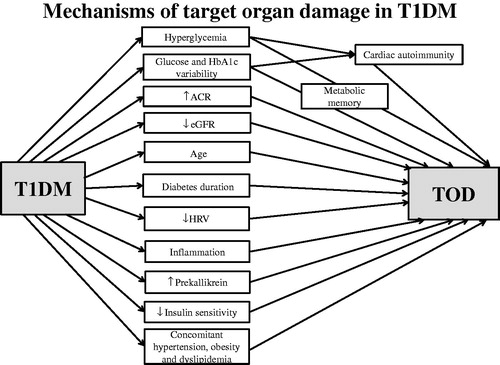
It should be noted that chronic hyperglycaemia in T1DM causes myocardial injury which induce persistent cardiac autoimmunity (). Furthermore, cardiac autoantibodies potentiate the development of CVD, possibly through inflammatory pathways [Citation96]. In addition to the above mechanisms, the influence of metabolic memory on the relationship between risk factors and atherosclerosis (including subclinical) should be taken into account. In metabolic memory, the body’s tissues (including arteries) continue to respond to poor or good glycemic control for years after glycaemia has improved or worsened [Citation97].
In conclusion, assessment of IMT, CAC score and PWV can identify subclinical organ damage preceding CV events in T1DM patients. Whether wider application of these methods could improve T1DM management and reduce the diabetes-related burden of CVD is unclear. Therefore, further research, including randomised clinical trials, is needed to confirm the role of routine assessment of subclinical CV damage in daily practice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Swerdlow AJ, Jones ME. Mortality during 25 years of follow-up of a cohort with diabetes. Int J Epidemiol. 1996;25(6):1250–1261.
- Soedamah-Muthu SS, Fuller JH, Mulnier HE, et al. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992-1999. Diabetologia. 2006;49(4):660–666.
- Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46(6):760–765.
- Laing SP, Swerdlow AJ, Slater SD, et al. The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet. Med. 1999;16(6):466–471.
- Huxley RR, Peters SA, Mishra GD, et al. Risk af all-cause mortality and vascular events in women versus men with type 1 diabetes: a systemic review and meta-analysis. Lancet Diabetes endocrinol. 2015;3(3):198–206.
- Soedamah-Muthu SS, Fuller JH, Mulnier HE, et al. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29(4):798–804.
- Soedamah-Muthu SS, EURODIAB Prospective Complications Study Group, Chaturvedi N, Toeller M, et al. Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care. 2004;27(2):530–537.
- Conway B, Costacou T, Orchard T. Is glycaemia or insulin dose the stronger risk factor for coronary artery disease in type 1 diabetes? Diab Vasc Dis Res. 2009; 6(4):223–230.
- Wadén J, on behalf of the Finnish Diabetic Nephropathy Study Group, Forsblom C, Thorn LM, et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58(11):2649–2655.
- Greenland P, American Heart Association, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2010;56(25):e50–103.
- Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51(4):527–539.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness:a systematic review and meta-analysis. J Am CollCardiol. 2010;55(13):1318–1327.
- Goff DC, Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63(25 Pt B):2935–2959.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34(39):3035–3087.
- Piepoli MF, ESC Scientific Document Group, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016;37(29):2315–2381.
- Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467.
- Giannattasio C, Failla M, Piperno A, et al. Early impairment of large artery structure and function in type 1 diabetes mellitus. Diabetologia. 1999;42(8):987–994.
- Atabek ME, Kurtoglu S, Pirgon O, et al. Arterial wall thickening and stiffening in children and adolescents with type 1 diabetes. Diabetes Res ClinPract. 2006;74(1):33–40.
- Krantz JS, Mack WJ, Hodis HN, et al. Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J. Pediatr. 2004;145(4):452–457.
- Järvisalo MJ, Raitakari M, Toikka JO, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109(14):1750–1755.
- El-Samahy MH, Tantawy AAG, Adly AAM, et al. Expression of CD4+ CD28null T lymphocytes in children and adolescents with type 1 diabetes mellitus: Relation to microvascular complications, aortic elastic properties, and carotid intima media thickness. Pediatr Diabetes. 2017;18(8):785–793.
- Frost D, Beischer W. Determinants of carotid artery wall thickening in young patients with type 1 diabetes mellitus. Diabet. Med. 1998;15(10):851–857.
- Giannattasio C, Failla M, Grappiolo A, et al. Progression of large artery structural and functional alterations in type 1 diabetes. Diabetologia. 2001;44(2):203–208.
- Vastagh I, Horvath T, Nagy G, et al. Evolution and predictors of morphological and functional arterial changes in the course of type 1 diabetes mellitus. Diabetes Metab. Res. Rev. 2010;26(8):646–655.
- Frost D, Beischer W. Progression of the carotid artery intima-media thickness in young patients with type 1 diabetes. Diabetes Care. 2003;26(2):545–545.
- Lorenz MW, Price JF, Robertson C, et al. Carotid intima-media thickness progression and risk of vascular events in people with diabetes: results from the PROG-IMT collaboration. Diabetes Care. 2015;38(10):1921–1929.
- Petrie JR, Chaturvedi N, Ford I, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):597–609.
- Polak JF, for the DCCT/EDIC Research Group, Backlund JC, Cleary PA, et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60(2):607–613.
- Orchard TJ, Nathan DM, Zinman B, et al. Association between seven years of intensive treatment of type 1 diabetes and long term mortality. JAMA. 2015;313(1):45–53.
- Tenjin A, Nagai Y, Yuji S, et al. Short-term change of carotid intima-media thickness after treatment of hyperglycemia in patients with diabetes: a cross-sectional study. BMC Res Notes. 2016;9(1):281.
- Shah AS, Dabelea D, Fino NF, et al. Predictors of increased carotid intima-media thickness in youth with type 1 diabetes: The SEARCH CVD Study. Dia Care. 2016;39(3):418–425.
- Distiller LA, Joffe BI, Melville V, et al. Carotid artery intima-media complex thickening in patients with relatively long-surviving type 1 diabetes mellitus. J Diabetes Complications. 2006;20(5):280–284.
- Cesana F, Giannattasio C, Nava S, et al. Impact of blood glucose variability on carotid artery intima media thickness and distensibility in type 1 diabetes mellitus. Blood Press. 2013;22(6):355–361.
- Gül K, Üstün I, Aydin Y, et al. Carotid intima-media thickness and its relations with the complications in patients with type 1 diabetes mellitus. Anadolu Kardiyol Derg. 2010;10(1):52–58.
- Głowińska-Olszewska B, Urban M, Urban B, et al. The association of early atherosclerosis and retinopathy in adolescents with type 1 diabetes: preliminary report. Acta Diabetol. 2007;44(3):131–137.
- Lee SH, Kim JH, Kang MJ, et al. Implications of nocturnal hypertension in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34(10):2180–2185.
- Atabek ME, Akyürek N, Eklioglu BS, et al. Impaired systolic blood dipping and nocturnal hypertension: an independent predictor of carotid intima-media thickness in type 1 diabetic patients. J Diabetes Complications. 2014;28(1):51–55.
- Kozera GM, Wolnik B, Kunicka KB, et al. Cerebrovascular reactivity, intima-media thickness, and nephropathy presence in patients with type 1 diabetes. Diabetes Care. 2009;32(5):878–882.
- Araszkiewicz A, Naskret D, Zozulinska-Ziolkiewicz D, et al. Skinautofluorescence is associated with carotid intima-media thickness, diabetic microangiopathy, and long-lasting metabolic control in type 1 diabetic patients. Results from Poznan Prospective Study. Microvasc Res. 2015;98:62–67.
- Basu A, Jenkins AJ, Stoner JA, et al. Plasma homocysteine and carotid intima-media thickness in type 1 diabetes: A Prospective Study. Atherosclerosis. 2014;236(1):188–195.
- Lilje C, Cardinale JP, Cronan J, et al. Femoral and aortic intima-media thickness but not carotid or brachial intima-media thickness are abnormal in children with type 1 diabetes. Cardiology. 2015;131(Suppl 02):179–179.
- Araszkiewicz A, Zozulinska-Ziolkiewicz D, Pilacinski S, et al. Baseline diabetic knowledge after 5-day teaching program is an independent predictor of subclinical macroangiopathy in patients with type 1 diabetes (Poznan Prospective Study). Adv Med Sci. 2014;59(2):240–244.
- Raggi P, Shaw LJ, Berman DS, et al. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43(9):1663–1669.
- Elkeles RS, for the PREDICT Study Group, Godsland IF, Feher MD, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29(18):2244–2251.
- Hoff JA, Quinn L, Sevrukov A, et al. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am CollCardiol. 2003;41(6):1008–1012.
- Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24(2):335–338.
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am CollCardiol. 1990;15(4):827–832.
- Djaberi R, Schuijf JD, de Koning EJ, et al. Usefulness of carotid intima-media thickness in patients with diabetes mellitus as a predictor of coronary artery disease. Am J Cardiol. 2009;104(8):1041–1046.
- Snell-Bergeon JK, Hokanson JE, Jensen L, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care. 2003;26(10):2923–2928.
- Cleary PA, DCCT/EDIC Research Group, Orchard TJ, Genuth S, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55(12):3556–3565.
- Snell-Bergeon JK, Roman R, Rodbard D, et al. Glycaemic variability is associated with coronary artery calcium in men with Type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med. 2010;27(12):1436–1442.
- Madrigal JM, Monson RS, Hatipoglu B, et al. Coronary artery calcium may stabilize following islet cell transplantation in patients with type 1 diabetes. Clin Transplant. 2017;31(10):e13059.
- Olson JC, Edmundowicz D, Becker DJ, et al. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49(9):1571–1578.
- Rodrigues TC, Veyna AM, Haarhues MD, et al. Obesity and coronary artery calcium in diabetes: the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. DiabetesTechnolTher. 2011;13(10):991–996.
- Maahs DM, Jalal D, Chonchol M, et al. Impaired renal function further increases odds of 6-year coronary artery calcification progression in adults with type 1 diabetes: the CACTI study. Dia Care. 2013;36(9):2607–2614.
- Maahs DM, Snell-Bergeon JK, Kinney G, et al. ACE-I/ARB treatment in type 1 diabetes patients with albuminuria is associated with lower odds of progression of coronary artery calcification. J. Diabetes Complicat. 2007;21(5):273–279.
- de Boer IH, Gao X, Cleary PA, et al. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. CJASN. 2016;11(11):1969–1977.
- Rodrigues TC, Ehrlich J, Hunter CM, et al. Reduced heart rate variability predicts progression of coronary artery calcification in adults with type 1 diabetes and controls without diabetes. Diabetes Technol. Ther. 2010;12(12):963–969.
- Mogensen UM, Jensen T, Køber L, et al. Cardiovascular autonomic neuropathy and subclinical cardiovascular disease in normoalbuminuric type 1 diabetic patients. Diabetes. 2012;61(7):1822–1830.
- Burge MR, Eaton RP, Schade DS. The role of a coronary artery calcium scan in type 1 diabetes. Diabetes Technol. Ther. 2016;18(9):594–603.
- Sehestedt T, Jeppesen J, Hansen TW, et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31(7):883–891.
- Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511.
- Prince CT, Secrest AM, Mackey RH, et al. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care. 2010;33(3):652–657.
- Prince CT, Secrest AM, Mackey RH, et al. Pulse wave analysis and prevalent cardiovascular disease in type 1 diabetes. Atherosclerosis. 2010;213(2):469–474.
- Jensen-Urstad K, Reichard P, Jensen-Urstad M. Decreased heart rate variability in patients with type 1 diabetes mellitus is related to arterial wall stiffness. J. Intern. Med. 1999;245(1):57–61.
- Lacy PS, O’Brien DG, Stanley AG, et al. Increased pulse wave velocity is not associated with elevated augmentation index in patients with diabetes. J Hypertens. 2004;22(10):1937–1944.
- Shah AS, Wadwa RP, Dabelea D, et al. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes. 2015;16(5):367–374.
- Theilade S, Lajer M, Persson F, et al. Arterial stiffness is associated with cardiovascular, renal, retinal, and autonomic disease in type 1 diabetes. Diabetes Care. 2013;36(3):715–721.
- Dabelea D, Talton JW, D'Agostino R, Jr, et al. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36(12):3938–3943.
- Shah AS, Black RS, Wadwa RP, et al. Insulin sensitivity and arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. J Diabetes Complications. 2015;29(4):512–516.
- Jaiswal M, Urbina EM, Wadwa RP, et al. Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36(8):2351–2358.
- Szczyrba S, Kozera GM, Neubauer-Geryk J, et al. Diabetic symmetric polyneuropathy is associated with increased aortal stiffening but not cerebral angiopathy in type 1 diabetes. J Diabetes Complications. 2015;29(1):73–76.
- Araszkiewicz A, Rogowicz-Frontczak A, Zozulińska-Ziółkiewicz D, et al. Presence of retinopathy in type 1 diabetic patients is associated with subclinical macroangiopathy. Scand. J. Clin. Lab. Invest. 2011;71(7):563–568.
- Roos CJ, Djaberi R, Schuijf JD, et al. Relationship between vascular stiffness and stress myocardial perfusion imaging in asymptomatic patients with diabetes. Eur J Nucl Med Mol Imaging. 2011;38(11):2050–2057.
- Bjornstad P, Maahs DM, Duca LM, et al. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the Coronary Artery Calcification in Type 1 Diabetes Study. J. Diabetes Complicat. 2016;30(4):586–590.
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948.
- Loh K, Deng H, Fukushima A, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10(4):260–272.
- Jaffa MA, DCCT/EDIC Research Group, Luttrell D, Schmaier AH, et al. Plasma prekallikrein is associated with carotid intima-media thickness in type 1 diabetes. Diabetes. 2016;65(2):498–502.
- Lp-PLA2 Studies Collaboration. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536–1544.
- Reddy KJ, Singh M, Bangit JR, et al. The role of lipoprotein-associated phospholipase A2 on cardiovascular disease risk assessment and plaque rupture: a clinical review. J ClinLipidol. 2009;3(2):85–93.
- Jarvie JL, Wang H, Kinney GL, et al. Lipoprotein-associated phospholipase A2 distribution among lipoproteins differs in type 1 diabetes. J ClinLipidol. 2016;10(3):577–586.
- Basu A, Jenkins AJ, Zhang Y, et al. Nuclear magnetic resonance-determined lipoprotein subclasses and carotid intima-media thickness in type 1 diabetes. Atherosclerosis. 2016;244:93–100.
- Keenan HA, Costacou T, Sun JK, et al. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care. 2007;30(8):1995–1997.
- Adamsson Eryd S, Svensson A-M, Franzén S, et al. Risk of future microvascular and macrovascular disease in people with type 1 diabetes of very long duration: a national study with 10-year follow-up. Diabet Med. 2017;34(3):411–418.
- Liu SL, Gurfinkel R, Spaic T, et al. The LONGevity & Type 1 Diabetes Macrovascular Epidemiology (LONGTIME) Study: carotid intima media thickness, LV strain and endothelial dysfunction in type 1 diabetes of 50 years duration. Canadian Journal of Diabetes. 2016;40(5):S8.
- Bain SC, Gill GV, Dyer PH, et al. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet. Med. 2003;20(10):808–811.
- Riddle MC, Bakris G, Blonde L, et al. American Diabetes Association Standards of Medical Care in Diabetes – 2018. Diabetes Care. 2018;41(Suppl. 1):86–104.
- Cosentino F, ESC Scientific Document Group, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41(2):255–323., Grant PJ, Aboyans V,.
- de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130(13):1110–1130.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314–340.
- Catapano AL, Graham I, De Backer G, ESC Scientific Document Group, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058.
- Djaberi R, Schuijf JD, Boersma E, et al. Differences in atherosclerotic plaque burden and morphology between type 1 and 2 diabetes as assessed by multislice computed tomography. Diabetes Care. 2009;32(8):1507–1512.
- Budoff M, Backlund JC, Bluemke DA, et al. The association of coronary artery calcification with subsequent incidence of cardiovascular disease in type 1 diabetes: the DCCT/EDIC trials. JACC Cardiovasc Imaging. 2019 ;12(7):1341–1349.
- Keshawarz A, Pyle L, Alman A, et al. Type 1 diabetes accelerates progression of coronary artery calcium over the menopausal transition: the CACTI study. Dia Care. 2019;42(12):2315–2321. Dec
- Alharby H, Abdelati T, Rizk M, et al. Association of lipid peroxidation and interleukin-6 with carotid atherosclerosis in type 2 diabetes. Cardiovasc Endocrinol Metab. 2019;8(3):73–76.
- Sousa GR, Pober D, Galderisi A, et al. Glycemic control, cardiac autoimmunity, and long-term risk of cardiovascular disease in type 1 diabetes mellitus. Circulation. 2019;139(6):730–743.
- Jenkins A, Januszewski A, O'Neal D. The early detection of atherosclerosis in type 1 diabetes: why, how and what to do about it. Cardiovasc Endocrinol Metab. 2019;8(1):14–27.