Abstract
Purpose
The Hypertension Optimal Treatment (HOT) Study investigated the relationship between target office diastolic blood pressure (BP) ≤80, ≤85 or ≤90 mmHg and cardiovascular morbidity and mortality in 18,790 patients aged 50–80 years. The home BP sub-study enrolled 926 patients and the aim was to clarify whether the separation into the BP target groups in the office prevailed in the out-of-office setting. The present study aimed to identify variables that characterised masked uncontrolled hypertension (MUCH) and white coat uncontrolled hypertension (WUCH).
Material and Methods
The sub-study participants took their home BP when office BP had been up titrated. The cut-off for normal or high BP was set to ≥135/85 mmHg at home and ≥140/90 mmHg in the office. We analysed data by using multivariate and stepwise multivariate logistic regression with home and office BP combinations as the dependent variables.
Results
WUCH was associated with lower body mass index (BMI) (odds ratio (OR) 0.92, 95% confident intervals (CIs) 0.88–0.96, p < 0.001). MUCH was associated with smoking (OR 1.89, 95% CIs 1.25–2.86, p = 0.0025) and with lower baseline heart rate (OR 0.98, 95% CIs 0.97–0.99, p = 0.03) and higher BMI (OR 1.03, CIs 1.00–1.06, p = 0.04). MUCH remained associated with smoking (OR 2.76, 95% CIs 1.76–4.35, p < 0.0001) also when using ≥140/90 mmHg as the cut-off for both home and office BP. MUCH was also associated with higher BMI (OR 1.05, 95% CIs 1.01–1.09, p = 0.009) while WUCH was associated with lower BMI (OR 0.93, 95% CIs 0.90–0.97, p = 0.0005) when using ≥140/90 mmHg as a cut-off.
Conclusion
Our data support that ‘reversed or masked’ treated but uncontrolled hypertension (MUCH) is common and constitutes about 25% of treated hypertensive patients. This entity (MUCH) is rather strongly associated with current smoking and overweight while uncontrolled white coat (office) hypertension (WUCH) is associated with lower BMI.
Introduction
The Hypertension Optimal Treatment (HOT) Study included patients in 26 countries. The rationale was described in detail [Citation1] and HOT was conducted in accordance with the Prospective Randomised Open Blinded Endpoint (PROBE) design [Citation2]. The main aim was to evaluate the relationship between three levels of target diastolic blood pressure (BP) (≤80, ≤85 or ≤90 mmHg) and cardiovascular morbidity (CV) and mortality in hypertensive patients. In addition, the study examined the effects of a low dose (75 mg daily) of acetylsalicylic acid vs. placebo. 18,790 patients between 50 and 80 years of age participated. Antihypertensive treatment commenced with a calcium antagonist. Additional antihypertensive therapy was given in accordance with a set protocol to reach target BPs. Details of the patient characteristics at randomisation, cardiovascular risk profiles, early BP results and CV outcomes were published [Citation1,Citation3,Citation4].
Home BP, or self-monitoring of BP, can easily be learned and it has high reproducibility and sensitivity of measurement [Citation5–7]. Because of limited prospective mortality/morbidity data in clinical outcome trials, home BP monitoring cannot be used alone to decide whether treatment is indicated; treatment decisions must usually be based on repeated standard clinic BP readings [Citation8]. Taking home BP by patients was particularly feasible in the HOT Study because of the standardisation of measurements of BP with a semiautomatic device and the subsequent possibility to train subjects at every office visit. Therefore, the aim of the home BP sub-study was to compare home BP with office BP in a fairly large and representative subsample of the HOT Study population after the up-titration of antihypertensive treatment. The two BPs in the office and at home were on average nearly identical [Citation9]. Heart rate averaged 1.7 ± 8.6 beats/min higher in the office compared to at home (p < 0.0001) [Citation9]. The sub-study also aimed to clarify whether the separation of subjects into the three main groups (≤80, ≤85 or ≤90 mmHg) based on office readings prevailed into the out-of-office setting [Citation9]. The present sub-study data were presented at the 14th European Hypertension Meeting [Citation10]. For many years the HOT sub-study on home BP was the only large study of home BP in treated hypertensive patients [Citation9]. However, in light of the increasing interest in uncontrolled reversed office (‘masked’) hypertension (MUCH), and the new nomenclature recently introduced [Citation11–14], we aim to fully publish the presented data [Citation10]. The aim is to identify variables and clinical characteristics found to be associated with various combinations of home and office BP being high or normal, and in particular, the combination of high home BP/normal office BP, namely uncontrolled reversed office (‘masked’) hypertension (MUCH).
Material and methods
Patients
In the main study, 18,790 patients of any race of 50–80 years were randomised in 26 countries. Details of their characteristics at randomisation were published [Citation3]. The average mean ± SD randomisation BP in patients untreated at enrolment was 169 ± 14/106 ± 3 mmHg (n = 8917) and in the previously treated patients, after at least 2 weeks of washout, BP averaged 170 ± 14/105 ± 3 mmHg (n = 9873). The three target BP groups were well-matched at the outset of the study [Citation3].
There were no additional criteria for participation in the home BP sub-study except for investigator interest and patient willingness and 88 of the 1921 participating centres in the HOT Study participated. The sample (n = 926) that participated in the sub-study contained a higher percentage of previously treated patients (66 vs. 52%); otherwise, characteristics were comparable with patients in the main study [Citation9]. The distribution of the 926 patients between countries was as follows: Canada 72, Greece 34, Hungary 36, Israel 10, The Netherlands 19, Norway 109, Spain 124, Sweden 82 and the United States 440 patients.
Data sharing
Data sharing is not applicable to this article as the database was closed for further analyses more than 10 years ago and no new data were created.
Protocol
The ethics committees in all participating countries approved the study. Patients gave informed consent. Blood pressure was measured at enrolment and then at two qualifying visits at least 7 days apart. The diastolic BP had to be in the range of ≥100 to ≤115 mm Hg at both qualifying visits. The number of exclusion criteria were specified [Citation1]. All patients started active antihypertensive treatment with the calcium antagonist felodipine 5 mg once daily. Additional antihypertensive therapy was given with either an angiotensin-converting enzyme inhibitor or a β-adrenoceptor blocker if the target BP was not reached. Dosage adjustments were, if needed, made in accordance with a set protocol. As a final step, a diuretic was added if it was needed. After 6 months [Citation3], the percentages of patients who had achieved their randomised target clinic diastolic BP were 57, 71 and 83% for the target groups at ≤80, ≤85, or ≤90 mmHg, respectively, and after 12 months they were 57, 72 and 84%, respectively. The distributions of the drug dose steps in the three target groups were rather similar.
Blood pressure and heart rate were measured in the sitting position with a newly calibrated semiautomatic oscillometric device with a digital readout (Visomat OZ, D2 International, Hestia Pharma GmbH, Mannheim, Germany). The accuracy of this device was validated in 407 normotensive and hypertensive people against standard sphygmomanometer readings according to the recommendations of the British Hypertension Society, with the conclusion that this device could provide accurate and reliable measurements of BP [Citation10]. Blood pressure measurements were taken in the same arm each time and with a cuff of appropriate size relative to the patient’s arm. The cuffed arm was kept at the heart level, and the arm was supported at the time of the measurement. In the office, three measurements were taken at least 15 s apart, after 5 min of rest, and the averages were calculated for statistical analysis. Visits took place at the same time of the day, usually in the morning. Measurements were performed at the end of the dosing interval (‘at trough’) and preferably by the same person.
Home BP measurements were performed through seven consecutive days with the same type of semiautomatic device after appropriate supplemental training of the participating patients. After they sat for 5 min, both in the morning before leaving home and in the afternoon after returning home, BPs and heart rates were taken three times, and the measurements were registered on special case record forms. Conditions for measurements were thus comparable at home and in the investigator’s office though measurements at home per protocol were less standardised related to the intake of the antihypertensive medication. The averages of all BP measurements at home, in most patients 42 measurements, were calculated for comparisons with the respective office measurements.
Assessments of BP and heart rates at home were performed at a time when the titration of antihypertensive medication in the study had been finalised, that is, at least 6 months after randomisation [Citation9]. schematically illustrates the design of the study. An automatic device was given to the patient after a scheduled visit for the majority of patients at 6 (n = 168), 12 (n = 462), 18 (n = 191), or 24 (n = 67) months; the home measurements were then performed shortly thereafter (on average 4 days later) and in most patients (n = 890) on consecutive days. The data from the home assessments in each patient was compared with BP and heart rate taken at the regularly scheduled visit to the office of the investigator that was nearest in time to and usually preceded the home measurements. Nine patients were excluded from the home-office comparison because the exact time for home measurements had not been written on the recording form. One patient had not written the diastolic BPs on the form, and four had not registered heart rates.
Figure 1. Design of the HOT study with the design of the sub-study on home BP measurements embedded.
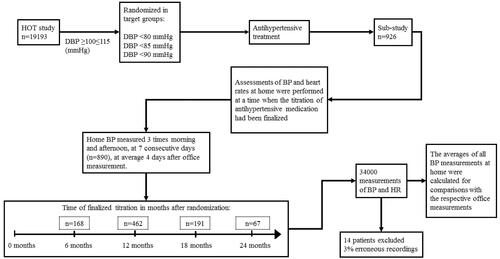
The home BP data was edited by one of the investigators (S.E.K.) according to an a priori protocol for the purpose of cleaning the data for clearly erroneous recordings [Citation9]. This was done before any statistical analysis. Approximately 3% of all recordings were considered erroneous (single numbers incompatible with life or way out of range with all others in the same patient, diastolic BP almost identical to systolic BP or higher, heart rates compatible with a paroxysmal tachycardia); the accompanying BPs and heart rates taken simultaneously were deleted. Not all patients had all measurements taken at all 14 occasions, leaving nearly 34,000 measurements of both BPs and heart rates for analysis.
Statistics
The patients were divided into four categories according to their average home and office BPs. This was done to investigate the patients’ characteristics and identify the variables influencing their home BP. Terms like ‘white coat’ hypertension for the isolated elevation of BP in the office and ‘masked hypertension’ for the isolated elevation of BP out of the office, were previously reserved for people without antihypertensive treatment. But these terms are now also used for people who are treated for hypertension [Citation11–14].
We have therefore used the following corresponding terms: Controlled home and office BP (controlled BP), controlled home but not office BP (white coat uncontrolled hypertension, WUCH), controlled office but not home BP (masked uncontrolled hypertension, MUCH) and uncontrolled office and home BP (uncontrolled BP). The cut-off for normal (‘controlled’) or high BP was set to ≥135/85 mmHg for home BP and ≥140/90 mmHg for office BP. The groups were compared by using multivariate logistic regression and stepwise multivariate logistic regression with the dependent variables being home BP normal/high and office BP normal/high. The analyses were carried out with all baseline variables including age, previous diseases and treatments included in the models. Data are shown as mean ± SD or percent. P-value <0.05 (two-tailed) was considered statistically significant.
Results
Distribution of the treated home/office BP categories
The distribution of participants in the four categories () was the following when using cut off ≥135/85 mmHg for home BP and cut off ≥140/90 mmHg for office BP (). Normal both home and office BP (controlled) 31% (n = 280), normal home and high office BP (WUCH) 11% (n = 103), normal office and high home BP (MUCH) 25% (n = 232) and high both office and home BP (uncontrolled) 33% (n = 300). Age ranged from 59.8 to 62.1 years in the four categories without significant differences ().
Figure 2. Distribution of the home BP sub-study population in categories depending on control of home BP <135/85 mmHg and office BP <140 mmHg. WUCH: white coat uncontrolled hypertension; MUCH: masked uncontrolled hypertension; CONTROLLED: controlled home and office BP; UNCONTROLLED: uncontrolled office and home BP.
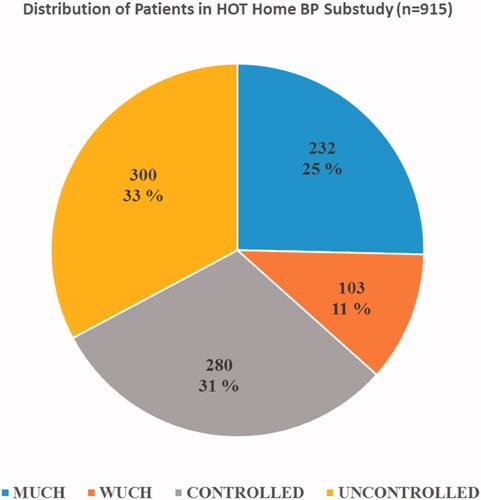
Table 1. Distribution of HOT sub-study participants (n = 915) according to their home and office BPs during treatment.
Table 2. Baseline characteristics of participants in the HOT home BP sub-study (n = 915).
Findings in patients with masked uncontrolled hypertension (MUCH)
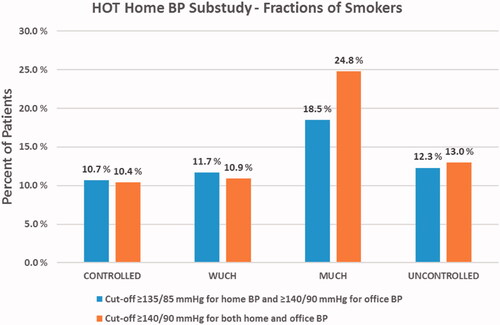
MUCH had the highest mean body mass index (BMI) for males (30.1 kg/m2) and for females (29.7 kg/m2) and lower office heart rate 79 (11) beats/min (). MUCH had the highest portion of current smokers (18.5%, ). These findings were statistically significant and the logistic multivariate models showed that the MUCH patients (high home, normal office BP) were associated with more smoking (OR 1.89, CIs 1.25–2.86, p = 0.0025), with lower baseline office heart rate (OR 0.98, CIs 0.97–0.99, p = 0.03) and higher BMI (OR 1.03, CIs 1.00–1.06, p = 0.04).
When cut-off was set to ≥140/90 mmHg, for both home and office BP (n = 137, 59% males, 41% females), the portion of current smokers was 24.8% (n = 34, ) and smoking remained highly significantly associated with MUCH (OR 2.76, CIs 1.76–4.35, p < 0.0001). Higher BMI (OR 1.05, CIs 1.01–1.09, p = 0.009) was also associated with MUCH while there was a borderline significant association for baseline heart rate (OR 0.98, CIs 0.97–1.00, p = 0.049).
Figure 3. Fractions of smokers in the various HOT study home BP sub-study categories when using ≥135/85 mmHg and ≥140/90 mmHg as cut-offs for elevated home BP, respectively. CONTROLLED: controlled home and office BP; WUCH: white coat uncontrolled hypertension; MUCH: masked uncontrolled hypertension; UNCONTROLLED: uncontrolled office and home BP.
Findings in patients with uncontrolled white coat hypertension (WUCH)
With cut-off set to ≥135/85 mmHg for home BP and ≥140/90 mmHg for office BP, the group with normal home and uncontrolled high office BP (WUCH) had the lowest mean BMI for females (27.3 kg/m2) (). In multivariate models, this group was associated with lower BMI (OR 0.92, CIs 0.88–0.96, p < 0.001). When cut-off was set to ≥140/90 mmHg for both home and office BP (n = 165, 53% males, 47% females, n = 18 smokers, 10.9%), this group (WUCH) remained associated with lower BMI (OR 0.93, CIs 0.90–0.97, p = 0.0005). Chronic pulmonary disease was also associated with WUCH at the same cut-off (≥140/90 for both home and office BP) (OR 3.26, CIs 1.23–8.65, p = 0.0125, n = 7) but with this low n (4.2%) we consider this results as a likely chance finding.
Discussion
We investigated variables that characterised various combinations of home and office BP being high or normal in patients with treated hypertension and in particular the combination of high home BP/normal (controlled) office BP. Our data support that such ‘reversed or masked’ treated but uncontrolled hypertension (MUCH) is common and constitutes about 25% of treated hypertensive patients. Heart rate may be involved, but firstly MUCH is rather strongly associated with current smoking and overweight. These findings in MUCH were confirmed and extended by applying a more conservative approach using ≥140/90 mmHg as cut off for both home and office BP. Patients with normal home and uncontrolled high office BP (WUCH) were associated with lower body weight whether using ≥135 or ≥140 mmHg as cut off for home BP.
When evaluating the distribution of participants, it is important to note that the size of the four fractions may depend on several conditions. The majority of the HOT Study patients (66%) were previously treated while approximately one third was previously untreated. The choice of using diastolic BP as a target, as well as separating the patients into target groups of ≤80, ≤85 or ≤90 mmHg, without attention to systolic BP might also have had an impact on our results of fraction size. That is to say, the distribution and the high MUCH of 25% and WUCH of 11% may not necessarily correspond with findings in other studies. A more recent publication from the large ELSA study [Citation11] aiming to evaluate the long-term reproducibility of MUCH and WUCH assessed by average 24-h ambulatory BP, found that after 1 year of antihypertensive treatment 21.1 and 17.8% of patients were classified as MUCH and WUCH, respectively. Although these numbers are fairly comparable to ours, the study also uncovered that despite the prevalence for both conditions being similar in the following years, there was a large change in patient composition, and only 4.5% and 6.2% of the patients remained in MUCH and WUCH throughout the treatment period of 4 years. Thus, by 24-h ambulatory BP monitoring the fraction findings for MUCH demonstrated poor reproducibility [Citation11,Citation12]. However, it appears that when analysing the presence of antihypertensive drugs or drug metabolites in urine samples, poor medication adherence did not explain the existence of MUCH [Citation13,Citation14]. It is more likely that MUCH is representing a form of hypertension that is worsening with time, and if no further intervention (adding more medication to gain BP control), there is a transition from MUCH to established uncontrolled hypertension [Citation11].
A high prevalence of MUCH was also recently described in the Spanish Registry [Citation15]; of 14,840 patients with treated and controlled office BP, as many as 4608 patients (31%) had MUCH according to 24-h ambulatory BP criteria. The prevalence of MUCH was higher in patients at high CV risk (smokers, diabetes and obesity). However, MUCH appeared most often because of poor control of nocturnal BP. Approximately 14% of their patients had MUCH if assessed with daytime ambulatory BP. Their fraction of 14% is lower than our finding of 25% in the HOT Home BP sub-study and the finding of 21% in the ELSA Study [Citation11]. The explanation is most likely that the Spanish Registry data had been collected and analysed during years with generally more aggressive BP-lowering therapy (after 2010). The HOT Home BP and the ELSA Study data were collected approximately 20 years ago [Citation11,Citation16] though the ELSA Study data on reproducibility was analysed more recently [Citation11].
Other studies have compared the characteristics of hypertensive patients and found that variables such as obesity, relatively higher office systolic BP, habitual drinking, and the use of two or more antihypertensive drugs are associated with MUCH [Citation17,Citation18]. Another study has shown that passive smoking in a dose-related manner is associated with masked hypertension [Citation19] and a study using ambulatory BP measurements showed already in 1997 that smokers, and in particular elderly men, had less white coat effect [Citation20]. Smoking may also raise ambulatory BP [Citation21,Citation22].
The detailed effects of smoking on BP are unknown though it is likely that the haemodynamic effects of tobacco may contribute. It has since long been known that blood pressure and heart rate increase during smoking [Citation23]. These effects are specifically associated with nicotine while the other components of tobacco, of which more than a thousand have been isolated, seem to be of minor importance [Citation24,Citation25]. The rise in blood pressure is due both to an increase in cardiac output and total peripheral vascular resistance. The blood pressure rise appears immediately and occurs before any increase in circulating plasma catecholamines.
It is a paradox that while smoking acutely increases blood pressure a slightly lower blood pressure level has been found among smokers than non-smokers in larger epidemiological and cross-sectional studies [Citation26,Citation27]. The mechanisms have been unknown though the development of chronic pulmonary disease and weight loss could be possible mechanisms [Citation24,Citation25]. Longitudinal studies have been scarce; however, in the Oslo Ischaemia Study 7 years of follow up of 1393 apparently healthy middle-aged men showed that smoking predicted a highly significant rise of 4.3 mmHg (p < 0.001) in the prognostic important peak systolic BP on 600 kpm/min for 6 min during ergometer exercise [Citation28]. These findings rather strongly suggest that smoking is a causative factor for high blood pressure when concomitant diseases are not involved or adjusted for in statistical multivariate analysis [Citation24,Citation25].
A high fraction of previous treatment for hypertension may potentially have influenced our results in the HOT Home BP sub-study. It is however unknown why patients participated in the sub-study beyond willingness. Our data are contradictory to the notion that smokers have normal or even low BP. On the contrary, our results add to a body of evidence that smoking is associated with higher BP. Possibly MUCH represents a clustering of CV risk factors [Citation29] and that the BP component in this ‘metabolic syndrome’ develops with time with the transition to uncontrolled BP as shown in the ELSA Study [Citation11]. We cannot distinguish whether our findings of smoking associated with MUCH is just an expression of chronic smoking or whether the patients are smoking prior to or during BP measurements at home. Smoking could potentially directly increase the participants’ home BP and contribute to the MUCH status of the patients.
Overweight and obesity were significant determinants of masked hypertension in general hypertension as well as in people with high normal BP [Citation30]. Our analysis suggests that MUCH is associated with higher BMI. This is compatible with the findings of other investigators [Citation31]. A review of the literature stated that studies have found patients with masked hypertension to be associated with higher BMI or waist to hip ratio compared to normotensives [Citation32]. As mentioned another large study suggested an association with obesity, compared to controlled hypertension [Citation15]. In contrast to MUCH, we found WUCH to be associated with less body weight, which remains unexplained but supported in the literature [Citation18,Citation33].
In the general population, age may be a significant determinant of masked and white coat hypertension [Citation30]. However, this is not necessarily a finding in drug-treated hypertensive patients, neither in the ELSA Study [Citation11] nor in the present HOT sub-study.
Our study has some limitations worth mentioning. The HOT Study and the home BP sub-study took place more than 20 years ago at a time when the evidence in support of aggressive BP lowering was less obvious. This was evident in the protocol [Citation1], which instructed investigators to treat patients to modest target diastolic BPs such as ≤80, ≤85 or ≤90 mmHg without emphasis on systolic BP control. The sub-study patients participated based on willingness, only, and potentially they are not ideally representative for the HOT Study population though their characteristics were similar [Citation9]. This subgroup of patients is likely to be more adherent to medication and lifestyle measures thus achieving better BP control. Thus, if less adherent to medication the CONTROLLED BP subgroup could potentially be even more limited than what we found in this study. On the other side, recent hypertension guidelines [Citation8] recommend to delete the home BP data of the first day and to include only days 2–7 into the analysis (i.e. 36 readings in our study). However, to achieve acceptable readings as previously detailed [Citation9], the patients went through the measurement procedure repeatedly at the clinic visits before taking home measurements. The accuracy of the home measurements minimally influenced by including day 1 in the analyses was supported by the low within-individual SDs for home BP and the consistency of the main results with a small variation of the home findings and SDs compared with the office readings.
As mentioned above, our data cannot discriminate between effects on home BP of acute and chronic smoking.
In conclusion, our data support that ‘reversed or masked’ treated but uncontrolled hypertension (MUCH) is common and constitutes about 25% of treated hypertensive patients. This entity (MUCH) is rather strongly associated with current smoking and overweight while uncontrolled white coat (office) hypertension (WUCH) is associated with lower BMI. It is obvious to us that the phenomenon of ‘reversed or masked’ treated but uncontrolled hypertension (MUCH) affects a major fraction of the general population. Consequently, MUCH must be the target for future research.
Acknowledgements
This study received support from Astra, Mölndal, Sweden. The authors are thankful to Nils-Gunnar Pehrsson at the HOT Coordinating Center at Sahlgrenska Hospital Östra, now at Statistiska Konsultgruppen, Gothenburg, Sweden, for data processing and statistical work.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- HOT Study Group. The Hypertension Optimal Treatment Study (the HOT Study). Blood Press. 1993;2:62–68.
- Hansson L, Hedner T, Dahlöf B. Prospective Randomized Open Blinded End-point (PROBE) Study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press. 1992;1(2):113–119.
- Hansson L, Zanchetti A. for HOT Study Group. The Hypertension Optimal Treatment (HOT) Study: patients characteristics – randomization, risk profiles, and early blood pressure results. Blood Press. 1994;3(5):322–327.
- Hansson L, Zanchetti A, Carruthers SG, et al. for the HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755–1762.
- Mejia AD, Julius S, Jones KA, et al. The Tecumseh Blood Pressure Study: normative data on blood pressure self-determination. Arch Intern Med. 1990;150(6):1209–1213.
- Kjeldsen SE, Moan A, Petrin J, et al. Evaluation of self-measured home vs. clinic intra-arterial blood pressure. Blood Press. 1993;2(1):28–34.
- Julius S. Home blood pressure monitoring: advantages and limitations. J Hypertens. 1991;9(3):S41–S46.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314–340.
- Kjeldsen SE, Hedner T, Jamerson K, et al. Hypertension Optimal Treatment (HOT) Study: home blood pressure in treated hypertensive subjects. Hypertension. 1998;31(4):1014–1020.
- Lithell H, Berglund L. Validation of an oscillometric blood pressure measuring device: a substudy of the HOT Study. Hypertension Optimal Treatment. Blood Press. 1998;7(3):149–152.
- Mancia G, Facchetti R, Cuspidi C, et al. Limited reproducibility of MUCH and WUCH: evidence from the ELSA Study. Eur Heart J. 2020;41(16):1565–1571.
- Kjeldsen SE, Os I. Poor reproducibility of masked and white coat un-controlled hypertension – important new information on MUCH and WUCH. Eur Heart J. 2020;41(16):1572–1574.
- Siddiqui M, Judd EK, Dudenbostel T, et al. Masked uncontrolled hypertension is not attributable to medication non-adherence. Hypertension. 2019;74(3):652–659.
- Kjeldsen SE, Os I. Are people with masked hypertension adherent to their antihypertensive medication? Hypertension. 2019;74(3):497–498.
- Banegas JR, Ruilope LM, de la Sierra A, et al. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35(46):3304–3312.
- Kjeldsen SE, Jamerson K, Julius S, et al. Characteristics of reversed office hypertension – may treatment resistant home hypertension be explained by smoking? J Hypertens. 2004;22(2):S138.
- Vriz O, Nesbitt S, Krause L, et al. Smoking is associated with higher cardiovascular risk in young women than in men: the Tecumseh Blood Pressure Study. J Hypertens. 1997;15(2):127–134.
- Obara T, Ohkubo T, Funahashi J, et al. Isolated uncontrolled hypertension at home and in the office among treated hypertensive patients from the J-HOME Study. J Hypertens. 2005;23:1653–1660.
- Makris TK, Thomopoulos C, Papadopoulos DP, et al. Association of passive smoking with masked hypertension in clinically normotensive nonsmokers. Am J Hypertens. 2009;22(8):853–859.
- Mikkelsen KL, Wiinberg N, Høegholm A, et al. Smoking related to 24-h ambulatory blood pressure and heart rate: a study in 352 normotensive Danish subjects. Am J Hypertens. 1997;10(5):483–491.
- Narkiewicz K, Maraglino G, Biasion T, et al. Interactive effect of cigarettes and coffee on daytime systolic blood pressure in patients with mild essential hypertension. Hypertension Ambulatory Recording VEnetia STudy (HARVEST) Study Group (Italy). J Hypertens. 1995;13(9):965–970.
- Bolinder G, de Faire U. Ambulatory 24-h blood pressure monitoring in healthy, middle-aged smokeless tobacco users, smokers, and nontobacco users. Am J Hypertens. 1998;11(10):1153–1163.
- Groppelli A, Giorgi DM, Omboni S, et al. Persistent blood pressure increase induced by heavy smoking. J Hypertens. 1992;10(5):495–499.
- Omvik P. How smoking affects blood pressure. Blood Press. 1996;5(2):71–77.
- Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Press. 2005;14(2):69–71.
- Green MS, Jucha E, Luz Y. Blood pressure in smokers and nonsmokers: epidemiologic findings. Am Heart J. 1986;111(5):932–940.
- Mann SJ, James GD, Wang RS, et al. Elevation of ambulatory systolic blood pressure in hypertensive smokers. A case control study. JAMA 1991;265(17):2226–2228.
- Mundal R, Kjeldsen SE, Sandvik L, et al. Predictors of 7-year changes in exercise blood pressure; effects of smoking, physical fitness and pulmonary function. J Hypertens. 1997;15(3):245–249.
- Mundal R, Kjeldsen SE, Sandvik L, et al. Clustering of coronary risk factors with increasing blood pressure at rest and during exercise. J Hypertens. 1998;16:19–22.
- Alwan H, Pruijm M, Ponte B, et al. Epidemiology of masked and white-coat hypertension: the family-based SKIPOGH Study. PLoS One. 2014;9(3):e92522.
- Mallion JM, Clerson P, Bobrie G, et al. Predictive factors for masked hypertension within a population of controlled hypertensives. J Hypertens. 2006; 24:2365–2370.
- Verberk WJ, Thien T, de Leeuw PW. Masked hypertension, a review of the literature. Blood Press Monit. 2007;12(4):267–273.
- Hwang ES, Choi KJ, Kang DH, et al. Prevalence, predictive factor, and clinical significance of white-coat hypertension and masked hypertension in Korean hypertensive patients. Korean J Intern Med. 2007;22(4):256–262.
