Abstract
Purpose
Blood pressure telemonitoring and remote counselling (BPTM) improves blood pressure (BP) control in patients with hypertension (HTN). Studies assessing the efficacy of BPTM from a value-based perspective are lacking. We investigated whether BPTM fits all principles of the value-based approach (clinical and economic effectiveness, improvement in patient-reported outcome/experience measures (PROM/PREM)).
Materials and methods
Two hundred and forty ambulatory patients with uncontrolled HTN were randomised in a 2: 1 manner to BPTM (n = 160, mean age 47 y.o.) and usual care (UC, n = 80; 49 y.o.) with baseline and 3-month follow-up clinic visits. BPTM employed a mobile application (for patients) and a desktop version (for clinician), which allowed communication and exchange of medical data. The main outcomes were changes in office and ambulatory systolic (S) BPs, rate of BP control. The incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratio (ICUR) were evaluated in economic analysis. The MOS SF-36 score was taken as a PROM, and the PEQ score was used as a PREM.
Results
Larger decreases in office and ambulatory SBPs (–16.8 and −8.9 mm Hg, respectively; p < .05) was achieved in BPTM group while the treatment intensity was equal (2.4 drugs). The ICER 11.1 EUR/–1 mm Hg 24-hour SBP/1 year was 75% effective as per willingness-to-pay threshold. BPTM improved PROM (+2.1 in mean MOS SF-36; p = .04), reduced long-term mortality (+0.11 life years gained), leading to +0.49 quality-adjusted life years (QALYs) gained as compared with UC. The ICUR was 4 169.4 EUR/QALY gained. Patient-reported experience was higher in the BPTM (+10 PEQ, p = .01). The UC group showed minor changes in MOS SF-36 and PEQ (+1.3; +6, respectively; p n.s.).
Conclusions
Being cost-effective, BPTM incorporates both clinical benefits and patient-perceived value. Larger randomised studies are needed to confirm our findings.
Introduction
High blood pressure (BP) appears to be the major risk factor responsible for increased global morbidity and mortality [Citation1]. Prevalence of hypertension (HTN) among the adult population varies between 32% and 46% across the globe, with 44% in the Russian Federation [Citation2]. Despite the effective therapeutic options and promising device-based interventions, HTN is still poorly controlled in the Russian population. The main reasons for this are therapeutic inertia and non-adherence [Citation3].
During the last few decades, global healthcare encountered multiple challenges, including an increasing prevalence of HTN and growing HTN-associated costs. Estimated annual costs of HTN management exceed 10% of healthcare expenditure in high-income countries, reaching almost $3.4 trillion [Citation4]. To overcome the economic burden and to improve quality of care, organisational reforms that emphasise value over volume are urgently needed. The paradigm is shifting from evidence-based to value-based medicine (VBM) in the majority of industrialised countries [Citation5]. VBM combines the best evidence-based data with data from cost–utility analysis based on the patient-reported outcome measures (PROMs) that are recommended by the regulatory bodies [Citation6]. Generic and disease-specific PROMs may evaluate the effectiveness of new interventions from a patient’s perspective, and this evaluation is undoubtedly crucial. VBM aims at encouraging the use of medical services to derive maximum benefit without serious healthcare expenditures.
It is worth noting that information and communication technologies (ICTs) are considered to be promising in solving the problem of increased medical indigence and untenably high costs. e-Health and m-Health solutions provide greater access to medical care. Available evidence shows that telehealth simplifies self-monitoring of BP, improves adherence, ensures patient centeredness, and ultimately gives higher value at a lower cost [Citation7].
This study aimed to investigate whether BP telemonitoring with remote counselling (BPTM) goes in line with all the principles of VBM in producing meaningful clinical benefit, patient centeredness, and cost effectiveness.
Materials and methods
Study population
The study was a three-month open-label prospective two-arm randomised study. Patients were screened for eligibility according to inclusion criteria: uncontrolled HTN with a systolic BP (SBP) level ≥ 140 mmHg (measured at least on two prestudy office visits), ambulatory mean 24-hour SBP ≥ 130 mm Hg or/and home SBP ≥ 135 mm Hg, and ongoing treatment with at least one antihypertensive drug in the last 3 months.
Exclusion criteria were as follows: symptomatic cardiovascular or other major comorbidities requiring close medical monitoring, pregnancy, significant cognitive impairment, and active or acute mental problem. All of the patients were to have a personal computer or a smartphone/tablet with instant Internet access and video-link. Eligible patients underwent a training session with a technician. Then the patients were randomised using an online random number generator to BPTM and usual care (UC) groups at a 2: 1 ratio.
All patients signed the informed consent document at the randomisation visit. The study was carried according to the ICH GCP standards and the Helsinki Declaration of the World Medical Association. The protocol was approved by the local ethics committee.
All participants were given an information package, which contained publicly available leaflets on lifestyle modification. During the baseline and end-of-study visits, the patients were asked to fill in the Medical Outcome Study 36-Item Short Form Survey (MOS SF-36) and Patient Experience Questionnaire (PEQ), which represented PROM and PREM, respectively.
Office blood pressure measurement
Office BP was measured conventionally (attended) at baseline and after three months, using an expert-level device (Omron M3 Expert, OMRON Healthcare, Kyoto, Japan). Each time, office BP was measured after 5 min of quiet rest in sitting position with the back and arm supported. An appropriate bladder cuff was used, encircling at least 80% of the arm. Three serial BP readings were taken 1–2 min apart, and the average of the last two readings was displayed.
Ambulatory blood pressure monitoring
We performed 24-hour ABPM at baseline and after three months, using a validated, automated, non-invasive, oscillometric device (BPLab, Petr Telegin Ltd., Russian Federation). The procedure described in detail in the (Online Supplement).
Blood pressure telemonitoring and remote counseling intervention
A free simple website and a mobile application were developed for patient–physician communication as well as storage and exchange of medical information. Patients in BPTM group used the mobile application, while web-based software was installed on office computers at the clinical site. Hardware-based security was maintained by a double-encryption protocol. Each BPTM patient was managed by the same physician throughout the follow-up period. Full-time technical support was available ().
Figure 1. Blood pressure telemonitoring system. The icon “Doctor” was created by Wilson Joseph and the Authors purchased the license at www.thenounproject.com to use this icon for unlimited amount of time.
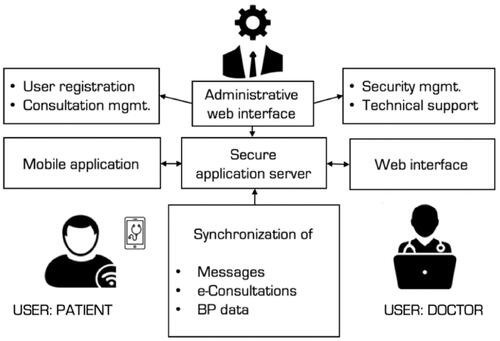
During the baseline visit, all BPTM patients underwent a brief training session on the home BP monitoring (HBPM) technique. Their HBPM device had to be listed in the ‘STRIDE BP’ database [Citation8] and was checked for accuracy. The initial guidance was to record BP measurements bis in die (morning and evening) until SBP reached the on target level [<135 mm Hg]. Then the patients had to self-measure BP monthly for 7 consecutive days with 3 readings obtained twice daily. Instructions were given in a written form duplicated with a supporting sheet. Patients were instructed to measure their BP and then to enter the data manually into the BP diary. Under the protocol, the supervising physician had to have checked the BP readings on a weekly basis. The number of BP readings transmitted by each patient was totalled and averaged separately for SBP and DBP. A traffic light coding was applied to average BP readings as per current guideline-recommended HTN grading [Citation1] (‘green’ for normotension, ‘yellow’ for HTN grade 1 or 2, and ‘red’ for HTN grade 3. In case a consultation was needed, a patient could opt to use the chat by texting questions (i.e. regarding symptoms, BP data, laboratory or instrumental findings). Physicians would then reply to the query. Alternatively, physicians could initiate a consultation themselves based on the results of weekly BP reports. For the BPTM group, the physician was asked to use the HBPM readings to guide antihypertensive treatment decisions. Additional information on BPTM intervention can be found in the (Online Supplement).
Patients allocated to the UC group were asked to continue regular office check-ups according to the routine practice with an intended three-month follow-up visit. If they were already monitoring their home BP, they were not discouraged from continuing. If questions arose regarding medications or general health, they could telephone or e-mail a doctor. For UC, physicians were asked to base their treatment decisions on the initial clinic BP readings.
Treatment adherence
Screening for adherence was performed by witnessed drug intake (directly observed therapy, or DOT) in all patients at the baseline visit (Online Supplement). Each time when there was a change in treatment (dose adjustment or switching to another medicine), only the BPTM patients were invited for a video call to ensure drug intake (by FaceTime or Skype). Self-reported home BP data input was an additional indicator of adherence in the BPTM group. The criteria for treatment adherence were defined as follows: ‘good’- BP data entered every week and a video call every time the medication regimen changed; ‘moderate’ – BP data entered inconsistently (not every week, but fairly regularly or after the doctor’s notification) and video calls made half of the time; and ‘poor’ – BP data not entered and video calls not made.
The main outcomes were reductions in office, ambulatory, and home SBPs. Additional outcomes included the number of patients with target SBP (<140 mm Hg, <130 mm Hg, <135 mm Hg for office, 24-hour, and home SBP, respectively), number of patients with fully controlled HTN (both <140/90 mm Hg and <130/80 mm Hg for office and 24-hour SBP, DBP, respectively), changes in quality of life (using generic PROM, MOS SF-36 assessments) and quality of care (generic ambulatory PREM, PEQ assessments), number of remote consultations, and medication changes.
Cost effectiveness modelling and evaluation
This study used Markov cohort-level simulation to analyse the hypothetical cohort of patients (n = 1000 per each arm) in the initial health state (uncontrolled HTN) and then distributed through various health states (complications) annually (cycle length = 1 year). The model was run over a 10-year time horizon to capture all relevant long-term costs and consequences. More information presented in the (Online Supplement), the structure of the Markov model is provided in (Supplemental Figure 1).
An ICER was expressed as a cost of an additional −1 mm Hg of mean 24-hour SBP per year per patient. A cost–utility analysis was performed to estimate the incremental cost–utility ratio (ICUR), which is the difference in costs divided by the difference in quality-adjusted life years (QALYs), expressed as life years gained multiplied by utility value. The results are presented as additional cost per 1 QALY gained per patient. A cost-effectiveness acceptability curve was built to reflect the probability for the BPTM intervention to be cost effective in relation to the International Monetary Fund (2 138 235 RUB or 30 358.57 EUR for the Russian Federation).
Statistical analyses
Our main outcome was the difference in the changes in SBP over 3 months. A randomised clinical trial of a home BP monitoring service for hypertensive patients suggests a 3-month reduction in SBP between the intervention and UC groups is approximately 4.8 mm Hg with a standard deviation of approximately 13 mm Hg [Citation9]. Based on these values, the sample sizes of 172 patients in the BPTM group and 86 in the UC group (including 10% dropout) ensured 80% power at 95% confidence for a two-sample t-test at a 2: 1 allocation.
The Student t-test and Wilcoxon rank-sum test were used to conduct intergroup comparisons of SBP, DBP, PROM, PREM, and number of antihypertensive medications. We evaluated the differences between intervention and control groups with continuous primary outcomes (office and 24-hour SBP, DBP changes) using linear regression models adjusted for baseline using F-tests (ANCOVA). Categorical variables, such as target BP achievement and differences in medications prescribed, were compared using the Pearson χ2 test. Results were considered significant at a p-value < .05. Statistical analyses were carried out using SPSS version 23 (IBM SPSS, Chicago, IL, United States); economic analyses employed PCT Mathcad 15.0 (PTC Inc., Boston, MA, United States).
Results
Study population
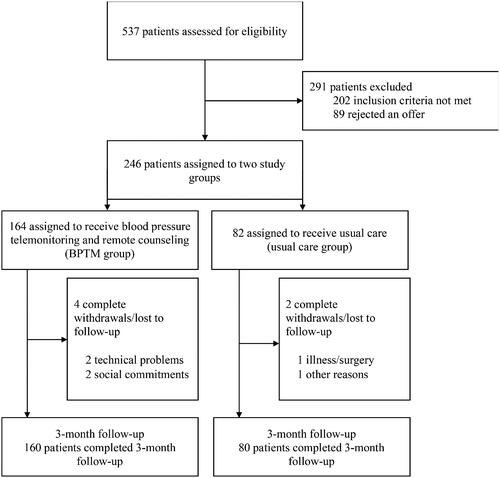
Of 537 patients who took part in the initial survey and were assessed for eligibility, 291 patients were excluded: 202 did not meet the inclusion/exclusion criteria and 89 rejected the offer to participate. Of those not meeting inclusion criteria, 65 had white coat HTN, 56 had established cardiovascular diseases, 38 had no or little digital literacy and 43 achieved the target BP in 3 months prior to randomisation. The remaining 246 patients were trained and randomised to the BPTM (n = 164) or UC (n = 82) group. No follow-up data were available for 4 patients in the BPTM group and 2 patients in the control group (). The groups were well matched with respect to baseline parameters ().
Table 1. Baseline data.
Clinical effectiveness
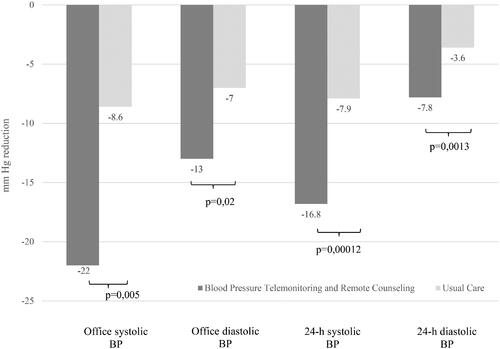
After 3 months, there was a significant reduction in office SBP in the BPTM group (from 157.5 ± 16.5 to 134.0 ± 13.0 mm Hg; Δ = −22.1 ± 12.4 mm Hg, 95% CI [–18.0 to −26.2 mm Hg], p = .0001). The DBP level decreased from 94.4 ± 9.3 mm Hg at baseline to 80 ± 8 mm Hg with the reduction Δ = −13.0 ± 10.8 mm Hg (95% CI [–10.0 to −17.1 mm Hg], p = .0001). The UC group similarly showed moderate reductions in office SBP (from 164.9 ± 27.3 to 156.2 ± 25.6 mm Hg, Δ = −8.6 ± 22.4 mm Hg, 95% CI [+0.3 to −17.0 mm Hg], p = .11) and office DBP (from 98.1 ± 13.1 to 91.2 ± 12.3 mm Hg, Δ = −7.1 ± 11.3 mm Hg, 95% CI [–3.0 to −11.1 mm Hg], p = .002). After adjustment for baseline office BP, the mean between-group differences in office SBP and office DBP were Δ = −16.1 ± 6.2 mm Hg, p = .005 and Δ = −8.4 ± 3.4 mm Hg, p = .02, respectively.
The mean baseline 24-hour SBP was 159.3 ± 9.1 mm Hg in the BPTM group and 158.8 ± 10.1 mm Hg in the UC group (p = .86). Mean SBP decreased to 141.2 ± 10.2 and 149.5 ± 8.4 mm Hg in the two groups, respectively, at the end of follow-up (adjusted mean SBP Δ = −16.8 ± 2.9 and 7.9 ± 3.9 mm Hg, p = .0001). The mean 24-hour DBP was 89.2 ± 4.3 mm Hg in the BPTM group and 92.1 ± 4.1 mm Hg in the UC group at baseline (p = .81) and decreased to 82.1 ± 3.2 and 88.5 ± 2.6 mm Hg, respectively, after 3 months, resulting in Δ = −7.8 ± 3.0 and 3.6 ± 4.2 mm Hg, respectively (p = .001 for interaction when adjusted for baseline ambulatory DBP).
By the end of the study, home SBP values decreased from 151.0 ± 12.2 to 142.7 ± 10.1 mm Hg and DBP values decreased from 83.4 ± 6.1 to 79.8 ± 4.7 mm Hg in the BPTM group. The home BP reduction was Δ = −9.1 ± 1.3 mm Hg (95% CI [–7.3 to −11.2 mm Hg], p < .0001) for SBP and Δ = −5.4 ± 0.9 mm Hg (95% CI [–3.0 to −8.1 mm Hg], p < .0001) for DBP ().
The patients of the BPTM group were more likely to achieve the target office and 24-hour SBPs at 3 months (110 patients [69%] versus 20 patients [25%] in the UC group [25%]; odds ratio (OR) 6.60; 95% CI [3.59–12.15]; χ2=55.2, p = .0013). The target office SBP was reached by 120 (75%) patients in the BPTM group and 23 (29%) in the UC group (OR 7.4, 95% CI [4.1–13.6]). When both, office and ambulatory SBPs and office and 24-hour DBPs were analysed, there were 102 (64%) and 11 (14%) patients with fully controlled HTN in the BPTM and UC groups, respectively (OR 11.0, 95% CI [5.4–22.5]).
Of all 160 BPTM patients, 117 (72%) patients were fully adherent to BPTM procedures; 35 (22%) were deemed to be moderately adherent (27 of them did not comply fully with the HBPM protocol and 8 made only one video call after the medication regimen was changed); and the other 8 (5%) patients had poor adherence, that is, they did not made a video call after a change in treatment scheme (while continuing to enter BP data). Thus, there were 125 patients with good adherence to HBPM in the active group.
A total of 42 UC patients provided the study team with their HBPM logs at the final clinic appointment (34 patients shared paper copies and the other 8 patients had digital logs in the memory of a mobile device). When interviewed retrospectively, 16 of these patients performed HBPM accurately and in line with given recommendations; and 22 HBPM logs had sufficient quality to facilitate clinical decision making. Because of the small number of suitable logs (<30% of the UC patients), the results of HBPM are not presented for this group.
Health-related quality of life (HRQoL) increased slightly but significantly for physical health (SF-36 score 2.9 ± 1.3, 95% CI [1.6 to 4.2], p = .04) and nonsignificantly for mental health (MOS SF-36 score 1.4 ± 1.7, 95% CI [–0.3 to 3.1], p = .15) in the BPTM patients. Patient-perceived quality of care also improved in the active arm (PEQ score 10 ± 5.6, 95% CI [4.4–15.6], p = .001). The UC group showed minor improvements by both MOS SF-36 and PEQ (physical health score 1.3 ± 2.3, mental health score 0.8 ± 2.4, p > .5 in both cases; PEQ score 6.1 ± 7.1, p = .534). None of these qualitative changes was associated with office, ambulatory, and home BP reductions in the BPTM and UC groups.
Each of the 160 patients in the BPTM group received at least one remote consultation during the study. The number of consultations was the highest at the beginning of the study (n = 87 new requests in the first week). The total number of requests was 438 (approximately 3 per patient, 95% CI [1–11]). The requests concerned technical issues (105, 24%), general health (158, 36%), and antihypertensive therapy (175, 40%). In the UC group, 7 patients received telephone consultations from the study team. All of these patients complained of a persistent BP elevation detected while performing HBPM and were advised to up-titrate the medicines they were already taking. None of the patients in the BPTM and UC groups had additional clinic visits during the study. Certain laboratory tests were recommended to patients in both groups at the baseline visit (mean number of tests per patient 3.2 ± 1.6). Due to the nature of the study, some of the BPTM patients were recommended additional laboratory tests (2.1 ± 0.8 tests per patient) in the first month of the study.
Antihypertensive treatment adjustments were performed in 44 patients (27.5%) of the BPTM group. The changes included up- or down-titration (20 patients), class-to-class switching (15 patients), and original-to-generic switching (9 patients). Despite these treatment decisions, the number of treatments prescribed remained largely unchanged by the end of the study (2.1 ± 1.5 to 2.4 ± 1.5 (+0.3 drugs, 95% CI [–0.2 to 1.1 drugs], p = .115)). Due to the study design, the UC patients received medications prescribed at the start of the study, and treatment adjustments made in 7 UC patients did not affect the mean number of antihypertensive drugs (2.5 drugs, 95% CI [1–4 drugs], p = .87 for a between-group comparison).
Cost-effectiveness and cost-utility analyses
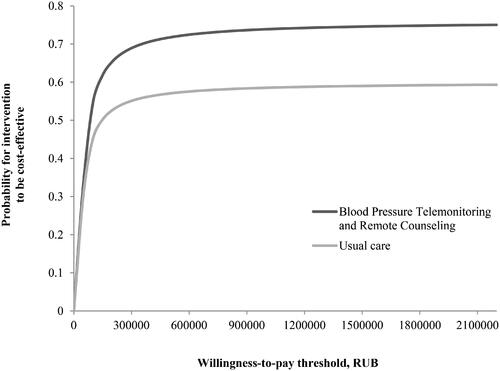
Model inputs, quality, and cost parameters are shown in the (Supplemental Table 1). The overall costs for one year amounted to 21 220.6 and 14 714.2 RUB (301.3 and 208.9 EUR) for BPTM and UC, respectively. An ICER of BPTM resulted in additional 731.1 RUB (11.1 EUR)/1 mm Hg/year. The cost-effectiveness analysis and the corresponding CEAC show the BPTM service would be cost effective in 76% of iterations. This result is in line with the willingness-to-pay threshold assumed for the Russian Federation (). More details on cost-effectiveness analysis are presented in the (Online Supplement).
Figure 4. According to the results the derived cost-effectiveness acceptability curves show that with BPTM strategy is almost 10% more effective than usual care (55% versus 45%) at a willingness-to-pay threshold of 100 000 RUB. When this threshold comes to 300 000 RUB the gap between two strategies comes to a linear function, while the probability of effectiveness also reaches a plateau. It appears from the curves that there is a probability of BPTM being cost-effective is 76% if decision makers were willing to pay at least 300 000 RUB per 24-hour BP decrease.
The QALYs gained per patient were estimated at 8.31 for BPTM and 7.82 for UC (+0.49 QALYs gained in favour of BPTM). The cost of 1 QALY gained per patient was 275 178.98 RUB, or 4 169.4 EUR (134 837.70 RUB [2 043 EUR] per 0.49 per patient), being fully affordable within the given the willingness-to-pay threshold.
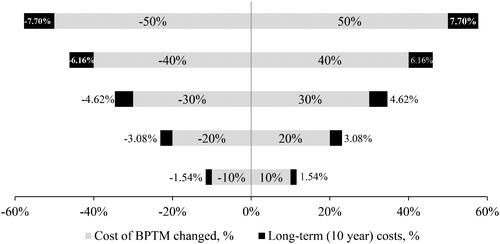
Possible uncertainties in the model results were assessed using univariate deterministic sensitivity analysis (the impact of variation of the cost of BPTM up to ±50% in increments of 10%). The cumulative costs of BPTM in a 10-year period was found to be ±7.70% in the case of a 50% change, suggesting sustainability and accessibility for the intervention ().
Figure 5. The sensitivity analysis was performed with the single but crucial parameter – the cost of BPTM service. The results were robust when increasing or decreasing the cost of BPTM by 50% because these changes lead to only 7.7% change in cumulative costs. BPTM strategy therefore remained cost-effective. BP: blood pressure; BPTM: blood pressure telemonitoring and remote counseling.
Discussion
The HTN burden has grown dramatically, and the trend is predicted to continue at least in the next 20 years [Citation10]. Addressing long-term (improved prognosis) and short-term (improved HRQoL) concerns should be affordable and attractive to healthcare decision makers and government high-ranking executives. Due to melting budgets and a widening gap between demand and spending, the current status of evidence-based medicine is unsustainable and it is prime time for an integrated ‘value-based’ approach [Citation11]. This could hardly be achieved without new technologies, particularly digital health [Citation12]. Telemonitoring with or without remote counselling may complement conventional care and improve BP control and adherence [Citation13] and becomes even more valuable in case of force majeure events. During COVID-19 pandemic many patients with chronic diseases are left alone but exactly cardiovascular diseases (notably HTN) are frequent comorbidities in COVID-19 increasing risks of adverse outcomes [Citation14]. Hypertensive patients require professional guidance, and in the majority of cases the help can be easily provided remotely without loss in privacy and security [Citation15].
In this article, we report the three-month results of an intervention based on home BP telemonitoring and additional counselling. Clinical, patient-centered and economic benefits were demonstrated to affirm that BPTM is consistent with the value-based paradigm.
The evidence supports that HBPM can lead to favourable clinical effects among hypertensives without telemonitoring [Citation16]. But a crucial aspect for conventional HBPM is data reporting and interpretation (often handwritten and inaccurate). In our study, more than a half of UC patients (n = 42) underwent HBPM but with different accuracy. Telemonitoring may enhance the quality of reported information and facilitate its interpretation. In our study manual transcription of measured BP values in the app presents a challenge of inaccurate BP data input leading to a terminal (end) digit bias [Citation17]. Further improvement of our system is to add the option of automated data transmission from HBPM device to the app and then to a clinician to ensure all the measurements will be stored and analysed properly [Citation18]. Despite telemonitoring, a more pronounced effect is seen when HBPM is combined with the additional support or counselling [Citation19]. Our study showed that BPTM led to greater decreases in office and ambulatory BPs as compared with UC. The result is consistent with recently published data. Omboni and Guarda have shown modest reductions of SBP and DBP (mean difference −5.6 mm Hg and −2.8 mm Hg, respectively) and a 31% increase in BP control rate in the telemonitoring group [Citation13]. Likewise, a 30% gap in BP control rate, favouring telemonitoring, has been reported by Verberk et al. [Citation20]. On the other hand, Wiedmer et al. have obtained contradictory results with a slight SBP reduction (–1.18 mm Hg; p = .19) still associated with an impact on morbidity [Citation21].
Despite extreme importance of adequate compliance, there is still no ‘gold standard’ test [Citation22]. Our study used the widely recognised DOT technique [Citation23]. We observed that over two thirds of patients had good adherence in the BPTM group. This result is consistent with the findings reported by Kerby et al. who have shown a 73% rate of adherence to a telemonitoring protocol [Citation24].
Importantly, HTN is often asymptomatic and the patient may attach little, if any, importance to HRQoL, but the latter is usually affected significantly [Citation25]. This notion prompted us to explore the effect of BPTM on the patient’s HRQoL and experience of care. We showed a significant improvement of PROM and PREM in the BPTM group. Data on HRQoL from modern telehealth trials are limited, and the results published to date are rather contradictory. In their systematic review, Purcell et al. did not find any improvement in patient-perceived HRQoL [Citation26]. On the contrary, Madsen et al. reported that bodily pain and general health were slightly better in the telemonitoring group after 6 months [Citation27].
We suggest that both, clinical and patient-reported outcome changes cannot be explained by treatment decisions alone. Some the studies reported an increased number of drugs with the intervention [Citation13], while a few studies have not [Citation28]. Antihypertensive treatment was not hugely intensified during our study, although more fixed-dose combinations and, possibly, more potent drugs were prescribed to some of the patients in the BPTM group. Importantly, active medical supervision stimulated the BPTM patients to adhere with treatment intake and BP monitoring. However, any intervention per se encourages patients to perform better in clinical trials. The general Hawthorne effect [Citation29] cannot therefore be ruled out, and the results should be interpreted in this context. As the present trial was not blinded, it is prone to bias, specifically selection bias (the subgroup performance bias). Masked telehealth studies with some sort of active interventions in control groups (i.e. mailing or texting BP data without counselling) are urgently needed.
Even a slight BP reduction may lead to an enormous economic effect. We revealed that there is no short-term economic benefit of BPTM but the investments will pay off in the long term. Both ICER and ICUR fit well within the cost-effectiveness margins applied in the Russian Federation [Citation30]. In the 6-month HITS trial, BP telemonitoring was more expensive though more effective with an ICER of £25.60/mm Hg (95% CI [£16.05 to £46.69]) [Citation31]. The landmark TASMINH2 trial showed that a 12-month telehealth intervention is lifesaving (+0.18 QALYs gained) and at least 99% cost-effective in the long term [Citation32]. Other potential strengths and novelties are discussed in the (Online Supplement).
Study limitations
Firstly, the small population size introduces potential selection bias. More digitally literate and technically equipped patients were included, and this is a case for ascertainment bias. It is expected that the digital divide will largely disappear in the future [Citation33]. Randomisation seemed to work well, the mean baseline cardiovascular risk was moderate to high in both groups, increasing the homogeneity and facilitating further cost analysis.
Secondly, study duration is 3 months. There is a strong prejudice against short-term trials because the majority of already completed lasted 6 to 12 months [Citation31,Citation32]. Three months is the minimum time interval required by the Guidelines to perform clinic visits [Citation1]. The key idea was to provide the initial support for a patient who is a ‘newcomer’ in an referral centre to improve the odds of treatment success.
Thirdly, our economic analysis based on hypothetical long-term probabilities of an effective but short program. We evaluated costs in close to real-world setting. Patients were recruited at an outpatient department, medical therapy was not standardised (a quasi-experimental model). We did not include the costs of additional phone consultations performed in the UC group because these were few and of an unknown duration. The costs of BPTM service were based on the prospective financial estimates according to Compulsory Medical Insurance Fund. Nowadays, the doctor-to-patient telehealth is gaining momentum in Russian Federation and the tariffs for remote monitoring and counselling are to be formed on the basis of such small studies predominantly for high-risk working population. Other barriers, limitations and their potential solutions are discussed in the (Online Supplement).
Conclusion
The results demonstrate that a protocol utilising simple telehealth software is capable of performing better than the usual approach. The implementation of this user-friendly technique led to PROM and PREM improvements independently of a BP reduction. Both clinical benefits and patient-perceived value suggest that BPTM is cost-effective in the long-term. Therefore, BPTM fits well within the value-based healthcare paradigm. Larger trials with long-term follow-up are needed to confirm our findings.
Online_Supplement2.docx
Download ()Disclosure statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Additional information
Funding
References
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018; 27(6):314–340.
- Boytsov SA, Balanova YA, Shalnova SA, et al. Arterial hypertension among individuals of 25–64 years old: prevalence, awareness, treatment and control. By the data from ECCD. Cardiovasc Ther Prev. 2014;13(4):4–14.
- Ahluwalia M, Bangalore S. Management of hypertension in 2017: targets and therapies. Curr Opin Cardiol. 2017;32(4):413–421.
- Foy AJ, Mandrola JM. Heavy heart: the economic burden of heart disease in the United States now and in the future. Prim Care. 2018;45(1):17–24.
- Bae J-M. Value-based medicine: concepts and application. Epidemiol Health. 2015;37:e2015014.
- US Department of Health and Human Services. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. 2009. [cited 2020 Aug 21]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf.
- Omboni S, Ferrari R. The role of telemedicine in hypertension management: focus on blood pressure telemonitoring. Curr Hypertens Rep. 2015;17(4):535.
- STRIDE BP (Science and Technology for Regional Innovation and Development in Europe). Valid blood pressure monitors (section Home). [cited 2020 Aug 21]. Available from: https://www.stridebp.org/bp-monitors.
- Rogers MA, Small D, Buchan DA, et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med. 2001;134(11):1024–1032.
- Forouzanfar MH, Liu P, Roth GA, et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317(2):165–182.
- Kelly MP, Heath I, Howick J, et al. The importance of values in evidence-based medicine. BMC Med Ethics. 2015;16(1):69.
- Milani RV, Bober RM, Milani AR. Hypertension management in the digital era. Curr Opin Cardiol. 2017;32(4):373–380.
- Omboni S, Guarda A. Impact of home blood pressure telemonitoring and blood pressure control: a meta-analysis of randomized controlled studies. Am J Hypertens. 2011;24(9):989–998.
- Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547.
- Nittari G, Khuman R, Baldoni S, et al. Telemedicine practice: review of the current ethical and legal challenges. Telemed J E-Health Off J Am Telemed Assoc. 2020.
- Uhlig K, Patel K, Ip S, et al. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185–194.
- Parker RA, Paterson M, Padfield P, et al. Are self-reported telemonitored blood pressure readings affected by end-digit preference: a prospective cohort study in Scotland. BMJ Open. 2018;8(1):e019431.
- Parati G, Dolan E, McManus RJ, et al. Home blood pressure telemonitoring in the 21st century. J Clin Hypertens. 2018;20(7):1128–1132.
- Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389
- Verberk WJ, Kessels AGH, Thien T. Telecare is a valuable tool for hypertension management, a systematic review and meta-analysis. Blood Press Monit. 2011;16(3):149–155.
- Widmer RJ, Collins NM, Collins CS, et al. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc. 2015;90(4):469–480.
- Carey RM, Calhoun DA, Bakris GL, American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53–e90.
- Hjørnholm U, Larstorp ACK, Andersen MH, et al. Directly observed therapy prior to ambulatory blood pressure measurement (DOT-HTN) in uncontrolled hypertensive patients - Effect on blood pressure, safety and patient perception. Blood Press. 2019;28(5):327–335.
- Kerby TJ, Asche SE, Maciosek MV, et al. Adherence to blood pressure telemonitoring in a cluster-randomized clinical trial. J Clin Hypertens (Greenwich). 2012;14(10):668–674.
- Trevisol DJ, Moreira LB, Kerkhoff A, et al. Health-related quality of life and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2011;29(2):179–188.
- Purcell R, McInnes S, Halcomb EJ. Telemonitoring can assist in managing cardiovascular disease in primary care: a systematic review of systematic reviews. BMC Fam Pract. 2014;15:43.
- Madsen LB, Kirkegaard P, Pedersen EB. Health-related quality of life (SF-36) during telemonitoring of home blood pressure in hypertensive patients: a randomized, controlled study. Blood Press. 2008;17(4):227–232.
- Zahr RS, Anthony CA, Polgreen PM, et al. A texting-based blood pressure surveillance intervention. J Clin Hypertens (Greenwich). 2019;21(10):1463–1470.
- Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:h4672.
- World Bank National Accounts Data, and OECD National Accounts Data Files. GDP per Capita. [cited 2020 Aug 21]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
- Stoddart A, Hanley J, Wild S, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): cost and cost-effectiveness analysis of a randomized controlled trial. BMJ Open. 2013;3(5):e002681.
- Kaambwa B, Bryan S, Jowett S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a cost-effectiveness analysis. Eur J Prev Cardiol. 2014;21(12):1517–1530.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154–161.