Abstract
Purpose
We hypothesise that dietary sodium intake interacts with serum uric acid to influence blood pressure (BP) in children and adolescents. In the present study, we investigated ambulatory BP in relation to hyperuricaemia, dietary sodium intake and their interaction in children and adolescents with hypertension.
Materials and methods
A total of 616 study participants were 10–24 years old and had primary hypertension diagnosed after admission in a specialised inpatient ward. Ambulatory BP monitoring was performed during hospitalisation. 24-h urine was collected for measurements of electrolytes. Hyperuricaemia was defined as a serum uric acid of ≥327.25 μmol/L in patients <18 years old and of ≥420 and ≥360 μmol/L, respectively, in male and female patients ≥18 years old.
Results
In adjusted analyses, patients with hyperuricaemia (n = 283), compared with those with normal serum uric acid, had similar 24-h systolic BP (131.7 mmHg, p = 0.54) and a significantly (p ≤ 0.005) lower 24-h diastolic BP (77.5 vs. 80.9 mmHg) and higher 24-h pulse pressure (54.2 vs. 51.7 mmHg). In similar adjusted analyses, 24-h ambulatory pulse pressure, but not systolic/diastolic BP (p ≥ 0.12), significantly differed across the quartile distributions of urinary sodium excretion (p for trend ≤ 0.04). Further adjusted analyses showed significant (p ≤ 0.04) interaction between serum uric acid and urinary sodium excretion in relation to 24-h systolic BP. In patients with hyperuricaemia (p = 0.04), but not those with normal serum uric acid (p = 0.13), 24-h systolic BP was significantly associated with urinary sodium excretion, with a 6.5 ± 2.1 mmHg difference between quartiles 4 and 1. Similar results were observed for daytime and night-time BP and pulse pressure.
Conclusions
Both hyperuricaemia and higher dietary sodium intake were associated with higher pulse pressure, and their interaction further heightened systolic BP.
Introduction
Although the 2018 European Society of Cardiology/European Society of Hypertension guidelines on the management of hypertension included hyperuricaemia as a factor for risk assessment [Citation1], it is still controversial whether hyperuricaemia is a causal risk factor of hypertension, and if yes, how it increases BP [Citation2,Citation3]. The results of several recent studies suggest that dietary sodium intake may interact with the urinary excretion of uric acid to influence BP regulation potentially [Citation4–6]. In short-term studies, sodium intake increases the urinary excretion and decreases the serum concentration of uric acid [Citation4,Citation6]. Long-term health consequences of such a phenomenon, however, are unknown. Indeed, in long-term studies, high dietary sodium intake is associated with an increased risk of hyperuricaemia and hypertension [Citation5].
A speculative mechanism is that high urinary excretion of uric acid induced by high dietary sodium intake in the long run leads to the decay of renal function in the excretion of uric acid and to high serum concentration of uric acid [Citation5,Citation6], which may activate sodium-uric acid co-transportation in the renal tubules and increase sodium reabsorption and BP [Citation6]. This mechanism may be relevant in susceptible populations, such as children and adolescents. At similar levels of perfusion pressure, children and adolescents have higher renal blood flow [Citation7–9] and higher glomerular filtration rate [Citation9,Citation10] and hence high sodium and uric acid load in the renal tubules. Allopurinol showed blood pressure lowering effect in children and adolescents with hypertension and hyperuricaemia [Citation11] but not in their adult counterparts [Citation12].
We hypothesise that dietary sodium intake interacts with serum uric acid to influence BP, especially in children and adolescents. In the present study, we investigated ambulatory BP in relation to hyperuricaemia, dietary sodium intake and their interaction in children and adolescents with hypertension.
Methods
Study participants
Our cross-sectional study included 898 children and adolescents (10–24 years old) with hypertension, who admitted in the Department of Hypertension, Ruijin Hospital, Shanghai, China from 2000 to 2020. We excluded from the present analysis 195 patients with secondary hypertension and 87 patients because of missing information. Thus, the total number of participants included in the present analysis was 616 patients with primary hypertension. The study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China. All study participants or their proxy gave informed written consent.
Ambulatory BP measurement
Ambulatory BP monitoring was performed during hospitalisation. We programmed validated oscillometric SpaceLabs 90217 monitors (SpaceLabs, Inc; Redmond, WA, USA) to obtain BP readings at 20-min intervals during daytime (06:00–22:00) and at 30-min intervals during night-time (22:00–06:00). All recordings covered >20 h and included ≥10 readings during the awake period and ≥5 readings during sleep. Mean values were weighed for the time interval between consecutive readings.
Questionnaire and anthropometric measurement
An experienced physician inquired into each patient’s medical history, intake of medications, and current smoking and alcohol intake. Anthropometric measurements included body weight, body height, and waist and hip circumferences. A nurse measured body height to the nearest 0.5 cm. Patients wore light indoor clothing without shoes for body weight measurement. Body mass index was calculated as the body weight in kilograms divided by the body height in metres squared.
Blood and urine measurements
Venous blood samples were taken after overnight fasting for the measurement of plasma glucose and insulin concentration, serum concentrations of triglycerides, total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol, creatinine and uric acid. A 24-h urine was collected for measurements of sodium and potassium during hospitalisation. A standardised hospital diet was supplied for all patients but not controlled for the quantity of meals or drinks.
Hyperuricaemia was defined as a serum uric acid concentration of 327.25 μmol/L (5.5 mg/dL) or higher in patients less than 18 years old [Citation13], and as a serum uric acid concentration of 420 μmol/L or higher and 360 μmol/L or higher, respectively, in male and female patients of at least 18 years old, similarly as adults [Citation2].
Statistical analyses
For database management and statistical analyses, we used SAS software (version 9.4, SAS Institute, Cary, NC, USA). Means and proportions were compared by the Student t-test and Fisher’s exact test, respectively. We performed analyses of covariance for between-group comparisons and multiple linear regression for continuous analyses. p values <0.05 were considered to be statistically significant.
Results
Characteristics of patients
A total of 616 children and adolescents had a mean age of 19.1 years (range, 13–24 years) and included 488 male and 128 female patients. Male and female patients differed significantly (p ≤ 0.04) in most of the characteristics, except age and fasting plasma insulin concentration (p ≥ 0.38, ).
Table 1. Characteristics of the patients by gender.
Male, compared with female patients, had a significantly (p ≤ 0.001) higher 24-h (133.9 ± 11.6 vs. 129.8 ± 14.3 mmHg), daytime (138.6 ± 12.9 vs. 133.0 ± 15.4 mmHg) and night-time (122.3 ± 13.9 vs. 121.4 ± 16.3 mmHg) systolic BP, lower 24-h (78.1 ± 9.5 vs. 83.9 ± 11.7 mmHg), daytime (82.6 ± 10.3 vs. 86.4 ± 12.3 mmHg) and night-time (70.4 ± 11.3 vs. 75.8 ± 14.0 mmHg) diastolic BP, and a lower proportion of antihypertensive treatment (71.1% vs. 81.3%).
Male, compared with female patients, had significantly higher 24-h urinary excretions of sodium (151 ± 68 vs. 126 ± 63 mmol) and potassium (36.9 ± 16.2 vs. 31.9 ± 14.3 mmol), and had a higher serum uric acid concentration (394 ± 81 vs. 298 ± 76 μmol/L) and prevalence of hyperuricaemia (51.2% vs. 25.8%),
Ambulatory BP in relation to hyperuricaemia
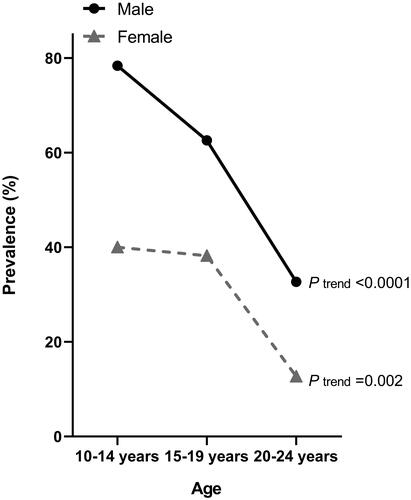
The prevalence of hyperuricaemia was higher with younger age in male (78.4, 62.6 and 32.7% in patients of 10–14, 15–19 and 20–24 years old, respectively, p for trend < 0.0001) as well as female patients (40.0, 38.2 and 12.7%, respectively, p for trend = 0.002, ).
Figure 1. Prevalence of hyperuricaemia by age and gender. Symbols represent the prevalence of hyperuricaemia in youths with primary hypertension. p values for trend are given for male and female patients separately.
After adjustment for sex, age, body mass index, current smoking and alcohol intake, serum creatinine and use of antihypertensive drugs, patients with hyperuricaemia (n = 283), compared with those with normal serum uric acid (n = 333), had similar 24-h systolic BP (131.7 mmHg, p = 0.54) and had a significantly (p ≤ 0.005) lower 24-h diastolic BP (77.5 vs. 80.9 mmHg) and higher 24-h pulse pressure (54.2 vs. 51.7 mmHg, ). Similar trends were observed for daytime and night-time BP and pulse pressure ().
Table 2. Ambulatory blood pressure and 24-h urinary electrolytes by hyperuricaemia.
With similar adjustments applied as above, patients with hyperuricaemia had significantly (p ≤ 0.01) higher urinary excretions of sodium (153.3 ± 4.1 vs. 138.6 ± 3.9 mmol) and potassium (38.3 ± 1.0 vs. 34.0 ± 0.9 mmol) than patients with normal serum uric acid, but similar sodium/potassium ratio ().
Ambulatory BP in relation to urinary sodium excretion
In similar adjusted analyses according to the quartile distributions of urinary sodium excretion, ambulatory systolic and diastolic BP were not significantly (p ≥ 0.12) associated with urinary sodium excretion except for daytime diastolic BP (p = 0.04). However, ambulatory pulse pressure was significantly higher with higher urinary sodium excretion (p trend ≤ 0.04, ). 24-h, daytime and night-time pulse pressure were 4.4, 3.0 and 2.2 mmHg higher in quartile 4 than quartile 1 of urinary sodium excretion (p ≤ 0.04).
Table 3. Ambulatory blood pressure according to the quartile distributions of 24-h urinary sodium excretion.
Ambulatory BP in relation to the interaction between serum uric acid and urinary sodium excretion
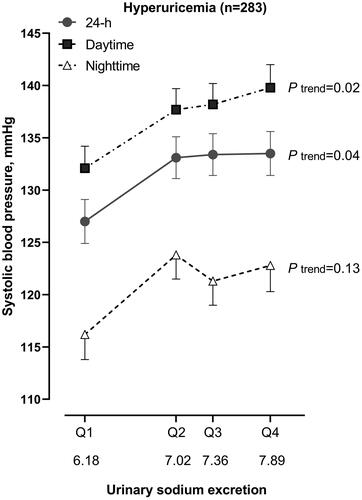
Further adjusted analyses demonstrated significant (pint ≤0.04) interaction between urinary sodium excretion and serum uric acid in relation to 24-h systolic BP. Indeed, in patients with hyperuricaemia (p = 0.04) but not those with normal serum uric acid (p = 0.13), 24-h systolic BP was significantly higher with higher urinary sodium excretion, with a 6.5 ± 2.1 mmHg of difference between quartiles 4 and 1 of urinary sodium excretion (p = 0.03, ). Similar trends were observed for daytime and night-time systolic BP. The corresponding differences between quartiles 4 and 1 of urinary sodium excretion were 7.7 ± 2.2 mmHg (p = 0.01) and 6.5 ± 2.4 mmHg (p = 0.07), respectively. When the analysis was performed according to the tertile distributions of serum uric acid, the results were reproducible. Only in the top tertile of serum uric acid, 24-h systolic BP was higher in quartile 4 than quartile 1 of urinary sodium excretion (7.3 ± 2.3 mmHg, p = 0.02).
Figure 2. 24-h, daytime and night-time systolic blood pressure according to the quartile distributions of 24-h urinary sodium excretion in patients with hyperuricaemia. Symbols represent mean value adjusted for age, sex, body mass index, current smoking and alcohol intake and use of antihypertensive drugs. The vertical lines denote standard error. p values for trend are given.
Discussion
Our study in children and adolescents showed that hyperuricaemia was prevalent in young hypertensive patients, especially boys of 10–14 years old. Both hyperuricaemia and higher dietary sodium intake were associated with higher ambulatory pulse pressure, and their interaction further heightened ambulatory systolic BP. Our study demonstrated clinical relevance of hyperuricaemia in the pathogenesis of primary hypertension in children and adolescents. Both dietary sodium intake and hyperuricaemia are typically lifestyle-related. Fructose intake is a proven risk factor of hyperuricaemia [Citation14]. Dietary salt and sugar restriction might have to be implemented already early in childhood for the prevention of hypertension and cardiovascular complications in adulthood [Citation15].
Our observation on the high prevalence of hyperuricaemia in 10–14 years old children and adolescents with primary hypertension is in keeping with the results of several previous studies [Citation14,Citation16–18]. In a group of 6–18 years old untreated children with newly diagnosed hypertension (n = 125), the prevalence of hyperuricaemia, defined as a serum uric acid concentration of ≥327.25 μmol/L (5.5 mg/dL), was higher in patients with primary hypertension (n = 56, 89%) than those with secondary hypertension (n = 12, 30%) [Citation14]. Similarly, in the National Health and Nutrition Examination Survey (NHANES) 1999–2006 [Citation18], adolescents (12–17 years old) with an elevated BP (≥95th percentile for age, sex and height, n = 209) had a significantly higher prevalence of hyperuricaemia (≥327.25 μmol/L [5.5 mg/dL]) than those with normal BP (50% vs 33%, p < 0.001). Hyperuricaemia is probably particularly important for the so-called ‘primary’ hypertension in children and adolescents.
The observed widening pulse pressure associated with hyperuricaemia and dietary sodium intake in the present study is resulted from the lower diastolic BP instead of higher systolic BP. This phenomenon in the young is different from the widening of pulse pressure in elderly people with isolated systolic hypertension, in whom it is the consequence of arterial stiffness and higher systolic BP [Citation19]. The mechanism of the widening of pulse pressure and lower diastolic BP in the young is incompletely understood. A speculative mechanism is that hyperuricaemia [Citation20] and excessive sodium intake [Citation21] trigger sympathetic activity, and in turn dilatates arterials, which reduces peripheral resistance and diastolic BP. However, the sympathetic overactivation in the long run will cause arterial stiffness and then higher systolic BP, as observed in those patients with the combination of hyperuricaemia and high dietary sodium intake.
Our finding on the interaction between dietary sodium intake and serum uric acid in relation to BP is in line with the results of a large (n = 4062) population-based prospective observational study [Citation5]. During 5–9 years of follow-up, dietary sodium intake at baseline was positively associated with serum uric acid concentration during follow-up (1.4 μmol/L per 1 g higher sodium intake at baseline, 95%CI 0.2–2.6). Dietary sodium intake was also associated with a higher incidence of hypertension but only in the study participants with the baseline serum uric acid concentration in the top tertile (hazard ratio 1.09 per 1 g higher sodium intake, 95% CI 1.02–1.16). Taken the results of this previous and our current study together, hyperuricaemia might be an indicator of renal dysfunction, which increases sodium sensitivity and BP in the presence of high dietary sodium intake. This mechanism might be particularly relevant for Asians, because they tend to be more likely salt-sensitive and have high dietary sodium intake [Citation22]. These factors not only increase the risk of hypertension, especially sodium related nocturnal hypertension [Citation23], but also render hypertension difficult to control to the target [Citation24,Citation25] and more harmful to the organs [Citation26].
The underlying mechanism for the relationship between BP, dietary sodium intake and hyperuricaemia is not completely understood. There is evidence that high serum sodium increases urinary uric acid excretion, and high serum and urinary uric acid impairs renal function [Citation27]. A speculative mechanism is therefore hyperuricaemia as a consequence of high dietary sodium intake in the long run impairs kidney function and finally leads to high BP in the presence of the continued high dietary sodium intake [Citation6]. This mechanism may be particularly relevant for children and adolescents. Glomerular filtration rate is high in the young. There is a high load of uric acid for the kidney to reabsorb or excrete. That is probably why there is a close association between hyperuricaemia and the onset and progression of primary hypertension in adolescents [Citation28,Citation29], and allopurinol lowers BP in adolescents with hypertension and hyperuricaemia but not in their adult counterparts [Citation12].
Our study should be interpreted within the context of its limitations. First, our study is cross-sectional and does not allow any causal inference. Second, our study had a relatively small sample size and included hypertensive patients only. Third, most of our study participants took antihypertensive drugs. These drugs not only lower BP but may also influence the metabolism of sodium and uric acid. Finally, although a standardised hospital diet was supplied for all patients, it was not controlled for the quantity of meals or drinks.
Conclusions
In children and adolescents with primary hypertension, both hyperuricaemia and higher dietary sodium intake were associated with higher ambulatory pulse pressure, and their interaction further heightened ambulatory systolic BP. If confirmed in future prospective studies, our finding is towards the definition of a special form of renal dysfunction, with significantly reduced function of sodium and uric acid excretions but with well-preserved glomerular filtration rate.
Disclosure statement
Dr Wang reports receiving lecture and consulting fees from Astra-Zeneca, Bayer, Novartis, Omron, Salubris, Servier and Takeda. The other authors declared no conflicts of interest.
Additional information
Funding
References
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36(12):2284–2309.
- De Becker B, Borghi C, Burnier M, et al. Uric acid and hypertension: a focused review and practical recommendations. J Hypertens. 2019;37(5):878–883.
- Sanchez-Lozada LG, Rodriguez-Iturbe B, Kelley EE, et al. Uric acid and hypertension: an update with recommendations. Am J Hypertens. 2020;33(7):583–594.
- Todd AS, Walker RJ, MacGinley RJ, et al. Dietary sodium modifies serum uric acid concentrations in humans. Am J Hypertens. 2017;30(12):1196–1202.
- Forman JP, Scheven L, de Jong PE, et al. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation. 2012;125(25):3108–3116.
- Lei L, Wang JG. Dietary sodium intake and serum uric acid: a mini-review. Pulse (Basel). 2018;6(1–2):124–129.
- Porter LE, Hollenberg NK. Obesity, salt intake, and renal perfusion in healthy humans. Hypertension. 1998;32(1):144–148.
- Price DA, Fisher ND, Osei SY, et al. Renal perfusion and function in healthy African Americans. Kidney Int. 2001;59(3):1037–1043.
- Hollenberg NK, Rivera A, Meinking T, et al. Age, renal perfusion and function in island-dwelling indigenous Kuna Amerinds of Panama. Nephron. 1999;82(2):131–138.
- Hilliard LM, Nematbakhsh M, Sampson AK, et al. Gender differences in pressure-natriuresis and renal autoregulation: role of the Angiotensin type 2 receptor. Hypertension. 2011;57(2):275–282.
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932.
- McMullan CJ, Borgi L, Fisher N, et al. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory BP: a randomized controlled trial. Clin J Am Soc Nephrol. 2017;12(5):807–816.
- Rodenbach KE, Schneider MF, Furth SL, et al. Hyperuricemia and progression of CKD in children and adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort Study. Am J Kidney Dis. 2015;66(6):984–992.
- Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252.
- Fujiwara T, Kikuchi K, Hoshide S, et al. Usefulness of a salt check sheet for elementary school and junior high school children. J Clin Hypertens. 2019;21(6):722–729.
- Round JM. Changes in plasma urate, creatinine, alkaline phosphatase and the 24 hours excretion of hydroxyproline during sexual maturation in adolescents. Ann Hum Biol. 1980;7(1):83–88.
- Wilcox WD. Abnormal serum uric acid levels in children. J Pediatr. 1996;128(6):731–741.
- Loeffler LF, Navas-Acien A, Brady TM, et al. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999–2006. Hypertension. 2012;59(4):811–817.
- Mattace-Raso FU, Verwoert GC, Hofman A, et al. Inflammation and incident-isolated systolic hypertension in older adults: the Rotterdam study. J Hypertens. 2010;28(5):892–895.
- Lambert EA, Eikelis N, Sari CI, et al. Serum uric acid and the relationship with subclinical organ damage in adults. J Hypertens. 2017;35(4):745–752.
- Babcock MC, Brian MS, Watso JC, et al. Alterations in dietary sodium intake affect cardiovagal baroreflex sensitivity. Am J Physiol Regul Integr Comp Physiol. 2018;315(4):R688–R695.
- Sun N, Mu J, Li Y, et al. Working committee of salt evaluation, blood pressure management, Chinese Medical Association Hypertension Professional Committee, hypertension group, Chinese Society of cardiology. An expert recommendation on salt intake and blood pressure management in Chinese patients with hypertension: a statement of the Chinese Medical Association Hypertension Professional Committee. J Clin Hypertens. 2019;21(4):446–450.
- Kario K. Nocturnal hypertension: new technology and evidence. Hypertension. 2018;71(6):997–1009.
- Guo QH, Zhang YQ, Wang JG. Asian management of hypertension: current status, home blood pressure, and specific concerns in China. J Clin Hypertens (Greenwich). 2020;22(3):475–478.
- Chia YC, Kario K, Turana Y, et al. Target blood pressure and control status in Asia. J Clin Hypertens (Greenwich). 2020;22(3):344–350.
- Kario K. The HOPE Asia Network activity for ‘zero’ cardiovascular events in Asia: overview 2020. J Clin Hypertens (Greenwich). 2020;22(3):321–330.
- Abou-Elela A. Epidemiology, pathophysiology, and management of uric acid urolithiasis: a narrative review. J Adv Res. 2017;8(5):513–527.
- Pan S, He CH, Ma YT, et al. Serum uric acid levels are associated with high blood pressure in Chinese children and adolescents aged 10–15 years. J Hypertens. 2014; 32:998–1003.
- Feig DI. The role of uric acid in the pathogenesis of hypertension in the young. J Clin Hypertens (Greenwich). 2012;14(6):346–352.
