Abstract
Purpose
Current evidence regarding renal involvement in pheochromocytoma and paraganglioma (PPGL) is scant. More accurate diagnostic methods, such as renal Doppler ultrasound for intrarenal hemodynamic studies, may provide more detailed information on renal function. It might be postulated that renal function in PPGL patients might be altered by high blood pressure and excess secretion of catecholamines. The aim of this prospective study was to assess intrarenal blood flow parameters in PPGL patients included in the prospective monoamine-producing tumour (PMT) study and to evaluate the effects of normalisation of catecholamine production after surgical treatment on long-term renal function.
Materials and methods
Seventy consecutive patients (aged 46.5 ± 14.0 years) with PPGL were included. Forty-eight patients from the PMT study cohort, matched for age, gender, blood pressure level and presence of hypertension, served as a control group. Renal artery doppler ultrasound spectral analysis included mean resistance index (RRI) and pulsatility index (PI). Forty-seven patients completed 12 months follow-up.
Results
There were no differences in renal parameters such as RRI, PI and kidney function between PPGL and non-PPGL patients as assessed by renal ultrasound, serum creatinine, eGFR and albumin excretion rate. No correlations between kidney function parameters, intrarenal doppler flow parameters and plasma catecholamines were observed in PPGL patients. At 12 months after surgery, no differences in creatinine level, eGFR, albumin excretion rate, RI and PI were found as compared to baseline results.
Conclusions
In contrast to patients with other forms of secondary hypertension, our study did not show differences in intrarenal blood flow parameters and renal function between PPGL and non-PPGL subjects. Intrarenal hemodynamics and renal function did not change after normalisation of catecholamine levels by surgical treatment.
Introduction
Pheochromocytomas and paragangliomas (PPGL) are rare catecholamine-producing tumours of chromaffin cell origin, which derive from the adrenal medulla or the extra-adrenal sympathetic nervous system respectively [Citation1,Citation2].
Clinically, PPGL present with symptoms associated with catecholamine excess and can also present with life-threatening complications resulting in acute end organ damage. Cardiovascular events, such as hypertension crisis, stroke, myocardial infarction and catecholamine-induced Takotsubo cardiomyopathy are known to be frequent potential consequences of high levels of circulating catecholamines in PPGL patients [Citation1–3].
Current evidence regarding renal involvement in PPGL is scant and is mostly limited to few experimental and clinical studies performed between 1950 and 1970, which indicated preserved renal function under those study conditions [Citation4]. These early clinical studies included small numbers of patients and used relatively simple techniques. In addition, although renal failure is not major clinical problem in PPGL patients, subclinical renal dysfunction may occur and go undetected in regular clinical care. It is also not established, to what extent causal treatment of PPGL may affect renal function and hemodynamics in long-term follow-up.
Therefore, more accurate available methods can provide better insight into renal function and renal hemodynamics. Doppler duplex ultrasonography has been proven as a useful and non-invasive tool for evaluating renal vascular function in several pathological conditions.
Among intrarenal blood flow parameters, the renal resistance index (RRI) derived from the Doppler pulsatile flow-velocity waveform correlates significantly with renal structural changes and outcomes in patients with essential hypertension, atherosclerotic renal artery stenosis and primary aldosteronism [Citation5–11].
It might be postulated that renal function in PPGL patients might be altered by high blood pressure and excess secretion of catecholamines. Since no data are available on intrarenal hemodynamics in subjects with PPGL, the aim of our prospective study was to evaluate intrarenal blood flow parameters in patients included in the prospective monoamine-producing tumour (PMT) study [Citation12]. We also evaluated the effects of normalisation of catecholamine production after surgical treatment of PPGL on long-term renal function.
Materials and methods
Subjects
Our prospective cross-sectional study included 70 consecutive patients with PPGL who were diagnosed and treated in the Department of Hypertension, National Institute of Cardiology, Warsaw, Poland in years 2009–2017 (). These patients participated in the PMT study (https://pmt-study.pressor.org), which has been reported previously [Citation12].
Forty-eight patients from the PMT study cohort in whom a PPGL was excluded based on the criteria described previously served as a control group (non-PPGL group) [Citation12]. They were matched for age, gender, blood pressure (BP) level and presence of hypertension.
Exclusion criteria included a body mass index (BMI)>40 kg/m2, symptomatic coronary artery disease, heart failure with reduced ejection fraction of less than 40%, valvular heart disease, aortic aneurysm and alcohol abuse.
Demographic and clinical data (i.e. sex, age, weight, height, BP, heart rate, signs and symptoms and antihypertensive medications) were recorded at study entry according to the study protocol [Citation12].
Hypertension was defined as either a known history of hypertension, current use of antihypertensive drugs or systolic BP above or equal to 140 mm Hg or diastolic BP above or equal to 90 mm Hg on office measurements or daytime systolic BP above or equal to 135 mmHg or diastolic BP above or equal to 85 mm Hg on 24-h ambulatory BP monitoring (ABPM) [Citation13]. Known duration of hypertension was assessed from patients interviews and defined as the period between first diagnosis and/or treatment of hypertension and study entry. Paroxysmal hypertension was defined as episodic increments of BP of >40–50 mm Hg over usual patient’s BP values.
Of the 70 PPGL patients who entered the study, 65 underwent surgical treatment and in 63 subjects plasma catecholamine levels had normalised after surgery. Forty-seven of them completed 12 months follow-up visit with renal Doppler ultrasound (). There were no statistical differences in baseline clinical characteristics between patients with (n = 47) and without (n = 23) follow-up.
Renal ultrasound and doppler studies
For renal ultrasound investigation a Logiq E9 (GE, USA) ultrasound unit with multiphase 2–4 MHz convex array transducer was used. Measurements were obtained from interlobular arteries (on the level of edge of pelvis and parenchyma). Doppler US spectral analysis included mean resistance index (RRI = peak systolic velocity − end-diastolic velocity/peak systolic velocity) and pulsatility index (PI = peak systolic velocity − end-diastolic velocity/time averaged velocity) obtained from three Doppler curves at different sites of each kidney. For calculation, duplex scanner software was used. For each patient mean RRI and mean PI based on indices calculated in the left and right kidney were also calculated. Measurements were performed by two experienced investigators with interobserver and intraobserver coefficients of variance of RRI were 5.6% and 4.7%, respectively (n = 12).
Biochemical tests of catecholamine excess
Biochemical testing at study entry included mass spectrometric-based measurements of catecholamine metabolites (normetanephrine, metanephrine, methoxytyramine) in their free form in plasma and 24-h urine collections. The latter also allowed for measurements of catecholamines (adrenaline and noradrenaline) [Citation12,Citation14,Citation15].
Catecholamine biochemical phenotypes were assessed based on relative tumour-derived increases in plasma concentrations of normetanephrine, metanephrine, and methoxytyramine. Tumour-derived increments were calculated by subtracting from the concentration of each metabolite in each patient with a PPGL the mean concentration of the corresponding metabolites- normetanephrine (52 pg/mL), metanephrine (26 pg/mL) and methoxytyramine (5 pg/mL) in a previously described reference group [Citation14]. We defined adrenergic tumours as those exhibiting both an increase in plasma metanephrine above a predefined cut-off (62 pg/mL) and a tumour-derived increment of metanephrine larger than 5% of combined increments of all O-methylated metabolites. We defined all other tumours as non-adrenergic, including both noradrenergic and dopaminergic tumors [Citation15]. Within the non-adrenergic group only one patient was characterised by solitary increased secretion of dopamine but not of noradrenaline nor adrenaline.
Office and ambulatory BP measurements
BP was measured on hospital admission, before any modification of BP lowering medications, by a trained nurse, using a validated oscillometric an automated device (Omron 705IT, Omron Co., Kyoto, Japan), after 5 min of rest with patients in the sitting position. A cuff adapted to the arm circumference was placed on the arm with the lower edge of the cuff 2 cm above the antecubital fossa. Three consecutive readings were performed. In cases where the difference between readings was >10 mm Hg, further measurements were taken so as to obtain three consecutive consistent readings, the average of which was then used for analysis.
ABPM was carried out in all patients using a Spacelabs 90207 or 90217 device (Redmond, WA, USA). Readings were obtained every 15 min during the day (06:00–22:00) and every 30 min during the night (22:00–06:00). Recorded data included averaged 24-h, daytime and night-time systolic blood pressure and diastolic blood pressure and averaged 24-h heart rate (HR). The nocturnal decrease in BP was quantified as the relative decrease in nocturnal BP for both systolic and diastolic BP: [daytime pressure − night-time pressure)/daytime pressure] × 100% [Citation16].
Results
The 70 patients with PPGLs were matched for age, sex, BMI and office and 24-h BP values with the 48 non-PPGL subjects (). There were no differences in use of any particular antihypertensive drugs between groups (Supplementary Table 1) nor in the known duration of hypertension (including paroxysmal blood pressure). Office and 24-h heart rates were significantly higher in patients with than without PPGL. Patients with PPGLs were also characterised by less pronounced BP decline at night as compared to controls.
Table 1. Clinical and biochemical characteristics of patients in PPGL and non-PPGL groups.
In addition, there were no differences in renal parameters such as RRI, PI and kidney function between PPGL and non-PPGL patients as assessed by renal ultrasound, serum creatinine, estimated glomerular filtration rate (eGFR) and albumin excretion rate (), (Supplementary Table 2). An eGFR < 60 mL/min was found in the minority of patients in both PPGL and non-PPGL groups (10.3% vs 4.3%, p = .23, respectively). Moderately increased albuminuria was present in 11.4% patient in PPGL group and 13.3% patients in non-PPGL group (p = .82).
Figure 2. Renal parameters in PPGL and non-PPGL patients and changes of parameters in PPGL group after surgery treatment. Data are shown as mean values and standard deviations (whiskers). Abbreviations: eGFR, estimated glomerular filtration rate; PPGL, pheochromocytoma and paraganglioma.
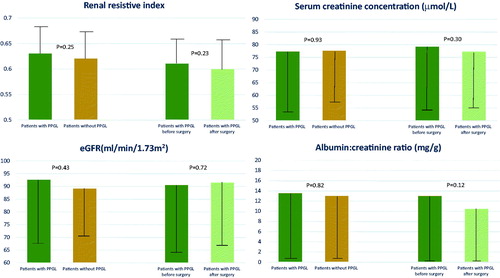
Table 2. Clinical, biochemical and renal characteristics of patients with adrenergic and non-adrenergic tumours.
In both groups of PPGL and non-PPGL patients there were significant though low correlations between RRI and age (r = 0.335; p = .020 and r = 0.347; p = .003, respectively), office diastolic BP (r= −0.288; p = .047 and r= −0.235; p = .05, respectively), 24-h diastolic BP (r= −0.485; p = .001, r= −0.279; p = .021, respectively), office pulse pressure (r = 0.265; p = .027 and r = 0.346; p = .016, respectively) and 24-h pulse pressure (r = 0.364; p = .002 and r = 0.276; p = .074, respectively). There was no significant correlation between kidney function parameters, intrarenal doppler flow parameters and plasma catecholamines concentration and urinary adrenaline and nor adrenaline excretion. There were no correlations between office and ABPM heart rate and parameters of renal function.
Among PPGL patients, 57 patients (84.1%) had pheochromocytoma, 8 patients (11.4%) a sympathetic paraganglioma and 5 patients (7.1%) both pheochromocytoma and paraganglioma. There were no differences in kidney function indices and intrarenal parameters between patients with adrenergic and non-adrenergic tumours ().
Patients with PPGL on alphablockade were characterised by lower eGFR as compared to PPGL subjects without alphablockade (82.98 vs 97.98 mL/min/1.73 m2, p = .017). Within the PPGL group, there were no differences in age, sex, blood pressure values, nor intrarenal parameters between those with and those without alphablockade. There were no differences in renal function parameters between patients with PPGL who were on alphablockade and non-PPGL patients who were treated with alphablockers.
After a median of 12 (IQR 11–12) months after surgery, 47 patients were re-evaluated. Compared to baseline, the frequency of hypertension was significantly lower at follow-up as were office and ambulatory BP, pulse pressure and HR levels. There was also a significant decrease in frequency of type 2 diabetes from 17.9% to 7.1% (p = .02) (Supplementary Table 3). In operated patients there were no differences in creatinine level, eGFR, albumin excretion rate, RRI and PI at baseline and after surgical treatment (), (Supplementary Table 4). There were no correlations between changes in BP and changes in renal parameters in the PPGL patients after surgery.
Discussion
To our best knowledge, this is the first study that has systematically evaluated intrarenal blood flow parameters in PPGL patients before and after surgery. An age, sex and BP matched cohort of non-PPGL patients served as a control group.
Our study shows that in PPGL patients the RRI, PI and renal function were within the range of normal values and were not different from those in non-PPGL subjects. This was also the case for the number of patients with moderately increased albuminuria. These data suggest that excessive amounts of catecholamines do not result in detrimental changes in intrarenal blood flow and vascular compliance in patients with a PPGL. This is supported by our finding that normalisation of catecholamine levels at 12 months after surgical treatment was not associated with any changes in RRI, PI, renal function or moderately increased albuminuria.
As no other studies have reported evaluation of renal function and intrarenal hemodynamics in subjects with PPGLs, it is not possible to compare our data with those of previous studies. However, an earlier report based on a small number of patients showed also that in the vast majority of patients with PPGL, serum creatinine levels were within normal limits and only occasionally slight elevations were noted [Citation4].
The outcome of our study is different from those in primary aldosteronism, another type of hormonal hypertension. In primary aldosteronism, increased renal perfusion pressure and glomerular hyperfiltration with decreased vascular resistance and pulsatility indices have been reported [Citation10].
In our study, a significant correlation was found between RRI values and both office diastolic BP and 24-h ABPM diastolic BP in PPGL patients. Also, a significant correlation was evident between RRI value and office pulse pressure and ambulatory 24-h pulse pressure, which may suggest a relationship between RRI value and PP, factors known to be associated with vascular stiffness. However, these findings also extend to a non-PPGL group, thus confirming renal hemodynamics in PPGL subjects is not specifically altered.
In our large series of PPGL patients, none demonstrated severe renal failure. Data regarding the incidence of acute renal failure in PPGL are scant. Pooled data analysis of 135 PPGL patients from three German tertiary hospitals showed that acute renal failure was present in 1.5% of patients [Citation17]. Also few case reports indicated that PPGL was accompanied by acute renal failure reversible after surgical treatment [Citation18,Citation19]. This may suggest that very high levels of circulating catecholamines may have detrimental effect on kidney function that was reversible after surgical treatment [Citation17–19]. These observations have been confirmed under experimental conditions indicating that increasing rates of infusion of catecholamines in dogs can cause a progressive fall in renal blood flow and decrease in glomerular filtration rate [Citation20–22].
The preserved renal function in patients with PPGL may be in part explained by the character of hypertension in those subjects. Of note, hypertension was generally well controlled both in office and on ABPM with a low median number of medications required for hypertension treatment. This may explain the preserved renal function and lack of subclinical organ damage in the kidney.
When evaluating the intrarenal hemodynamic parameters in PPGL subjects, also other factors with impact on RRI values should be considered [Citation8]. The location of the intrarenal Doppler measurement is known to influence RRI since it has been documented that RRI decreases from the hilum of the kidney towards the renal cortex. Therefore, if RRI is calculated from the flow pattern of the hilar artery, higher values of RRI are expected [Citation5–8]. While measurements of RRI in all consecutive patients were taken uniformly on the level of interlobular arteries, our results cannot be explained by the location of the intrarenal Doppler measurements.
Study limitations
Our study is based on a relatively large number of well-characterised patients with PPGL and an appropriate control group of patients without PPGL. In addition, we used contemporary imaging methods, including Doppler sonography. However, there are limitations to the data.
The potential impact of antihypertensive agents, particularly alphablockers, should be considered. However, in our study no difference was observed in intrarenal hemodynamics between patients treated with alphablockers and those without. Significantly lower eGFR in subjects on alphablockers was noted, however, the recorded values stayed within the normal range. Despite this limitation, our study provides for the first time, data regarding intrarenal hemodynamics and renal function in large group of PPGL subjects.
Conclusions
In conclusion, in contrast to patients with other forms of secondary hypertension, our study showed no differences in intrarenal blood flow parameters and renal function between PPGL and non-PPGL subjects. No changes in intrarenal hemodynamics and renal function were also found after normalisation of catecholamine levels by surgical treatment. This suggests there is no direct association between catecholamine excess and RRI and kidney function in these patients. Although PPGLs result in hypertension and cardiovascular alterations, there is no direct evidence that excessive catecholamine amounts lead to subclinical or more advanced kidney damage. Therefore, impairment of renal function should not be a specific major concern in PPGL patients.
Supplementary_Tables_03.11.2020.docx
Download MS Word (19.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Eisenhofer G, Pacak K, Huynh TT, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18(1):97–111.
- Prejbisz A, Lenders JW, Eisenhofer G, et al. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29(11):2049–2060.
- Geroula A, Deutschbein T, Langton K, et al. Pheochromocytoma and paraganglioma: clinical feature based disease probability in relation to catecholamine biochemistry and reason for disease suspicion. Eur J Endocrinol. 2019;181(4):409–420.
- Manger WM., Gifford RW, Jr. Clinical and experimental pheochromocytoma. Cambridge, MA: Blackwell Science; 1996.
- Krumme B, Hollenbeck M. Doppler sonography in renal artery stenosis-does the Resistive Index predict the success of intervention? Nephrol Dial Transplant. 2007;22(3):692–696.
- Krumme B, Blum U, Schwertfeger E, et al. Diagnosis of renovascular disease by intra- and extrarenal Doppler scanning. Kidney Int. 1996;50(4):1288–1292.
- Prejbisz A, Warchol-Celinska E, Florczak E, et al. Renal resistive index in patients with true resistant hypertension: results from the RESIST-POL study. Kardiologia Polska. 2016;74(2):142–150.
- Bardelli M, Veglio F, Arosio E, et al. New intrarenal echo-Doppler velocimetric indices for the diagnosis of renal artery stenosis. Kidney International. 2006;69(3):580–587.
- Monticone S, Sconfienza E, D'Ascenzo F, et al. Renal damage in primary aldosteronism: a systematic review and meta-analysis. J Hypertens. 2020;38(1):3–12.
- Sechi LA, Di Fabio A, Bazzocchi M, et al. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009;94(4):1191–1197.
- Ribstein J, Du Cailar G, Fesler P, et al. Relative glomerular hyperfiltration in primary aldosteronism. JASN. 2005;16(5):1320–1325.
- Eisenhofer G, Prejbisz A, Peitzsch M, et al. Biochemical diagnosis of chromaffin cell tumors in patients at high and low risk of disease: plasma versus urinary free or deconjugated O-methylated catecholamine metabolites. Clin Chem. 2018;64(11):1646–1656.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314–340.
- Eisenhofer G, Peitzsch M, Kaden D, et al. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clin Chim Acta. 2019;490:46–54.
- Eisenhofer G, Klink B, Richter S, et al. Metabologenomics of phaeochromocytoma and paraganglioma: an integrated approach for personalised biochemical and genetic testing. Clin Biochem Rev. 2017;38(2):69–100.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–2219.
- Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757–764.
- Fujiwara M, Imachi H, Murao K, et al. Improvement in renal dysfunction and symptoms after laparoscopic adrenalectomy in a patient with pheochromocytoma complicated by renal dysfunction. Endocrine. 2009;35(1):57–62.
- Celik H, Celik O, Guldiken S, et al. Pheochromocytoma presenting with rhabdomyolysis and acute renal failure: a case report. Ren Fail. 2014;36(1):104–107.
- Feigen LP, Coleman B, Glaviano VV. Effects of alpha-adrenergic blockade on renal function in hemorrhagic shock. Am J Physiol. 1977;232(5):F409–15.
- Berne RM, Hoffman WK, Jr., Kagan A, et al. Response of the normal and denervated kidney to L'epinephrine and L'nor-epinephrine. Am J Physiol. 1952;171(3):564–571.
- Lever AF, Mowbray JF, Peart WS. Blood flow and blood pressure after noradrenaline infusions. Clin Sci. 1961;21:69–74.