Abstract
Purpose
Available data of event-based clinical outcomes trials show that little evidence supports the guidelines recommendations to lower blood pressure (BP) to <130/80 mmHg in middle-aged and elderly people with type 2 diabetes mellitus and hypertension. We addressed this issue by post-hoc analysing the risk of cardiovascular (CV) events in mostly elderly high-risk hypertensive patients with type 2 diabetes mellitus participating in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial.
Material and methods
Patients (n = 5250) were divided into 4 groups according to the proportion of on-treatment visits before the occurrence of an event (<25% to ≥ 75%) in which BP was reduced to <140/90 or <130/80 mmHg.
Results
After adjustment for baseline demographic differences between groups, a reduction in the proportion of visits in which BP achieved <140/90 mmHg accompanied a progressive increase in the risk of CV mortality and morbidity as well as of cause-specific events such as stroke, myocardial infarction and heart failure. A progressive reduction in the proportion of visits in which BP was reduced <130/80 mmHg did not have any effect on CV risks.
Conclusion
In mostly elderly high-risk hypertensive patients with type 2 diabetes mellitus participating in the VALUE trial, achieving more frequently BP <140/90 mmHg showed a marked protective effect on overall and all cause-specific cardiovascular outcomes. This was not the case for a more frequent achievement of the more intensive BP target, i.e. <130/80 mmHg.
Introduction
Randomised event-based clinical outcomes trials [Citation1–4] show little evidence to support the recommendations of diabetes and hypertension guidelines [Citation5–7] that in patients with type 2 diabetes mellitus and hypertension, blood pressure (BP) should be treated to <130/80 mmHg rather than <140/90 mmHg. While in patients with diabetes, BP reductions to 130–139 mmHg systolic and 80–89 mmHg diastolic have usually been accompanied by reductions in cardiovascular (CV) and renal events, but on-treatment BP values <130/80 mmHg have usually shown no further protective effect as recently shown in a meta-analysis [Citation8]. This has been found also in post-hoc analyses of trials showing that in diabetes BP reductions <130/80 mmHg did not provide further CV or renal benefits than those obtained by reducing BP to <140/90 mmHg. Indeed, in some instances a trend to an increased CV outcome appeared [Citation9–11]. Thus, the optimal target BP has not been settled in patients with diabetes and hypertension, and this is particularly the case in the many elderly patients with the combinations of these diseases.
In the present study we investigated mostly elderly patients with type 2 diabetes mellitus and hypertension in the large database provided by the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial [Citation12]. We aimed to test the hypothesis put forward in recent guidelines [Citation5–7] that achieving a target BP <130/80 mmHg leads to a lower incidence of CV morbidity and mortality than achieving a target BP <140/90 mmHg in this population.
Material and methods
Participants
The design and the main results of the VALUE trial have been reported in detail previously [Citation12]. Briefly, VALUE was a multicenter, randomised, double-blind trial which compared the long-term effect of an antihypertensive treatment based on the angiotensin receptor blocker valsartan or the calcium antagonist amlodipine on cardiac morbidity and mortality in hypertensive patients of any ethnicity with an age ≥ 50 years and a high CV risk profile. The qualifying risk factors for recruitment were predefined combinations of male gender, age and other risk factors or the presence of ECG-based left ventricular hypertrophy (with or without a strain pattern), proteinuria, increased serum creatinine, diabetes or a verified but stable coronary, cerebrovascular or peripheral artery disease. Patients with renal artery stenosis, clinically relevant valvular disease, a recent (3 months) cerebrovascular event, coronary angioplasty or by-pass surgery, congestive heart failure requiring an ACE inhibitor and coronary disease requiring a beta-blocker were excluded from being randomised. Exclusion extended to pregnant women and individuals with severe hepatic disease.
Definition of type 2 diabetes mellitus
Type 2 diabetes mellitus was at the outset of the trial defined as the use of antidiabetic treatment or by the 1985 World Health Organisation (WHO) criteria (fasting glucose > 7.8 mmol/L [140 mg/dL] on ≥ 2 separate occasions). In 1999, during the course of the study, WHO changed the definition of type 2 diabetes mellitus to a fasting blood glucose of ≥ 7.0 mmol/L (126 mg/dL) and/or blood glucose ≥ 11.1 mmol/L (200 mg/dL) 2 h after oral intake of 75 g of glucose in venous plasma or serum (≥ 12.2 mmol/L [220 mg/dL] if capillary blood). During the blinded phase of the trial, the classification of new-onset diabetes was changed to adhere to the WHO 1999 criteria, and the new definition was explained in a study newsletter. Unfortunately, a 2006 VALUE paper [Citation13] did not catch the no. with type 2 diabetes mellitus according to 1999 WHO criteria, but this was corrected from 2007 [Citation14].
Blood pressure measurements and treatment
Both treated and untreated hypertensive patients were considered for inclusion into the trial. Untreated patients were recruited if their systolic BP was between 160 and 210 mmHg and diastolic BP was <115 mmHg. Treated patients were recruited if their systolic BP was <210 mmHg or diastolic BP <115 mmHg. The recruited patients were rolled-over into one or the other arm of the trial without a run-in phase. For valsartan treatment started with 80 mg daily and for amlodipine with 5 mg daily. The dose of either drug was doubled and hydrochlorothiazide (12.5 mg and 25 mg daily) and other antihypertensive drugs were added in sequential steps if BP was not reduced <140/90 mmHg. Angiotensin receptor blockers were excluded from the treatment algorithms and ACE inhibitors and calcium channel blockers only allowed if required for conditions other than hypertension. Patients were followed-up for 4–6 years with visits performed monthly during the initial 6 months of treatment and at 6 months intervals thereafter. Blood pressure was measured three times during each visit, with the patient in the sitting position, after 5 min rest and 24 h post-dose. Blood pressure was measured using a calibrated standard sphygmomanometer or a validated digital device, and mean BP was calculated as the mean of all three readings.
Outcomes
The primary endpoint of the study was time to first cardiac event, i.e. a composite of fatal or non-fatal myocardial infarction, sudden cardiac death and death from revascularization procedures or heart failure, heart failure requiring hospitalisation and emergency procedures to prevent myocardial infarction. Secondary endpoints were all events, fatal and non-fatal stroke, myocardial infarction, hospitalised heart failure, and CV, non-CV and all-cause mortality. An endpoint committee, blind to treatment allocation, adjudicated events.
Statistical analyses
Because the primary endpoint was not significantly different between the two treatment groups data were pooled for all analyses of the patients with type 2 diabetes mellitus (n = 5250). Four groups according to the percentage of on-treatment visits with BP <140/90 mmHg up to the occurrence of an event: <25%, 25–49%, 50–74% and ≥75% were considered as done in previous trials [Citation15–16] including in the overall VALUE population [Citation17]. The same four group subdivision was used for the percentage of visits with BP <130/80 mmHg, i.e. the target BP recommended by guidelines in a high CV risk condition [Citation5–7]. On the assumption that the BP found at a given visit reflected the value existing during the preceding between-visit interval data were expressed as the percentage of time in which BP was reduced below the higher or lower value. For each group calculation was made of the incidence of the primary and secondary endpoints. The relative risk of each endpoint was quantified separately for the higher and lower BP target, using the Cox proportional hazard model and taking the group in which BP control covered ≥ 75% of the on-treatment time as reference. To reduce the impact of potential confounders hazard ratios were adjusted for baseline covariates (age, gender, systolic BP and diastolic BP, body mass index, high serum total cholesterol [6 mmol/L or 240 mg/dl], smoking, proteinuria, history of CV events and left ventricular hypertrophy). For baseline systolic BP and diastolic BP, the 5th degree polynomials were used to capture an extended range of possible relationships between BP and events. Two-sided p-values were calculated for trends versus the subgroup with ≥ 75% of the time with BP control. p < 0.05 was considered statistically significant without adjustment for multiplicity. Data are shown as means ± standard deviations (SDs) or estimates with 95% confidence intervals (CIs).
Results
Baseline characteristics in relation to time achieving target <140/90 mmHg
shows the baseline characteristics of the diabetic patients achieving BP < 140/90 mmHg over different proportions of the on-treatment period prior to the occurrence of the primary endpoint. Systolic BP and diastolic BP were progressively greater, and most CV risk and disease factors progressively more common, from the longest to the shortest time (≥ 75% to <25%) with a BP <140/90 mmHg, with an expected concomitant progressive increase of average on-treatment BP.
Table 1. Baseline characteristics of the 4 groups of diabetic patients in which treatment reduced BP below 140/90 mmHg for different proportions (from < 25% to ≥ 75%) of the overall duration of treatment.
Fractions of smokers and fractions of study participants with coronary disease were inversed, while heart rate, baseline antihypertensive treatment, body mass index and fraction of patients with previous stroke or transient ischaemic attack were unchanged. Results were similar when groups were stratified according to BP values prior to the occurrence of secondary endpoints (data not shown).
Event incidence and risk for BP <140/90 mmHg
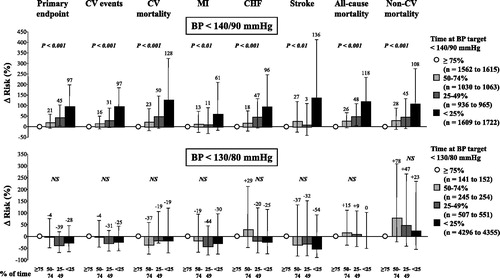
Both for the primary and for all secondary endpoints the event incidence increased progressively as the time with BP <140/90 mmHg decreased (, upper panel). The risk of any event also showed a steep progressive increase as the time with a BP below 140/90 mmHg decreased when adjusting the data for baseline covariates, including systolic BP and diastolic BP values (, upper panel).
Figure 1. Incidence of morbid and fatal events in groups of diabetic patients divided according to the proportion of the overall treatment duration (< 25% to ≥ 75%) in which BP was reduced < 140/90 mmHg (upper panel) or < 130/80 mmHg (lower panel) prior to the occurrence of an event. n refers to the number of patients in each group. BP: blood pressure; CV: cardiovascular; MI: myocardial infarction; CHF: congestive heart failure.
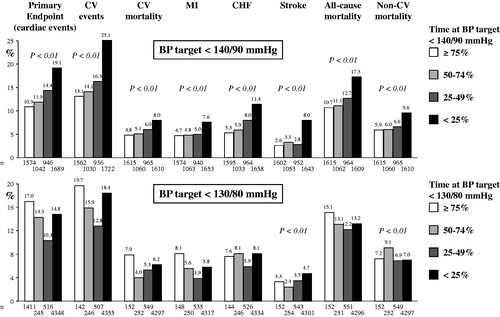
Figure 2. Percent (and 95% CI) change in the risk of events according to the proportion of time in which BP was reduced < 140/90 mmHg (upper panel) or < 130/80 mmHg (lower panel) in groups of diabetic patients. The group in which these BP targets were achieved for ≥ 75% of the time is used as reference and shown by the empty circle (minor variations in ‘n’ as some endpoints were composite and primary and other endpoints secondary). Data were adjusted for both baseline covariates and achieved average systolic BP and diastolic BP. P values refer to trend. NS: not significant. Other symbols as in .
Baseline characteristics in relation to time achieving target <130/80 mmHg
shows the baseline characteristics of the diabetic patients achieving BP < 130/80 mmHg over different proportions of the on-treatment period prior to the occurrence of the primary endpoint. Although the between-group differences were less pronounced and not invariably significant, baseline systolic BP and diastolic BP values as well as prevalence of several CV risk and disease factors increased progressively from the group with the longest to the group with the shortest time at BP <130/80 mmHg. There was an expected concomitant progressive increase of the on-treatment average BP values.
Table 2. Baseline characteristics of the 4 groups of diabetic patients in which treatment reduced BP below 130/80 mmHg for different proportions (from < 25% to ≥ 75%) of the overall duration of treatment.
At variance from the findings shown in , fractions with antihypertensive treatment at baseline increased with BP control ≥ 75%. This was also the case for fractions of participants with coronary disease but not for fractions of smokers, which was also at difference from the findings in . The results were similar when the groups were stratified according to BP values prior to the occurrence of secondary endpoints (data not shown).
Event incidence and risk for BP <130/80 mmHg
From the longest to the shortest time with BP <130/80 mmHg the incidence of stroke continued to show a progressive increase and non-CV mortality concomitantly exhibited a progressive reduction. The incidence of the primary and all other CV endpoints showed a J-curve pattern, i.e. an increase as the time under more intensive BP control decreased from ≥ 75% to 50–74%, with a decrease as control was achieved for times shorter than 50% (, lower panel). However, importantly the risk of all the various events did not show any consistent trend from the longest to the shortest time with a BP <130/80 mmHg when adjusting the data for baseline covariates, including systolic BP and diastolic BP values (, lower panel).
Discussion
The risk of CV morbidity and mortality as well as myocardial infarction, heart failure and stroke in mostly elderly patients with the combination type 2 diabetes mellitus and hypertension showed a progressive steep increase as the rate of BP control < 140/90 mmHg decreased from ≥ 75% to <25% of the on-treatment time. There was a concomitant steep increase in the risk of these events when adjusting for between-group differences in a large number of demographic and clinical baseline variables. This was not the case for the different rates of BP control <130/80 mmHg. For patients below these BP values, the adjusted overall morbidity and mortality risk, as well as the risk of cause-specific events of cardiac disease and stroke, were unaffected by the frequency of BP control. Thus, while more frequent BP reductions <140/90 mmHg were highly protective, no further protection was achieved by more frequent BP reductions <130/80 mmHg. Our findings provide evidence in support of BP target <140/90 mmHg but also against the need of pursuing an intensive BP target in middle-aged and elderly patients averaging about 67 years with type 2 diabetes mellitus and hypertension, as presently recommended by international guidelines [Citation5–7].
Several other results of our study are noteworthy. One, in the present large subgroup of 5250 diabetes mellitus hypertensive patients the relationship of the higher and lower BP targets with the incidence and adjusted risk of CV morbidity and mortality was principally similar to that of the entire VALUE population, i.e. CV protection was achieved by reducing BP <140/90 mmHg with no further protection <130/80 mmHg. We discuss the comparison of the diabetes mellitus patients with the entire population of high-risk hypertension in VALUE in more details in a companion article [Citation18].
Two, in a large trial of diabetes mellitus patients systolic BP reduction <120 mmHg did not show beneficial effects on CV morbidity and mortality except for stroke, the risk of which was reduced by 41% compared to patients remaining at SBP ≥ 130 mmHg [Citation4]. In post-hoc analyses of other large trials including hypertensive or normotensive patients, treatment-induced progressive systolic BP reduction to 120 mmHg or less was accompanied by no effect or even an increase of CV events and myocardial infarction, again with a progressive reduction in the incidence of stroke [Citation9–11]. We had 141 patients at the 120 mmHg level in systolic BP for ≥ 75% of the visits, but this number of patients is too low to achieve statistical power in the diabetic patients though there was less stroke with systolic BP <130 mmHg in the main VALUE study (17).
Three, only about one third of our patients with diabetes mellitus achieved BP <140/90 mmHg for ≥ 75% of the overall treatment duration, and in more than half of the patients this highly protective target BP remained unachieved for half of the treatment time. This confirms that consistent BP control is a difficult goal to reach even in the context of a randomised clinical trial, i.e. when the patients are care taken by expert investigators and the follow-up is more adequate than in regular clinical practice. Given the evidence that visit-to-visit BP variability may be an independent CV risk factor [Citation19–21] the inconsistency of BP control may be one of the factors responsible for the persistently high residual risk exhibited by treated hypertensive patients [Citation22].
Four, it is remarkable that non-CV mortality risk is highly significantly related to improved BP control over time when target BP is <140/90 mmHg. This finding suggests that in our patients with diabetes mellitus and high-risk hypertension, there is an extensive misclassification of CV death into non-CV death. In fact, the finding mirrors the relationship between time-dependent BP control and CV mortality with similar finding also for all-cause mortality.
Five, fractions of smokers and fractions of study participants with coronary disease were increasing with ≥ 75% of controls at BP target <140/90 mmHg. Further, fractions of participants with coronary disease but not fractions of smokers also increased with ≥ 75% of controls at BP target <130/80 mmHg. We report these findings in middle-aged and elderly patients with diabetes mellitus and hypertension, but we have not elucidated these findings in detail. Possibly, coronary patients with diabetes mellitus and hypertension receive the utmost attention for BP control with BP lowering medication.
Six, we have a future opportunity to provide data about the ‘very-early’ control of patients with diabetes mellitus in the trial (initial months) for the entire cohort. This analysis may be extended to the randomised arms with the achieved BP difference by randomised treatment arms reported as well. Further, if someone wants to argue in favour of or against a given statement, like the presence or absence of comorbidity to modulate outcomes, a better way is to compare what it is happening with and without the comorbidity (i.e. diabetes mellitus vs non-diabetes mellitus). This analysis may be done in the future also because we may then compare directly with how the National Heart, Blood and Lung Institute in the U.S. has organised their recent outcome trials (4).
Study limitations
Our study had some limitations. One, only a limited number of patients achieved BP <130/80 mmHg at rates greater than 50% or 75% of the treatment duration, which means that this target comparison involved groups of different sizes. This was particularly the case for the diabetes mellitus patients, in whom the low rate of intensified BP control for 50% or more of the overall treatment duration may have favoured chance findings such as the lack of relationship between the BP reduction and the CV risk compared to the overall trial population. In a previous analysis of achieved BP in VALUE the mathematical nadir for co-variate adjusted CV risk was 128/75 mmHg, which may suggest that there are patients who may benefit of target BP <130/80 mmHg [Citation23]. As there was neither any sign of a J-shaped curve (increased CV risk within the lower BP targets) achieved in VALUE [Citation23], any BP target <140/90 mmHg, even <130/80 mmHg may be safe from a CV risk point of view. However, too low achieved BP may potentially cause adverse events, typically dizziness, and patients may discontinue study treatment.
Two, because in post-hoc analyses comparisons involve non-randomised groups, the possibility that our results did not depend on the achievement rates of higher or lower BP values but rather on differences in baseline characteristics cannot be excluded. For example, in the patients who had known coronary disease at inclusion the mathematical nadir for risk of myocardial infarction was 128/76 vs. 128/79 mmHg, respectively, in non-coronary patients [Citation23]. However, our estimates of CV risk were adjusted for a large number of baseline variables, including markers of asymptomatic hypertension mediated organ damage (left ventricular hypertrophy and proteinuria), that have an important impact on CV risk. Furthermore, non-CV diseases like occult cancer with gastrointestinal or other occult bleeding not identified at baseline may potentially contribute to an achieved BP <130/80 mmHg in > 75% of the visits explaining the associated higher non-CV mortality in this group.
Three, the effect of more properly achieving higher and lower BP targets was different, although baseline differences between groups were qualitatively similar in either case. Four, unaccounted baseline differences are unlikely to explain the effect of BP reductions <140/90 mmHg on the various CV events. Thus, although interpretation of post-hoc data requires caution, it seems reasonable to conclude that baseline confounders did not play a major role in our results.
Implications
Our data suggest that in middle-aged and elderly patients with type 2 diabetes mellitus and hypertension a more consistent achievement of blood pressure target <140/90 mmHg leads to a major reduction in the risk of coronary events, heart failure, and stroke, protective effects extending to lower cardiovascular and all-cause mortality and even to non-cardiovascular mortality. This does not occur for a more frequent control of blood pressure <130/80 mmHg. Thus, guidelines should not recommend using this intensive blood pressure target in middle-aged and elderly patients with diabetes and hypertension. Our findings are in line with a recent Cochrane analysis [Citation24].
Acknowledgements
Dr. Eirik Olsen, MD, is supported by the Research Council of Norway. The VALUE trial (Valsartan Antihypertensive Long-Term Use Evaluation) was funded by an unrestricted grant from Novartis Pharma AG. The data file resides in the hands of the authors at Oslo University Hospital, Oslo, Norway. We are indebted to Dr. Tsushung A. Hua, PhD (deceased 2018), Unit of Biostatistics and Pharmacometrics, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA for invaluable help through many years.
Disclosure statement
S.E. Kjeldsen has received honoraria from Merck GBaA, Sanofi and Takeda. R. Mo has received honoraria from Novartis. B. Holzhauer and D. Zappe are employees of Novartis Pharma. The other authors report no relevant conflicts of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, S.E.K. upon dire need.
References
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755–1762.
- Holman RR, Paul SK, Bethel MA, et al. Long-term follow-up after tight control of blood-pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565–1576.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American diabetes association. Diabetes Care. 2017;40(9):1273–1284.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314–340.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324.
- Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
- Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304(1):61–68.
- Redon J, Mancia G, Sleight P, et al. Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). J Am Coll Cardiol. 2012;59(1):74–83.
- Sim JJ, Shi J, Kovesdy CP, et al. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J Am Coll Cardiol. 2014;64(6):588–597.
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031.
- Zanchetti A, Julius S, Kjeldsen S, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: an analysis of findings from the VALUE trial. J. Hypertens. 2006;24:2163–2168.
- Aksnes TA, Kjeldsen SE, Rostrup M, et al. Impact of new-onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial population. Hypertension. 2007;50(3):467–473.
- Mancia G, Messerli F, Bakris G, et al. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension. 2007;50(2):299–305.
- Mancia G, Schumacher H, Redon J, et al. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET). Circulation. 2011;124(16):1727–1736.
- Mancia G, Kjeldsen SE, Zappe DH, et al. Cardiovascular outcomes at different on-treatment blood pressures in the hypertensive patients of the VALUE trial. Eur Heart J. 2016;37(12):955–964.
- Olsen E, Holzhauer B, Julius S, et al. Cardiovascular outcomes at recommended blood pressure targets in middle-aged and elderly patients with type 2 diabetes mellitus compared to all middle-aged and elderly hypertensive study participants with high cardiovascular risk. Blood Press. doi:10.1080/08037051.2020.1856642.
- Mehlum MH, Liestøl K, Kjeldsen SE, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39(24):2243–2251.
- Mehlum MH, Liestøl K, Wyller TB, et al. Blood pressure variability in hypertensive patients with atrial fibrillation in the VALUE trial. Blood Press. 2019;28(2):77–83.
- Mehlum MH, Liestøl K, Kjeldsen SE, et al. Blood pressure–lowering profiles and clinical effects of angiotensin receptor blockers versus calcium channel blockers. Hypertension. 2020;75(6):1584–1592.
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 3. Effects in patients at different levels of cardiovascular risk. Overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2305–2314.
- Kjeldsen SE, Berge E, Bangalore S, et al. No evidence for a J-shaped curve in treated hypertensive patients with increased cardiovascular risk: The VALUE trial. Blood Press. 2016;25(2):83–92.
- Saiz LC, Gorricho J, Garjón J, et al. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database Syst Rev. 2020;9:CD010315. PMID: 32905623.
