Abstract
Purpose
Hypertension commonly co-exists with diabetes mellitus (DM), and both are closely related to adverse health outcomes. The activation of aldosterone and mineralocorticoid receptor (MR) may play important roles in this process. Therefore, we aim to evaluate the efficacy of MR antagonists on cardiovascular risk factors, including blood pressure (BP), glucose, lipids, renal function, fibrosis and inflammatory and its safety in patients with both hypertension and DM.
Methods
We searched PubMed, Embase, Web of Science and Cochrane databases for clinical trials published until December 31, 2019. Studies comparing the effect of spironolactone to placebo in patients with hypertension and DM were included. Mean difference with 95% confidence intervals was used to report outcomes.
Results
Eleven randomised placebo-controlled trials with 640 participants were finally included with mean follow-up of 5 months. Compared to placebo, spironolactone significantly reduced office systolic (–6.57, 95%CI: −9.21, −3.93) and diastolic BP (–2.63, 95%CI: −4.25, −1.02) as well as ambulatory BP; increased glycosylated haemoglobin by 0.3 but no clear effect on fasting glucose. Spironolactone induced a significantly reduction of urinary albumin but increased serum creatinine (7.60, 95%CI: 4.94, 10.27) and decreased glomerular filtration rate (–4.28, 95%CI: −6.38, −2.18). Markers of fibrosis and inflammation, including NIIINP, PICP, hs-CRP and TNF-α were also decreased after spironolactone therapy. For lipid metabolism, there was no significant difference between groups. Spironolactone mildly increased serum potassium (0.30, 95%CI: 0.23, 0.37). 2.5% subjects treated with spironolactone experienced mild to moderate hyperkalaemia and received medication or dietary advice and another 1.6% developed severe hyperkalaemia and withdrawn from the studies.
Conclusion
Spironolactone reduced BP and urinary albumin, improve fibrosis and inflammation, whereas slightly increases the glycosylated haemoglobin and serum creatinine in patients with hypertension and diabetes. Long-term RCTs to assess the effects of spironolactone on cardiovascular events in this population are warranted.
Introduction
Hypertension and diabetes mellitus (DM) are common comorbidities. Patients with hypertension show a twofold higher risk of having DM, compared to those without hypertension, making more than 20% of hypertensive patients have DM [Citation1]. The co-existence of the two conditions shows negative effects on blood pressure (BP) lowering, aggravate target organ damages and increase cardiovascular risk [Citation2,Citation3]. In this process, hypertension and DM share several common known pathogenesis including the overactivation of the sympathetic nervous system, renin-angiotensin-aldosterone system and insulin resistance [Citation4]. It is well known that patients with hypertension and DM usually need two or more medications to achieve target BP. However, poor BP reduction and disproportionate increase in cardiovascular events suggest that the influence of the co-existence of hypertension and DM may be far more complex than the simple combination of the two conditions [Citation5].
Aldosterone and MR activation play important roles in the increased cardiovascular risk by promoting endothelial dysfunction, inflammation, vascular oxidative stress and fibrosis [Citation6,Citation7]. Previous studies have shown that aldosterone concentrations are higher in patients with both hypertension and DM than in patients with hypertension alone [Citation8]. It has been also observed that the expression and sensibility of mineralocorticoid receptors (MR) are increased in diabetic patients [Citation9]. Except for increasing BP, aldosterone also causes the aggravation of insulin resistance and diabetes by damaging islet endothelial function [Citation10,Citation11]. Furthermore, aldosterone breakthrough, which is related to endothelial dysfunction, left ventricular function deterioration and renal damage occurs in about 50% of patients treated with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs), which are the preferred agents for patients with hypertension and DM [Citation12,Citation13]. Nonetheless, clinicians tend to pay more attention to the known pathogenesis whereas to ignore the role of aldosterone.
Several small sample clinical trials showed that add-on MR antagonists (MRAs) induce a reduction in BP and urinary protein in patients with hypertension and DM [Citation14–16]. Clinical trials have been indicating that MRAs reduce the incidence of cardiovascular events as well as the all-cause mortality in patients with chronic kidney disease or heart failure [Citation17,Citation18]. However, the anti-hypertensive efficacy and safety of MRAs in patients with hypertension and DM have not been demonstrated in large-sample studies, and whether MRAs bring cardiovascular benefits for this population remains unknown.
Therefore, we carried out the systematic review and meta-analysis of randomised placebo-controlled trials to evaluate the effect of MRAs on BP, glucose, lipid, renal function, fibrosis and inflammation, as well as the safety in patients with hypertension and DM.
Methods
Data source and search strategy
We searched PubMed, Embase, Web of Science and Cochrane databases for clinical trials published until December 31, 2019. The following search terms were used: MR antagonists, aldosterone antagonists, spironolactone, eplerenone, hypertension, hypertensive, blood pressure (BP), diabetes, diabetic, impaired glucose tolerance and trial. All of the searches were limited to English language and human studies. The study is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline [Citation19].
Eligibility criteria
Studies: randomised parallel-controlled or cross-over trials in which a spironolactone or eplerenone arm and a placebo arm were designed, or participants served as their own control.
Subjects: participants (aged ≥18 years old) with hypertension (diagnosed hypertension or under antihypertensive therapy) and diabetes were included.
Interventions: MRAs (spironolactone or eplerenone) or placebo administrated for at least two weeks.
Data extraction and quality assessment
The data were extracted using a pre-designed data extraction sheet by two investigators (Mengyue Lin and Mulalibike Heizati) independently. Information including publication details (author, year of publication), participant characteristics (age, sex, BP, glucose, etc.), interventions (MRA type, dose, and duration), and outcome measures (including BP, glucose, lipid, renal function, fibrosis, inflammation, and potassium) was extracted. The quality and potential bias of each study were also evaluated independently by two investigators (Lin Wang and Muyesaier Nurula) using the Cochrane Collaboration’s Risk of Bias Tool. Disagreements of extracted data were resolved through consensus or a third investigator (Zhikang Yang).
Statistical analysis
Meta-analyses were carried out for any factor if the values were available in three or more studies. Except for BP and potassium, which were reported in all studies, other cardiovascular risk factors were also reviewed descriptively, because of the small sample size. The main results were presented as the mean difference between MRAs and placebo group; forest plots were produced if the number of selected studies was ≥10. I2 value was used to assess the heterogeneity among studies. When the I2 value was less than 50%, fixed-effect model was used; otherwise, random-effect model was used. Subgroup analysis were performed according to study design (cross-over or parallel-control), follow-up period (≤3 months or >3 months), dose of spironolactone (25 mg/day or 25–50mg/day), and mean age (<60 years or ≥60 years). Publication bias was evaluated using the funnel plot. Sensitivity analysis was performed by excluding one study at a time from the model to evaluate whether any study had a substantial impact on the model. Statistical analyses were conducted using Revman 5.3 software (the Cochrane Collaboration, UK).
Results
Selection and characteristics of studies
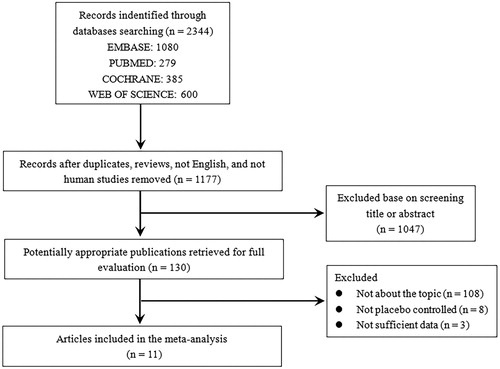
The detailed process of study search and selection was shown in . A total of 2,344 articles yielded in the initial search, of which 11 randomised double-blind placebo-controlled studies, with 640 participants met the inclusion criteria and were enrolled in this analysis [Citation14–16,Citation20–27]. The characteristics of the included studies and participants were summarised in and . In terms of study design, there were 4 parallel controlled studies and 7 cross-over studies. The type of MRAs in the included studies was spironolactone with 25 to 50 mg per day, and eplerenone was not administered. The mean age of the participants ranged from 43 to 66 years. Most participants took anti-hypertensive drugs ACEi/ARBs at baseline. The follow-up duration was 4 weeks to 1 year. The quality and potential biases were evaluated and showed in Table S1.
Table 1. Characteristics of studies including in the meta-analysis.
Table 2. Baseline information of included studies.
Effect of spironolactone on BP
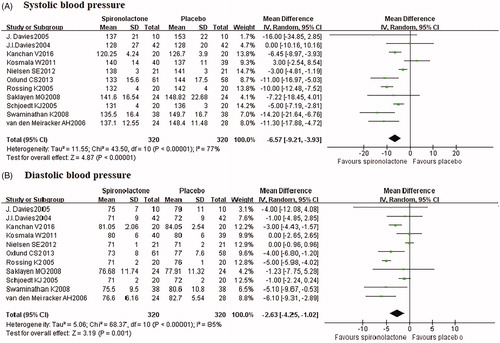
All included studies reported office BP and three studies reported 24 h ambulatory BP. The pooled analysis showed significant heterogeneity, and thus random-effect models were used. Meta-analysis () showed that office SBP was significantly decreased by spironolactone compared with placebo (–6.57 mmHg, p < 0.001). Spironolactone also reduced DBP by 2.63 (p = 0.001). Similar results were found for 24 h ambulatory SBP (–5.3 mmHg) and DBP (–2.68 mmHg) (Figure S1). Subgroup analysis showed that higher dose of spironolactone was more effective for BP lowering (Table S2 and S3). The sensitivity analysis showed no changes in the overall effect of the models. The funnel plot indicated little publication bias for SBP and DBP (Figure S2).
Glucose and lipid metabolism
For fasting glucose, there was no significant difference between spironolactone and placebo, whereas there seemed to be a decreased trend after spironolactone treatment compared to baseline (Table S4 and Figure S3). Five of the six studies showed significant increase in glycosylated haemoglobin (HbA1c) in spironolactone group (+0.2 to 0.4), and another study suggested a similar trend but not significant. Consistently, pooled analysis showed that spironolactone treatment increased HbA1c by 0.3% ( and Figure S3). In the two studies reported homeostasis model assessment of insulin resistance (HOMA-IR), no clear effect of spironolactone was found. In terms of lipid profiles, all of the five studies showed no significant differences between groups except one, which reported a slight but significant increase of total cholesterol (TG) in spironolactone (Table S5). However, the baseline TG between the two groups seems to be different in this study. Pooled analysis produced similar results that no differences between groups ( and Figure S4).
Table 3. Meta-analysis for effect of spironolactone on glucose, lipids, renal function.
Renal function and potassium
In the included studies, five of the eight studies showed significant higher creatinine in spironolactone group compared with placebo. The other three also reported similar trends but not significant (Table S6). In the three studies reporting baseline serum creatinine, post-treatment creatinine increased compared with baseline. Correspondingly, estimated glomerular filtration rate (GFR) decreased in spironolactone group. Also, pooled analysis suggested that spironolactone increased serum creatinine by 7.60, and decreases estimated GFR by 4.28 ( and Figure S5). Nevertheless, spironolactone significantly reduced urinary protein (Table S6). Compared to placebo, spironolactone decreased 24 h albuminuria by 30 to 33% and urinary albumin excretion rate by 60%.
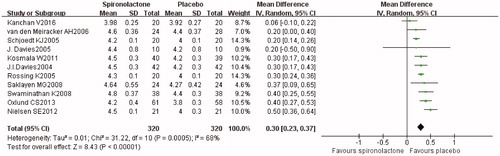
All of the included studies reported post-treatment serum potassium. Pooled analysis () showed that serum potassium in the spironolactone treatment group was significantly higher than in the placebo group (0.30, p < 0.001). Moreover, serum potassium was higher in high-dose group (Table S7). The sensitivity analysis showed no changes in the overall effect of the model. The funnel plot indicates little publication bias (Figure S2C). 2.5% (n = 16) participants treated with spironolactone experienced mild to moderate hyperkalaemia and received medication or dietary advice. Besides, 1.6% patients (n = 10) developed severe hyperkalaemia and were withdrawn from the studies.
Other outcome variables
Three studies reported N-terminal peptide of type III procollagen (PIIINP) and suggested that spironolactone induced a decrease of PIIINP. Also, pooled analysis produced a similar result that spironolactone reduced PIIINP by 0.77 (Figure S6). A parallel decrease in procollagen type I C-terminal peptide (PICP) was also found (Table S8). In terms of inflammatory reaction, high-sensitivity C-reactive protein (hs-CRP) and tumour necrosis factor α (TNF-α) were significantly decreased by spironolactone treatment compared with placebo; it was also reduced after spironolactone treatment compared to baseline. However, CRP showed no significant difference between groups.
Other reported adverse events
In the included studies, spironolactone was well tolerated with few other adverse effects. Five studies reported no other adverse events excepted for hyperkalaemia. Only one study reported mild gynaecomastia developed in 2 male patients. Other reported events included postural hypotension (n = 1), symptomatic hypotension (n = 1), mild diarrhoea (n = 1), and nausea (n = 1). In addition, there were three deaths have been reported, one of the cases was due to sepsis associated with diabetic foot ulcers (placebo group).
Discussion
This systematic review and meta-analysis suggest that spironolactone significantly reduces BP in patients with hypertension and DM, regardless of the patient’s basic antihypertensive regimen. Spironolactone slightly raises HbA1c but has no clear effect on fasting glucose and lipid metabolism. It also reduces urinary albumin excretion, whereas increases serum creatinine, and decreases estimated GFR. A potential improvement in fibrosis and inflammatory responses was found in the spironolactone treatment group. In terms of its safety, only a small part of the patients experienced potassium elevation, whereas no other severe events owing to spironolactone treatment.
Patients with hypertension and DM have a difficult-to-control BP and therefore treatment with two or more drugs with different blocking mechanisms for BP reduction is often recommended [Citation28]. Our study shows that add-on spironolactone may be effective in lowering BP in this specific patient population. Aldosterone concentration is higher in patients with hypertension and DM, and the activation of MR increases in this population [Citation8,Citation9]. Moreover, aldosterone breakthrough occurs in about 50% of patients treated with ACEi/ARBs [Citation12]. These may be the main cause of the poor BP control and serve as an explanation for the findings of this study at least in part.
The increase in aldosterone and the MR activation are closely related to endothelial damage, inflammation, and fibrosis [Citation6,Citation7]. Research on the diabetic animal has demonstrated that treatment with spironolactone reduces the production of inflammatory factors [Citation15]. In addition to lowering BP, MRAs may bring beneficial effects on cardiovascular morbidity and mortality through the relief of cardiovascular remodelling and vascular inflammation response [Citation29,Citation30], and the effects on oxidative stress as well as improving endothelial functions [Citation31]. In the present study, spironolactone significantly reduces inflammatory markers, including hs-CRP and TNF-α. PIIINP, a marker of cardiovascular fibrosis, is also reduced by spironolactone. These findings suggest that spironolactone may reduce cardiovascular risk by improving inflammatory response and fibrosis. Further study, however, are needed to verify.
The mechanism of the effect of spironolactone on glucose metabolism remains unclear. The level of cortisol increases after treatment with spironolactone [Citation32], which may in part explain why HbA1c elevated after treatment with spironolactone. However, the small sample size and short term follow-up of the included studies may not be able to account for the observed phenomenon, since spironolactone protects islet and increases circulating potassium, which may favour lowering glucose, at least theoretically. Nonetheless, larger sample studies with long term follow-up are needed to answer this part.
Albuminuria is a marker for renal impairment and aggravation. A significant reduction of cardiovascular mortality has been found in patients with a decrease of urinary albumin excretion [Citation33]. The present review showed that urinary protein was decreased, suggesting the beneficial effect of spironolactone on renal and cardiovascular prognosis. The potential mechanism of the anti-proteinuric effect of spironolactone may be related to the reduction of intraglomerular pressure by the decreased sensitivity of angiotensin II [Citation24]. Notably, the results of creatinine and estimated GFR are inconsistent with the improvement in renal function. The change in albuminuria is usually correlated with the decline of estimated GFR [Citation24]. However, the underlying mechanism of spironolactone on estimated GFR is not fully clear. It is probably due to the reversal of the effect of spironolactone on aldosterone on glomerular capillary pressure [Citation16]. Notably, studies have demonstrated that the initial GFR decline after treatment indicated a beneficial long-term renal function [Citation34].
Increased serum potassium or severe hyperkalaemia is one of the side effects of spironolactone and is also the reason for the limited application. Consistent with previous studies [Citation35], slight but significant increased serum potassium in the spironolactone group was observed in our study. Although a small number of patients were withdrawn in the study process due to severe hyperkalaemia, most participants had a serum potassium level in the low-risk range. In clinical practice, however, we should carefully evaluate the individual risk factors for patients and closely monitor serum potassium during the administration of spironolactone to prevent serious adverse events.
There are several limitations to our study. First, no study reported data of cardiovascular events, and only available risk factors were included as a surrogate; data on inflammatory factors and fibrosis are too scarce to make an accurate demonstration of the beneficial effect of spironolactone on cardiovascular risk. Large-sample clinical trials are needed to verify this. Second, some of the studies did not provide a detailed research protocol, and the quality of these studies could not be assessed accurately. Third, ten patients treated with spironolactone were excluded in the analysis due to hyperkalaemia, which may bias the results. Fourth, the power of our meta-analysis was limited because most of the included studies were small sample sizes and short-term follow-up; two studies with follow-up for 1 month should be interpreted with caution although the results were similar to other studies. Fifth, the sample size of type 1 diabetes was too small to draw a conclusion that whether spironolactone has similar effect on this population; it needs to be confirmed in future studies.
The study indicates that spironolactone reduces BP and urinary protein, improves fibrosis and inflammation in patients with hypertension and diabetes, regardless of the patient’s basic antihypertensive regimen. Nonetheless, uncertainty remains due to the small sample and short follow-up periods of included studies. Therefore, large sample trials with long-term follow-up periods are required to confirm the effects of spironolactone and especially on cardiovascular events in this specific patient population.
Authors’ contributions
Nanfang Li and Mengyue Lin contributed to the study conception and design. Literature search was performed by Mengyue Lin, Zhikang Yang and Zhongrong Wang. Data collection and analysis were performed by Mengyue Lin, Mulalibike Heizati, Lin Wang, Muyesaier Nurula, Reyila Abudoyreyimu and Zihao Wu. The first draft of the manuscript was written by Mengyue Lin and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
suppl_figures.zip
Download Zip (3.8 MB)Supplementary_material_Clean_version.docx
Download MS Word (47.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liu J, Zhao D, Liu J, et al. Prevalence of diabetes mellitus in outpatients with essential hypertension in China: a cross-sectional study. BMJ Open. 2013;3(11):e003798.
- Tian J, Sheng CS, Sun W, et al. Effects of high blood pressure on cardiovascular disease events among Chinese adults with different glucose metabolism. Dia Care. 2018;41(9):1895–1900.
- Liu HH, Cao YX, Li S, et al. Impacts of prediabetes mellitus alone or plus hypertension on the coronary severity and cardiovascular outcomes. Hypertension. 2018;71(6):1039–1046.
- Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584.
- American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989;12(8):573–579.
- Ohmine T, Miwa Y, Takahashi-Yanaga F, et al. The involvement of aldosterone in cyclic stretch-mediated activation of NADPH oxidase in vascular smooth muscle cells. Hypertens Res. 2009;32(8):690–699.
- Bender SB, McGraw AP, Jaffe IZ, et al. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62(2):313–319.
- Karashima S, Yoneda T, Kometani M, et al. Angiotensin II receptor blocker combined with eplerenone or hydrochlorothiazide for hypertensive patients with diabetes mellitus. Clin Exp Hypertens. 2016;38(7):565–570.
- Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25(5):514–523.
- Watanabe D, Yatabe M, Ichihara A. Evaluation of insulin sensitivity and secretion in primary aldosteronism. Clin Exp Hypertens. 2016;38(7):613–617.
- Wang J, Hu H, Song J, et al. Aldosterone induced up-expression of ICAM-1 and ET-1 in pancreatic islet endothelium may associate with progression of T2D. Biochem Biophys Res Commun. 2019;512(4):750–757.
- Sato A, Fukuda S. Effect of aldosterone breakthrough on albuminuria during treatment with a direct renin inhibitor and combined effect with a mineralocorticoid receptor antagonist. Hypertens Res. 2013;36(10):879–884.
- Schrier RW. Aldosterone 'escape' vs 'breakthrough'. Nat Rev Nephrol. 2010;6(2):61.
- Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens. 2013;31(10):2094–2102.
- Swaminathan K, Davies J, George J, et al. Spironolactone for poorly controlled hypertension in type 2 diabetes: conflicting effects on blood pressure, endothelial function, glycaemic control and hormonal profiles. Diabetologia. 2008;51(5):762–768.
- Saklayen MG, Gyebi LK, Tasosa J, et al. Effects of additive therapy with spironolactone on proteinuria in diabetic patients already on ACE inhibitor or ARB therapy: results of a randomized, placebo-controlled, double-blind, crossover trial. J Investig Med. 2008;56(4):714–719.
- Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–2463.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Davies JI, Band M, Morris A, et al. Spironolactone impairs endothelial function and heart rate variability in patients with type 2 diabetes. Diabetologia. 2004;47(10):1687–1694.
- Davies J, Gavin A, Band M, et al. Spironolactone reduces brachial pulse wave velocity and PIIINP levels in hypertensive diabetic patients. Br J Clin Pharmacol. 2005;59(5):520–523.
- Rossing K, Schjoedt KJ, Smidt UM, et al. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005;28(9):2106–2112.
- Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney International. 2005;68(6):2829–2836.
- van den Meiracker AH, Baggen RG, Pauli S, et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J Hypertens. 2006;24(11):2285–2292.
- Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, et al. A randomized study of the beneficial effects of aldosterone antagonism on lv function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging. 2011;4(12):1239–1249.
- Nielsen SE, Persson F, Frandsen E, et al. Spironolactone diminishes urinary albumin excretion in patients with type 1 diabetes and microalbuminuria: a randomized placebo-controlled crossover study. Diabet Med. 2012;29(8):e184–e190.
- Kanchan V, Pawan K, Sudhir V, et al. Effect of low-dose mineralocorticoid receptor antagonists on metabolic profile and endothelial dysfunction in metabolic syndrome. Diabetes Metab. 2016;42(1):65–68.
- Gu Q, Burt VL, Dillon CF, et al. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114.
- Iraqi W, Rossignol P, Angioi M, et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119(18):2471–2479.
- Edwards NC, Steeds RP, Stewart PM, et al. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54(6):505–512.
- Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459–469.
- McMurray EM, Wallace IR, Ennis C, et al. Effect of eplerenone on insulin action in essential hypertension: a randomised, controlled, crossover study. J Hum Hypertens. 2014;28(10):575–578.
- Schmieder RE, Mann JF, Schumacher H, ONTARGET Investigators, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22(7):1353–1364.
- Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int. 1997;51(3):793–797.
- Takahashi S, Katada J, Daida H, et al. Effects of mineralocorticoid receptor antagonists in patients with hypertension and diabetes mellitus: a systematic review and meta-analysis. J Hum Hypertens. 2016;30(9):534–542.