Abstract
Purpose
We investigated associations of blood pressure (BP) with albuminuria and left ventricular hypertrophy (LVH) in young, middle and older aged patients with hypertension and/or diabetes mellitus.
Materials & Methods
Study participants were treated patients with hypertension or diabetes, enrolled in a China nationwide registry. The 2510 patients were classified into young (<45 years, n = 345), middle (45–64 years, n = 1383) and older (≥65 years, n = 782) age groups. Clinic BP was measured three times consecutively on each of the two clinic visits. These six readings were averaged for analyses. Albuminuria was defined as a urinary albumin-to-creatinine ratio of ≥30 mg/g. LVH was assessed by the electrocardiogram (ECG) Cornell product and voltage methods.
Results
The prevalence of albuminuria and ECG-LVH was 17.8 and 6.5%, respectively. Mean (±SD) systolic/diastolic BP was 132.0 ± 16.5/85.2 ± 11.9 mmHg, 136.8 ± 17.9/81.7 ± 11.2 mmHg, and 139.8 ± 16.7/75.8 ± 10.4 mmHg in the young, middle and older age groups. In the young age group, the prevalence of albuminuria increased from 8.8% in systolic/diastolic BP <120/80 mmHg to 14.6, 16.0% and 16.5% in 120–129/80–84, 130–139/85–89 and ≥140/90 mmHg, respectively. The corresponding values were 8.9, 7.0, 18.1 and 22.2%, respectively, in the middle age group, and 21.2, 15.5, 16.4 and 24.4%, respectively, in the older age group. Adjusted analyses confirmed the J-shaped relation between BP and albuminuria in the older but not young age group. The prevalence of ECG-LVH was significantly (p for trend ≤0.04) higher with increasing BP similarly in all age groups.
Conclusions
The association between BP and organ damage seems to differ in young, middle and older aged patients for albuminuria but not ECG-LVH.
Introduction
Hypertension is a leading cause of cardiovascular morbidity and mortality [Citation1]. While more prevalent in older population, an increasing prevalence of hypertension is being observed in the young [Citation2,Citation3]. Blood pressure may influence target organs differently in different age groups, as blood perfusion to target organs may change with aortic stiffening, especially the kidneys [Citation4]. Regardless of blood pressure measurement technique, clinic or out-of-clinic, the relative risk of adverse health outcomes associated with blood pressure was higher in younger than older age groups, although the absolute risk was higher with increasing age [Citation5]. In the young age group, even mildly elevated blood pressure, such as prehypertension, confers higher risk of cardiovascular events [Citation6,Citation7]. Indeed, in a large Chinese population study (n = 45,641), prehypertension (systolic/diastolic blood pressure 120 to 139/80 to 89 mmHg) was associated with a 32% higher risk of total cardiovascular events than optimal and normal systolic/diastolic blood pressure (<120/80 mmHg) [Citation8].
In all but one recent hypertension guideline [Citation9], the therapeutic blood pressure target was recommended differently in elderly hypertensive patients [Citation10–13]. Indeed, in the 2017 American hypertension guidelines, 130/80 mmHg was recommended for all treated hypertensive patients, irrespective of age [Citation9]. In the other hypertension guidelines, a more conservative target, mostly <140/80 mmHg, was recommended for ‘older’ hypertensive patients, although ‘older’ might have been defined differently, 65 or 80 years or more [Citation10–13]. Too low blood pressure may compromise blood perfusion to some organs in older patients. Indeed, a recent meta-analysis suggested that intensive blood pressure control decreased major adverse cardiovascular events but increased risk of renal failure [Citation14].
We hypothesise that the association of blood pressure with some organ damage varies between young and older patients. In the present cross-sectional analysis, we investigated the association of blood pressure with albuminuria and left ventricular hypertrophy (LVH) in young, middle and older aged patients with hypertension and/or diabetes mellitus.
Methods
Study population
Our present ad hoc sub-analysis was based on data from a cross-sectional, multicenter registry in China, which was carried out in the departments of cardiovascular and endocrine medicine of hospitals from June 2011 to March 2012. The study protocol of the registry had been described in detail previously [Citation15,Citation16]. In brief, we registered consecutive patients with previously diagnosed hypertension from the departments of cardiovascular medicine and patients with previously diagnosed type 2 diabetes mellitus from the departments of endocrine medicine. The ethics committees of all participating hospitals approved the study protocol. All subjects gave written informed consent.
To be eligible for inclusion, a patient had to be at least 20 years old, and was able to participate in two clinic visits two to five days apart. Patients who were pregnant, had a history of type 1 diabetes mellitus or participated in other clinical research projects in the past three months were excluded from the study. At the first clinic visit, physicians administered a standardised questionnaire to collect information on medical history, lifestyle, and use of medications. Blood pressure and anthropometry were measured. At the second clinic visit, blood pressure was measured for the second time. Venous blood samples were drawn after overnight fasting for measurements of plasma glucose, glycosylated haemoglobin A1 (HbA1c) and serum lipids. Morning void urine samples were collected for urinary measurements.
Clinical and biochemical measurements
Blood pressure was measured using a validated automatic oscillometric blood pressure monitor (Omron HEM-7201, Omron Healthcare, Kyoto, Japan) at the first and second clinic visits. On each of the two occasions, three blood pressure readings were obtained in the seated position after the subjects had rested for at least five minutes. These six readings on two clinic visits were averaged for statistical analysis.
Standard 12-lead electrocardiogram (ECG) was recorded in all subjects. ECG-LVH was defined according to the Cornell product index, as (RaVL + SV3) × QRS duration >244 mV·ms and the Cornell voltage index, as RaVL + SV3 > 2.8 mV for men and RaVL + SV3 > 2.0 for women [Citation13].
Urinary routine test was performed on fresh spot urine samples at the laboratory of each participating hospitals. Urinary albumin and creatinine excretions were measured using the immunochemical method in a core laboratory certified by the College of American Pathologists (www.cap.org). In the absence of apparent urological infections on urine samples, albuminuria was defined as a urinary albumin-to-creatinine ratio ≥30 mg/g. Albuminuria included both microalbuminuria (30–299 mg/g) and macroalbuminuria (≥300 mg/g).
Statistical analysis
For database management and statistical analysis, we used SAS software (version 9.4, SAS Institute, Cary, NC, USA). Means and proportions across the groups were compared by the analysis of variance (ANOVA) and Fisher’s exact test, respectively. Continuous measurements with a skewed distribution were logarithmically transformed and represented by geometric mean and 95% confidence interval (CI). In logistic regression we defined dummy variables using the deviation from the mean coding approach to compare odds ratio for each subgroup, relative to the overall risk in the whole population or subpopulation.
Results
Clinical characteristics of patients
shows the characteristics of patients according to age group (<45, 45–64, and ≥65 years). Patients in the older aged group (≥65 years), compared to two younger aged groups (<45 and 45–64 years), included fewer men (44.0% vs. 61.7% and 45.1%, p < 0.0001), and had a smaller body mass index (24.8 kg/m2 vs. 25.8 and 25.4 kg/m2, p < 0.0001). Patients in these three age groups also differed significantly (p ≤ 0.04) in systolic and diastolic blood pressures, duration of hypertension, heart rate and serum total cholesterol and triglycerides concentrations. However, they had similar levels of serum HbA1c (p = 0.17).
Table 1. Characteristics of patients according to age group.
shows the data on antihypertensive and antidiabetic therapy according to age group. The use of antidiabetic therapy was not significantly different between the three age groups (p ≥ 0.12). The use of antihypertensive therapy was different, being highest in patients <45 years old for monotherapy (60.0% vs. 53.5% in two other subgroups, p < 0.0001), and highest in patients ≥65 years old for combination therapy (53.3% vs. 41.6% in two other subgroups, p < 0.0001). The use of renin-angiotensin system (RAS) inhibitors, however, was not significantly different between the three groups (p = 0.09).
Table 2. Treatment of hypertension and diabetes mellitus according to age group.
Overall, the prevalence of albuminuria and ECG-LVH was 17.8 and 6.5%, respectively, and the prevalence of both albuminuria and ECG-LVH was 1.6%.
Prevalence of albuminuria and ECG-LVH according to blood pressure and age groups
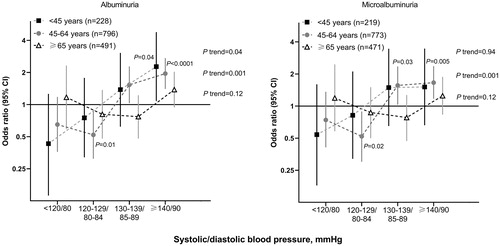
In the young aged group, the prevalence of albuminuria increased from 8.8% in systolic/diastolic blood pressure <120/80 mmHg to 14.6, 16.0 and 16.5% in systolic/diastolic blood pressure at 120–129/80–84, 130–139/85–89 and ≥140/90 mmHg, respectively (). The corresponding prevalence values were 8.9, 7.0, 18.1 and 22.2%, respectively, in the middle aged group, and 21.2, 15.5, 16.4 and 24.4%, respectively, in the older aged group. Similar results were observed in the analyses restricting to patients with microalbuminuria ().
Table 3. Prevalence of albuminuria according to blood pressure and age groups.
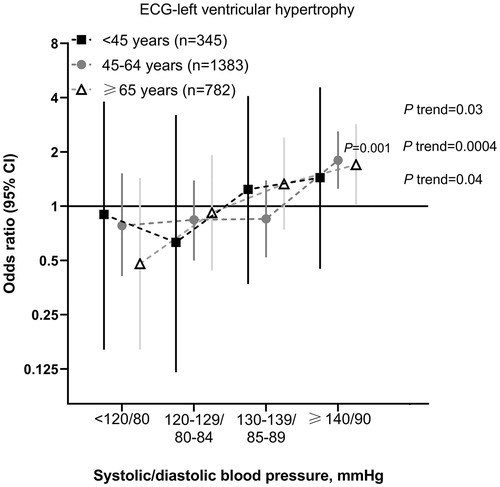
The prevalence of ECG-LVH was significantly (p for trend ≤0.04) higher with increasing blood pressure similarly in the three age groups ().
Table 4. Prevalence of ECG-left ventricular hypertrophy according to blood pressure and age groups.
We performed sensitivity analyses in patients with treated hypertension (n = 1713, Supplementary material Table S1), non-diabetic patients (n = 895, Supplementary material Table S2) and patients with the use of RAS inhibitors (n = 967, Supplementary material Table S3). The results of these analyses were confirmatory.
Adjusted age-stratified association of blood pressure with albuminuria and ECG-LVH
After adjustment for age, sex, body mass index, current smoking and alcohol intake, serum total cholesterol and triglycerides, heart rate, the presence of diabetes mellitus, HbA1c level, duration of hypertension and the use of RAS inhibitors, the odds ratio relative to the overall risk for albuminuria in the whole age subgroup was smallest in patients with a systolic/diastolic blood pressure of <120/80 mmHg in the young aged group, but in patients with a systolic/diastolic blood pressure of 120–129/80–84 mmHg in the middle and older aged groups (). A J-shaped relationship between the risk of albuminuria and blood pressure was observed in the older aged group. Similar results were observed in the analyses restricting to microalbuminuria ().
Figure 1. Odds ratio relative to the whole age subgroup for albuminuria (left panel) and microalbuminuria (right panel). The analysis was adjusted for age, sex, body mass index, current smoking and alcohol intake, serum total cholesterol and triglycerides, heart rate, the presence of diabetes mellitus HbA1c level, duration of hypertension and the use of RAS inhibitors. Vertical lines denote 95% confidence intervals (CI). The number of participants for each subgroup is given in the parentheses. p values for trend and for significant odds ratios are given alongside the symbols.
In similar adjusted analyses, the odds ratio relative to the overall risk for ECG-LVH in the whole age subgroup was smallest in those with a systolic/diastolic blood pressure of <120/80 mmHg consistently in the three age groups ().
Figure 2. Odds ratio relative to the whole age subgroup for left ventricular hypertrophy. The analysis was adjusted for age, sex, body mass index, current smoking and alcohol intake, serum total cholesterol and triglycerides, heart rate, the presence of diabetes mellitus HbA1c level, duration of hypertension and the use of RAS inhibitors. Vertical lines denote 95% confidence intervals (CI). The number of participants for each subgroup is given in the parentheses. P values for trend and for significant odds ratios are given alongside the symbols.
Discussion
Our study in patients with either hypertension and/or diabetes mellitus showed age-dependent relationship between blood pressure and albuminuria but not ECG-LVH. In the young age group, the prevalence of albuminuria was significantly higher with increasing blood pressure, even when blood pressure was at the high normal or mildly elevated range. While in the older age group, blood pressure below <120/80 mmHg was associated with a higher prevalence of albuminuria, as compared with a systolic/diastolic blood pressure of 120–129/80–84 mmHg.
There is a long-held belief that in the presence of albuminuria hypertension needs to be treated more intensively and earlier [Citation17–19]. In spite of some changes, most of current hypertension guidelines still recommend intensive blood pressure lowering in patients with albuminuria [Citation10]. Because of the positive results of the Systolic Blood Pressure Intervention Trial (SPRINT) [Citation20], including the subgroup of elderly people [Citation21], this notion has been reinforced. In fact, in SPRINT [Citation20,Citation21] and in a meta-analysis [Citation14], intensive blood pressure lowering was associated with a higher risk of renal damage. In the era that we define hypertension with an even lower threshold, and treat hypertension to an even tighter target, we might have to consider whether a universal definition of hypertension is still appropriate, and whether we should take into account age at least for some organ damages, such as albuminuria. One possible explanation is that low blood pressure induced renal hypoperfusion and therefore renal damage.
The prevalence of hypertension or prehypertension in young adults is increasing and the prognosis is often worse due to low awareness and inadequate treatment [Citation22,Citation23]. In young age group of our study, elevated blood pressure, high-normal blood pressure, or prehypertension, whatever the term is used, was already associated with a higher prevalence of albuminuria, which is in line with the results of several previous studies [Citation24,Citation25]. The Coronary Artery Risk Development in Young Adults (CARDIA) study demonstrated in 2582 young adults (18–30 years) that the prevalence of microalbuminuria (urinary albumin-to-creatinine ratio ≥25 mg/g) was higher with increasing blood pressure and the odds was significant (p = 0.0002) already at the systolic/diastolic blood pressure of 120–129/80–84 mmHg [Citation24]. In a study in 2430 Chinese children and adolescents, the urinary albumin-to-creatinine ratio was higher in moderate stable, high stable and moderate increasing blood pressure trajectories from 6–15 years at baseline to adulthood 30 years later than the low stable group [Citation25].
In spite of low prevalence of ECG-LVH, our study showed a significant positive association between blood pressure level and the prevalence of ECG-LVH in the overall population. Similar trends were observed in young, middle and older aged patients. This finding is in contrast to the results of age-dependent associations on albuminuria and is in line with the results of numerous previous studies [Citation26,Citation27]. High blood pressure confers risk of left ventricular hypertrophy, regardless whether diagnosed by ECG [Citation26], echocardiography [Citation27] or MRI in cross-sectional and longitudinal studies. Antihypertensive treatment, especially with the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, may prevent and regress left ventricular hypertrophy [Citation28].
The results of our study should be interpreted within the context of several limitations. First, our study had a cross-sectional design and does not allow any causal inference. Second, our study had a relatively small sample size with few measurements of biological markers. In particular, we did not measure serum creatinine and uric acid in this multicenter study. Kidney function may behave as a confounding factor. However, we performed urine routine testing and measured urinary albumin and creatinine excretion. According to a recent China multicentre registry, the prevalence of reduced glomerular filtration rate (≤60 mL/min/1.73 m2) in hypertensive outpatients was 22.0% [Citation29]. This relatively low prevalence makes a huge effect of kidney dysfunction on our study results less likely. Third, albuminuria was evaluated on a single spot urine sampling. However, urinary albumin and creatinine were measured in a core laboratory. A stringent quality assurance programme was implemented, including the exclusion of patients with suspected urinary tract infections. Fourth, our analysis was based on the mean of six blood pressure readings obtained at two clinic visits two to five days apart, which is usually 1–2 mmHg higher than the mean of the second and third readings [Citation30], and might hence slightly overestimate blood pressure. Finally, ECG, instead of echocardiography, was used to detect LVH, which has low sensitivity and may hence underestimate the prevalence of LVH [Citation31].
Conclusion
The association between blood pressure and organ damage seems to differ in young, middle and older aged patients for albuminuria but not ECG-LVH. This age-stratified cross-sectional association should be investigated in prospective observational and interventional studies.
Acknowledgements
The authors gratefully acknowledge the participation of the patients and the contribution of the investigators. The participating hospitals were listed in an Supplementary material Appendix 1 (http://links.lww.com/HJH/A634).
Disclosure statement
Dr. Wang reports receiving lecture and consulting fees from Novartis, Omron, Servier, and Takeda. The other authors declared no conflicts of interest.
Additional information
Funding
References
- Lacey B, Lewington S, Clarke R, et al. China Kadoorie Biobank collaborative group. Age-specific association between blood pressure and vascular and non-vascular chronic diseases in 0.5 million adults in China: a prospective cohort study. Lancet Glob Health. 2018;6(6):e641–e649.
- Zhang YY, Moran A. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension 2017;70(4):736–742.
- Wang J, Zhang L, Wang F, et al.; China National Survey of Chronic Kidney Disease Working Group. Prevalence, awareness, treatment, and control of hypertension in China: results from a national survey. Am J Hypertens. 2014;27(11):1355–1361.
- Franklin SS, Gustin W, IV, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96(1):308–315.
- Li Y, Thijs L, Zhang ZY, et al.; On behalf of the International Database on Ambulatory and Home Blood Pressure in Relation to Cardiovascular Outcome Investigators. Opposing age-related trends in absolute and relative risk of adverse health outcomes associated with out-of-office blood pressure. Hypertension 2019;74(6):1333–1342.
- Yano Y, Reis JP, Colangelo LA, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA. 2018;320(17):1774–1782.
- Ishikawa Y, Ishikawa J, Ishikawa S, et al. Jichi Medical School Cohort Investigators Group. Prehypertension and the risk for cardiovascular disease in the Japanese general population: the Jichi Medical School Cohort Study. J Hypertens. 2010;28:1630–1637.
- Wu S, Huang Z, Yang X, et al. Cardiovascular events in a prehypertensive Chinese population: four-year follow-up study. Int J Cardiol. 2013;167(5):2196–2199.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115.
- Williams B, Mancia G, Spiering W, et al.; Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
- Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–1481.
- Qaseem A, Wilt TJ, Rich R, et al.; for the Clinical Guidelines Committee of the American College of Physicians and the Commission on Health of the Public and Science of the American Academy of Family Physicians. Clinical Guidelines Committee of the American College of Physicians and the Commission on Health of the Public and Science of the American Academy of Family Physicians. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430–437.
- Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the Revision Committee of Chinese guidelines for prevention and treatment of Hypertension. J Geriatr Cardiol. 2019;16:182–241.
- Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69(5):486–493.
- Song J, Sheng CS, Huang QF, et al. Management of hypertension and diabetes mellitus by cardiovascular and endocrine physicians: a China registry. J Hypertens. 2016;34(8):1648–1653.
- Zhang W, Liu CY, Ji LN, et al.; for the ATTEND investigators. Blood pressure and glucose control and the prevalence of albuminuria and left ventricular hypertrophy in patients with hypertension and diabetes. J Clin Hypertens. 2020;22(2):212–220.
- Leitão L, Soares-Dos-Reis R, Neves JS, et al. Intensive blood pressure treatment reduced stroke risk in patients with albuminuria in the SPRINT trial. Stroke 2019;50(12):3639–3642.
- Viazzi F, Muiesan ML, Schillaci G, et al. Changes in albuminuria and cardiovascular risk under antihypertensive treatment: a systematic review and meta-regression analysis. J Hypertens. 2016;34(9):1689–1697.
- Jiang X, Srinivasan SR, Radhakrishnamurthy B, et al. Microalbuminuria in young adults related to blood pressure in a biracial (black-white) population. The Bogalusa Heart Study. Am J Hypertens. 1994;7(9_Pt_1):794–800.
- The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;22:2103–2116.
- Williamson JD, Supiano MA, Applegate WB, et al.; for the SPRINT Research Group. SPRINT Research Group. Intensive vs. standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: A randomized clinical trial. JAMA. 2016;315(24):2673–2682.
- Haggart RC, Bartels CM, Smith MA, et al. Sociodemographics and hypertension control among young adults with incident hypertension: a multidisciplinary group practice observational study. J Hypertens. 2018c;36(12):2425–2433.
- Johnson HM, Thorpe CT, Bartels CM, et al. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens. 2014;32(1):65–74.
- Murtaugh MA, Jacobs DR, Jr, Yu X, et al. Coronary Artery Risk Development in Young Adults Study. Correlates of urinary albumin excretion in young adult blacks and whites: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2003;158(7):676–686.
- Zheng W, Mu J, Chu C, et al. Association of blood pressure trajectories in early life with subclinical renal damage in middle age. J Am Soc Nephrol. 2018;29(12):2835–2846.
- Cox J, Amery A, Clement D, et al. Relationship between blood pressure measured in the clinic and by ambulatory monitoring and left ventricular size as measured by electrocardiogram in elderly patients with isolated systolic hypertension. J Hypertens. 1993;11:269–276.
- Urbina EM, Mendizábal B, Becker RC, et al. Association of blood pressure level with left ventricular mass in adolescents. Hypertension 2019;74(3):590–596.
- Soliman EZ, Prineas RJ. Antihypertensive therapies and left ventricular hypertrophy. Curr Hypertens Rep. 2017;19(10):79.
- Xie K, Bao L, Jiang X, et al. The association of metabolic syndrome components and chronic kidney disease in patients with hypertension. Lipids Health Dis. 2019;18(1):229.
- Chen X, Li Y, Hu Z, et al. May Measurement Month 2018: an analysis of blood pressure screening results from China. Eur Heart J Suppl. 2020; 22(Suppl H):H40–H42.
- Wang D, Xu JZ, Zhang W, et al. Performance of electrocardiographic criteria for echocardiographically diagnosed left ventricular hypertrophy in Chinese hypertensive patients. Am J Hypertens. 2020;33(9):831–836.
