Abstract
Background: Acute increases of high blood pressure values, usually described as ‘hypertensive crises’, ‘hypertensive urgencies’ or ‘hypertensive emergencies’, are common causes of patients’ presentation to emergency departments. Owing to the lack of ad hoc randomized clinical trials, current recommendations/suggestions for treatment of these patients are not evidenced-based and, therefore, the management of acute increases of blood pressure values represent a clinical challenge. However, an improved understanding of the underlying pathophysiology has changed radically the approach to management of the patients presenting with these conditions in recent years. Accordingly, it has been proposed to abandon the terms ‘hypertensive crises’ and ‘hypertensive urgencies’, and restrict the focus to ‘hypertensive emergencies’.
Aims and Methods: Starting from these premises, we aimed at systematically review all available studies (years 2010-2020) to garner information on the current management of hypertensive emergencies, in order to develop a novel symptoms- and evidence-based streamlined algorithm for the assessment and treatment of these patients.
Results and Conclusions: In this educational review we proposed the BARKH-based algorithm for a quick identification of hypertensive emergencies and associated acute organ damage, to allow the patients with hypertensive emergencies to receive immediate treatment in a proper setting.
Introduction
Uncontrolled arterial hypertension is a common cause of admittance to the Emergency Departments. The patients presenting with acute increases of high blood pressure (BP) values are commonly labelled as having hypertensive ‘crises’, ‘urgencies’, or ‘emergencies’, which entail highly heterogeneous clinical profiles, ranging from absence of symptoms to non-specific symptoms or to life-threatening conditions because of concomitant hypertension-mediated organ damage.
As to date there is no evidence that the treatment of patients without acute organ damage should differ from that of patients with asymptomatic uncontrolled arterial hypertension (HT) a Task Force of the European Society Cardiology recently proposed that the terms ‘hypertensive crises’, and ‘hypertensive urgencies’, being misleading and useless, should be abandoned and attention should be focussed on recognising the patients presenting with hypertensive emergencies (HEs), as defined below [Citation1]. Nevertheless, the terms ‘crises’ and ‘urgencies’ are still frequently used, and it is common experience that these outdated definitions often translate into a clinical management that does not comply with the 2019 ESC Position document [Citation1]. We therefore conceived this minireview as an educational paper to provide concise information on proper terminology, basic pathophysiology and principles of treatment of hypertensive patients presenting with high BP values at the Emergency Departments.
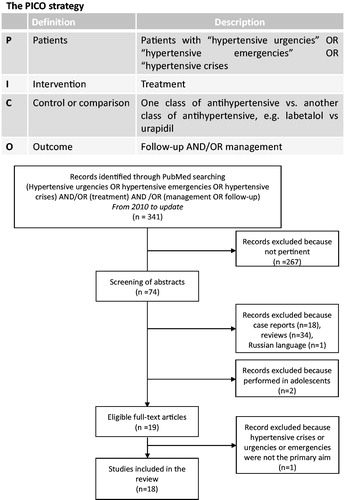
Starting from these premises, we have used a PICO strategy and the boolean operators: [‘hypertensive urgencies’ OR ‘hypertensive emergencies’ OR ‘hypertensive crises’], AND [‘follow-up’ OR ‘management’ OR ‘treatment’] to search the PubMed database from year 2010 to 2020 () and garner information on the current management of these patients. A total of 341 papers were originally identified; however, after screening abstracts and full-texts for relevance and study design independently by two investigators (TMS, GPR), eighteen papers were judged to be eligible and included in this review ().
Definition and epidemiology of hypertensive emergencies
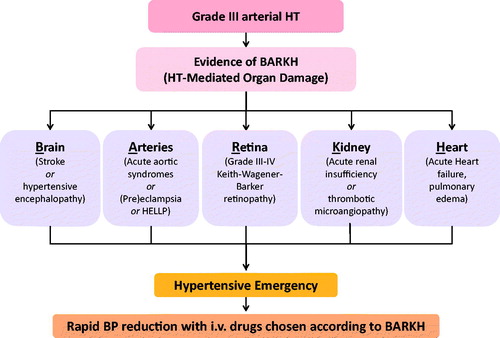
HEs are situations where high BP values are associated with acute life-threatening organ damage involving (any of) the following key organs: Brain, Arteries, Retina, Kidney, and/or Heart (). This led to conceive the BARKH acronym as a widget allowing not only a swift identification of HEs, but also to focus treatment on the affected organ(s).
Figure 2. Simplified brain, arteries, retina, kidney and/or heart (BARKH)-based algorithm for a quick identification of the hypertensive emergencies (HEs) and the associated acute organ damage. If BARKH involvement is detected, the reduction of BP values should be undertaken with i.v. treatment; in any other case, an oral treatment is recommended. BP: blood pressure; HT: hypertension; HELLP: haemolysis elevated liver enzymes low platelets.
An acute rise of BP is a common motive of presentation to the emergency departments: in a survey of administrative data collected over 3 years in 1,290,804 adult patients at 114 acute care hospitals, systolic BP values ≥180 mmHg involved 13.8% of the cases [Citation2]. The rate of HEs, as defined above, was, however, much lower as it entailed only one every 200 patients [Citation3]. Interestingly, this rate has remained relatively stable over the past two decades [Citation4–11]; however, it seems to be higher in developing countries, where over 5% (1.04 billion) of the patients with high blood pressure live.
Mortality for HEs is held to be substantial, i.e. about 4%; moreover, the high BP values are instrumental in driving organ damage, and thus in determining prognosis. Accordingly, to save the patient’s life, an immediate decrease of BP values to limit the extension of organ damage, is mandatory [Citation12]. Consensus exists (Class of Recommendation I, Level of Evidence B) that the BP reduction should be achieved through patient’s admission to an acute care unit, or at least to a unit with a specific expertise in the management of hypertension to allow for continuous monitoring of BP values and organ damage during administration of the appropriate treatment [Citation1].
The choice of the antihypertensive agent to be used and the time course for BP reduction are dictated by type of organ damage and the presence of contraindications to specific agents (). At variance, in patients without acute organ damage, who do not have a HE, acute BP reduction is not necessary and can actually be contraindicated. These patients are better treated with drugs, as long-acting calcium channel blockers, α-1 blockers [Citation13] and mineralocorticoid receptor antagonists [Citation14], that do not interfere with the diagnostic work-up and permit identification of secondary forms of hypertension, a common cause of HEs. This is a key issue as in tertiary HT centres secondary hypertension involves up to 35% of the patients referred for evaluation of HT, about 50% of those with drug-resistant hypertension [Citation15], and 20–40% of those with HEs [Citation16,Citation17].
Table 1. Drugs that can be used intravenously for treatment of hypertensive emergencies (HEs).
Pathophysiological considerations and implication for management
The speed and severity of BP elevation are the main factors driving the onset of a HE: a rapid severe BP increase activates the renin-angiotensin-aldosterone system (RAAS). This raises peripheral vascular resistances in the kidney and other vital organs, thus altering the autoregulation process. Furthermore, RAAS activation causes oxidative stress, formation of peroxinitrite with ensuing impaired nitric oxide bioactivity and, thereby endothelial dysfunction and damage [Citation18,Citation19]. The dislodging of endothelial cells and exposure of subendothelial tissues to blood lead to activation of platelet aggregation and the clotting cascade ().
Figure 3. Mechanisms that lead to loss of autoregulation and injury to microcirculation in severe uncontrolled hypertension (HT). A sudden increase in the vascular resistances induces natriuresis that activates the renin–angiotensin-aldosterone system (RAAS), thus increasing blood pressure and augmenting the microvascular damage. Disruption of the endothelial lining, causing exposure of the sub-endothelial tissue, triggers the clotting cascade leading to thrombotic microangiopathy. Modified from van den Born et al. [Citation1].
![Figure 3. Mechanisms that lead to loss of autoregulation and injury to microcirculation in severe uncontrolled hypertension (HT). A sudden increase in the vascular resistances induces natriuresis that activates the renin–angiotensin-aldosterone system (RAAS), thus increasing blood pressure and augmenting the microvascular damage. Disruption of the endothelial lining, causing exposure of the sub-endothelial tissue, triggers the clotting cascade leading to thrombotic microangiopathy. Modified from van den Born et al. [Citation1].](/cms/asset/6baeacec-f802-4e6f-8f68-4f248677ea77/iblo_a_1917983_f0003_c.jpg)
The hallmark of HEs is the loss of autoregulation, the phenomenon whereby blood flow and organ perfusion are maintained in spite of conspicuous changes of the perfusion pressure. For example, in the brain of normal subjects the range of autoregulation is relatively wide and comprises the BP values that occur in everyday life (). When the autoregulation is lost, an acute increase in BP can lead to cerebral oedema; conversely, a swift reduction of BP can lead to cerebral hypoperfusion. Under both circumstances, the detrimental consequences leading to organ damage in organs (BARKH) as, for example, in the brain are obvious.
Figure 4. Cerebral autoregulation of blood flow in normotensive subjects (continuous line) and in hypertensive patients (dotted lines) with and without ischaemic brain damage. Cerebral blood flow is physiologically maintained at a constant level with mean arterial pressure between 70 and 90 mmHg, below which it dramatically drops. In hypertensive patients (violet dotted line) the autoregulation range of BP is shifted to right towards higher values, between 110 and 150 mmHg, and is narrowed (central shaded green area). At lower and higher cerebral perfusion pressure levels, a fall or an abrupt pressure rise can induce ischaemia (left shaded violet area) or oedema (right shaded pink area). After an ischaemic injury, blood flow blunts proportionally to the injury severity (red and blue dotted lines). Modified from Blumenfeld and Laragh [Citation20].
![Figure 4. Cerebral autoregulation of blood flow in normotensive subjects (continuous line) and in hypertensive patients (dotted lines) with and without ischaemic brain damage. Cerebral blood flow is physiologically maintained at a constant level with mean arterial pressure between 70 and 90 mmHg, below which it dramatically drops. In hypertensive patients (violet dotted line) the autoregulation range of BP is shifted to right towards higher values, between 110 and 150 mmHg, and is narrowed (central shaded green area). At lower and higher cerebral perfusion pressure levels, a fall or an abrupt pressure rise can induce ischaemia (left shaded violet area) or oedema (right shaded pink area). After an ischaemic injury, blood flow blunts proportionally to the injury severity (red and blue dotted lines). Modified from Blumenfeld and Laragh [Citation20].](/cms/asset/fb8ec727-42f4-458a-832b-b7c83997ad3e/iblo_a_1917983_f0004_c.jpg)
The full-blown picture of HEs is seen clinically, for examples, in hypertensive encephalopathy, malignant hypertension, and thrombotic microangiopathy. The narrowing of the autoregulatory range developing with chronic HT, alongside the loss of autoregulation occurring with severe elevations of BP, drive ominous consequences in the brain. This explains why a swift reduction of BP values usually leads to prominent amelioration of the clinical picture, but also why it should be undertaken cautiously. Regardless of which BARKH is involved, the loss of autoregulation has similar detrimental consequences: for example, in the retina acute reductions of the high BP can cause acute optic ischaemia with vision loss; in the coronary vascular bed acute reduction of high BP can induce irreversible ischaemic changes [Citation21].
Hypertensive encephalopathy
In patients with chronic HT the range of autoregulation is not just reset towards higher values, but markedly narrowed and, therefore, when BP is reduced, patients may experience organ hypoperfusion. In the presence of stroke, the loss of autoregulation and the steeper relationship between BP and flow implies that any reduction of BP, even not in the hypotensive range, will translate into a reduction of blood flow (). These pathophysiological considerations explain why an acute BP lowering in the first 5–7 days of a stroke was associated with worse neurological outcome [Citation22,Citation23] and, therefore, is no longer recommended by the ESC/ESH guidelines [Citation24,Citation25] (Class of Recommendation III, Level of Evidence B).
Conversely, increases of BP values exceeding the upper of the autoregulatory range, particularly if abrupt, can raise intracranial pressure leading to cerebral oedema, especially in the posterior areas of the brain where BP oscillations are less effectively damped because sympathetic innervation is less pronounced [Citation26]. Hypertensive encephalopathy can thus cause the posterior reversible leukoencephalopathy syndrome, a condition featuring headache, vision abnormalities, paresis, hemianopsia, nausea, altered mental status, white matter lesions, and vasogenic oedema in the posterior brain regions.
Focal neurological lesions are rare in hypertensive encephalopathy; they should raise the suspicion of an acute stroke, but focal regions of symmetric hemispheric oedema are usually seen on CT/MR imaging, mostly in the parietal and occipital lobes, but also in the frontal lobes, the inferior temporal-occipital junction, and the cerebellum [Citation27,Citation28]. As oedema increases, lesions can become confluent, and small haemorrhages and infarctions can occur, unless BP is rapidly lowered.
In fact, if not adequately treated, hypertensive encephalopathy [Citation29] and posterior reversible leukoencephalopathy syndrome [Citation30] can progress to cerebral haemorrhage, coma, and death. However, an appropriate and prompt treatment can be followed by a complete recovery [Citation31], which emphasises the key role of an immediate diagnosis and an effective BP-lowering treatment.
Retinopathy
Grade III retinopathy, characterised by flame-shaped haemorrhages and cotton wool spots, and grade IV, which also includes papillooedema, may be frequently found in HE patients. Concomitant arteriolar narrowing with an increased light axial reflex, and the arterio-venous nicking (Salus Gunn sign) are common and denote long-standing hypertension. Fundoscopy should be an essential step of the examination, because besides the high BP, no other signs or symptoms can predict retinopathy [Citation32]. Nonmydriatic ocular fundus digital photography, which can be implemented for use in smartphones, is a valuable addition to direct ophthalmoscopy. It can facilitate the examination and has the undubious advantage of an objective documentation of the rethinopathy [Citation33].
Thrombotic microangiopathy
The aforementioned endothelial damage occurring in HEs triggers a cascade of events that begins with platelets activation and thrombi formation, microvessels obliteration, disseminated intravascular coagulation (DIC), and progression to thrombotic microangiopathy, with erythrocytes bridling and platelet consumption. The HE involving thrombotic microangiopathy resembles thrombotic thrombocytopenic purpura and hemolytic-uremic syndrome; however, differentiation of this form from the others, albeit sometimes possible only post hoc, is crucial, as summarised in [Citation1,Citation34].
Table 2. Criteria for differential diagnosis between hypertensive emergencies (HE) due to thrombotic microangiopathy and thrombotic thrombocytopenic purpura.
Pre-eclampsia/eclampsia
The ESC defines pre-eclampsia as gestational hypertension associated with proteinuria ≥0.3 g/day in a 24 h urine collection or ≥30 mg/mmol urinary creatinine in a spot random urine sample [Citation35]. The NICE guidelines adopted a tighter definition entailing new onset hypertension after 20 weeks of pregnancy, proteinuria, and maternal organ dysfunction or utero-placental dysfunction [Citation36]. In both definitions hypertension is defined as systolic BP >140 mmHg and/or diastolic BP >90 mmHg; oedema is not considered as a conditio sine qua non criterion of pre-eclampsia because it occurs in up to 60% of the normal pregnancies [Citation36].
Pre-eclampsia develops in 5–7% of pregnancies [Citation37], with a 3–5 fold higher rate in women with pre-existing hypertension, and even more commonly in first pregnant, diabetic, multiple foetuses or hydatidiform mole [Citation38]. As pre-eclampsia implies severe complications for both the mother and the foetus, appropriate risk assessment and management are indispensable.
Consensus exists that to prevent hypertensive complications in the mother, BP should be lowered to <160/105 mmHg [Citation1,Citation39] with intravenous labetalol or nicardipine, along with magnesium sulphate for prevention of seizures and convulsions. As regards labetalol, monitoring of foetal heart rate is needed to avoid bradycardia, and its cumulative daily dose should not exceed 800 mg (Class of Recommendation II, Level of Evidence B) [Citation1].
When switching to oral treatment is feasible, methyldopa and long-acting nifedipine are first choice [Citation1]; in contrast, ACE inhibitors and angiotensin-receptor blockers should be avoided because of potential teratogenity, and diuretics are not recommended because they reduce amniotic fluid and placental blood flow [Citation1].
Acute aortic syndromes
Aortic dissection, intramural haematoma, and penetrating atherosclerotic ulcers are inter-related life-threatening conditions whose incidence ranges from 4 to 6 cases per 100,000 persons/years, but increases up to 30 or more in those who are older than 65 years [Citation40–42]. High BP values are the force driving towards a dreadful outcome in these acute aortic syndromes. Hence, rapid lowering of systolic BP with i.v. drugs is necessary to <120 mmHg, or less if tolerated, preferably with drugs that lower dP/dt without causing reflex tachycardia () [Citation1].
Acute coronary syndrome (ACS)
HEs in the setting of an ACS mandate administration of i.v. nitroglycerine with up-titration to control pain and lower systolic BP to less than 140 mmHg. Considering that these patients usually receive antiplatelet drugs, as aspirin, ticagrelor, or clopidogrel, that raise the risk of cerebral haemorrhage if BP is not well controlled, the lowering of high BP is an obligatory step. β blockers should be used to lower cardiac work and myocardial oxygen consumption and to control nitroglycerine-induced reflex tachycardia [Citation43]. If they are contraindicated, a non dihydropyridine calcium channel blocker as verapamil or diltiazem, represent a reasonable alternative.
Acute heart failure
In patients presenting with a HE associated with acute heart failure treatment has the goals of lowering afterload, thus improving the ejection fraction, and of resolving lung congestion. Hence, i.v. loop diuretics, along with nitroglycerine uptitrated to the highest tolerated dose to decrease afterload and preload, are necessary.
The mineralocorticoid receptor antagonists, which have neglibile acute BP lowering effects, can be effectively combined with loop diuretics as they help preventing the hypokalaemia that often occurs as a result of increased delivery of Na+ to the epithelial Na channel (eNaC) in the distal tubule and collecting ducts of the nephron.
The lowering of BP values in HEs of acute coronary syndromes associated decreases afterload and cardiac work with ensuing decrease of myocardial oxygen consumption, thus contributing to resolve chest pain and limit both the extent of myocardial necrosis and the risk of myocardial rupture.
Pheochromocytoma/paraganglioma (PPGL)
PPGL are rare causes of high BP, but in about half of the cases they can present with very severe and acute increases of BP values and evidence of organ damage. For example, an acute coronary syndrome and/or acute heart failure, aortic dissection, stroke or eclampsia, can be the first clinical manifestation of an undetected PPGL. The presence of affected relatives in the pedigree and the detection at physical examination of signs pointing to a syndromic form of PPGL, for example neurofibromatosis, or of midriasis indicating catecholaminergic excess, are strong clues of a PPGL that should not be disregarded.
Administration of α1 blocking agents, as fentolamine or doxazosin, followed by a β blocker, but not in the opposite order to avoid enhancing α1-mediated vasoconstriction, are essential to achieve rapid control of BP. Among β blockers, labetalol, which has an α1 blocking effect when administered intravenously [Citation44], is the only one that can be used i.v. without prior α1 blockade for the treatment of HEs in PPGL. As PPGL patients have relative hypovolemia, owing to a shift of blood volume from the periphery to the cardiopulmonary district, rapidly acting diuretics should be avoided unless strictly necessary to control congestion [Citation45].
Diagnostic work-up to identify hypertensive emergencies
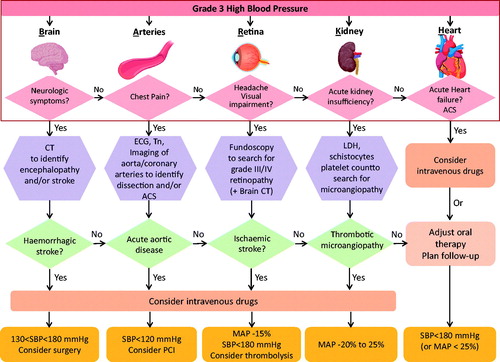
‘Time is life’ for HE patients; this means that a swift identification of the underlying conditions immediately followed by appropriate treatment is vital. The hierarchical algorithm shown in uses well-defined symptoms to immediately identify the BARKH involvement, and the underlying acute life-threatening organ damage, thus allowing physicians to undertake a simple and swift selection of the diagnostic work up to be immediately commenced.
Figure 5. The cartoon illustrates the BARKH approach to identification and treatment of HEs, a simplified symptom-based hierarchical algorithm to assist physicians in the rapid evaluation of patients presenting with suspected HEs. See text for the details. BARKH: brain, arteries, retina, kidney and/or heart; SBP: systolic BP; MAP: mean arterial pressure; LDH: lactic dehydrogenase.
Treatment of hypertensive emergencies
For a proper management of HEs the pathophysiological considerations on autoregulation of blood flow in vital organs are central, given that a prompt initiation of an i.v. treatment to achieve a rapid BP reduction is mandatory. Hence, when undertaking acute BP reduction with intravenous agents, utmost attention should be given to preserve vital organs perfusion.
Drugs with hardly titratable effects, for example immediate-release and swiftly acting agents given sublingually [Citation8], should be avoided (Class of Recommendation III, Level of Evidence C). Conversely, in patients who do not have a HE at BARKH assessment, administration of drugs that lower BP acutely is contraindicated (Class of Recommendation III, Level of Evidence B). Antianxiety drugs, as diazepam, which can lower BP and do not preclude the search for secondary forms of hypertension, were suggested to lower BP in a small prospective study [Citation46].
As a general principle, the choice of the drug to use in HEs patients is defined by the type of organ damage and the presence of contraindications to specific drug(s) or a class of agents (). In our experience labetalol was proved to be an effective all-round agent, well tolerated and with few contraindications. When given intravenously, it has alpha-blocking activity, which is useful in most HEs, including patients with PPGL. The onset of its antihypertensive effect occurs within 2–5 min and peaks over 5–15 min, which provides a good time window for titrating its rate of i.v. administration. A 20 mg priming i.v. bolus is usually followed by a continuous infusion, the rate of which can be up- or down-titrated to reach and maintain the desired BP target values. In the patients with hypertensive encephalopathy or stroke, labetalol should be preferred to nitrates, as nitroglycerine and nitroprusside, because it leaves the cerebral blood flow unaffected and does not increase intracranial pressure [Citation47].
The BP level that should be maintained in patients with acute ischaemic stroke to ensure the best outcome is not known. Caution shoud be exercised in ischaemic stroke to avoid hypotension and worsening of neurologic defects [Citation23,Citation48]. Mean arterial pressure should be reduced by 15% during the first hour; however, a faster BP decrease could be appropriate in certain conditions [Citation49]. For example, a swift reduction of BP is particularly important if patients are candidate to emergency reperfusion therapy with i.v. alteplase, because this is feasible only if their BP is not severely elevated, i.e. systolic BP <185 and diastolic BP <110 mmHg (class of recommendation I, level of evidence B) [Citation49]. The same is recommended in patients for whom mechanical thrombectomy is planned and who have not received i.v. thrombolytic therapy (class of recommendation IIa, level of evidence B).
In haemorrhagic stroke, the elevation of BP is usually greater than in patients with ischaemic stroke, owing to stress-induced activation of the sympathetic nervous system, the RAAS and cortisol release, and also to increased intracranial pressure. Moreover, high BP is the driving force for expansion of the haematoma with possible rebleeding. Therefore, although an acute lowering of systolic BP to < 140 mmHg is probably safe, a more modest reduction of BP (e.g. MAP of 110 mm Hg or target BP of 160/90 mmHg) is recommended by the American Heart Association (AHA)/American Stroke Association (ASA) guidelines if patients have no evidence of increased intracranial pressure [Citation50]. In both ischaemic and haemorrhagic strokes, labetalol is the preferred drug, but nicardipine or clevidipine can be alternatives in those countries where such drugs are available [Citation49].
For a detailed treatment of high BP in ischaemic and haemorrhagic stroke syndromes the reader is referred to the available AHA/ASA 2019 guidelines that updated the 2018 guidelines [Citation49] and to the AHA/ASA guidelines, respectively [Citation50].
summarises first line and alternative treatments for the HEs with different types of organ damage. With its rapid onset of action, ranging from 5 to 15 min, and marked coronary and cerebral vasodilator effects that result in increased local blood flow, nicardipine is also a first-line treatment for HEs. However, owing to such actions, it can induce reflex tachycardia and is contraindicated in liver failure. Despite being approved for HEs by both European Medicines Agency (EMA) and Food and Drug Administration (FDA), its use remains confined to US and some European countries. As reported above, nitroglycerine is the first line drug for acute heart failure and coronary syndrome.
Table 3. First line and alternative treatments for hypertensive emergencies.
For the general treatment of the acute coronary syndromes the reader is referred to the ESC guidelines, which, however, devoted scant attention to the management of high BP [Citation51]. The BP target to be attained in the different BARKH HEs are illustrated in .
Hypertensive emergencies caused by cocaine abuse are becoming more common. They can present with chest pain, tachycardia and altered mental status, and less frequently with aortic dissection, cerebral haemorrhage, seizures, arrhythmias and even sudden cardiac death [Citation52–54]. Dilated pupils (bilateral midriasis) are the typical sign of the hyperadrenergic state that associates with cocaine abuse.
The mechanism of cocaine cardiovascular toxicity entails a sympathomimetic effect that implies increased oxygen demand along a decreased oxygen delivery and the blockade of voltage-dependent K+ and Na+ channels. A hypersensitivity to drug or contaminants, such as amphetamine or talc, used to adulterate cocaine can also play a role in enhancing or masking the clinical picture. Hypertension secondary to cocaine is responsive to i.v. benzodiazepines that minimise the stimulant effects of cocaine on the central nervous system and to mixed β and α-blocker labetalol that, as mentioned above, acts as α-blocker when i.v. infused [Citation1]. Nitroglycerine or nitroprusside can be also administered if further therapy is indicated for chest pain.
Non-selective beta-blockers should be avoided because of the risk of an abrupt rise in blood pressure as well as coronary vasoconstriction due to the exaggerated effect of catecholamines on unblocked alpha-receptors.
Conclusions
Markedly raised BP values cause anxiety in the patient, her/his family, and physicians. However, true HEs are rare. The vast majority of the patients presenting at the EDs with high BP values do not have a HE and do not need acute BP lowering, but rather referral to a hypertensive specialist/center for proper work-up aimed at discovering the underlying cause. Patients with HEs are identified by the presence of organ damage, which foretells life-threatening complications, and not just of high BP values.
In this educational review, we proposed the BARKH-based algorithm for a quick identification of HEs and associated acute organ damage, to allow that the patients with HEs receive immediate treatment in a proper setting. Once the high BP values have been controlled, one should consider that, the acute elevation of BP causing organ damage can be due to a secondary form of hypertension that need to be searched for, and diagnosed timely, in order to improve the otherwise slim prognosis. In line with this contention, in patients with drug-resistant hypertension, a cohort presenting every now and then to the EDs, about 25% were recently found to have unrecognised primary aldosteronism that could be cured with adrenal vein sampling-guided unilateral adrenalectomy [Citation55].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- van den Born B-JH, Lip GYH, Brguljan-Hitij J, et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur Hear J – Cardiovasc Pharmacother. 2019;5(1):37–46.
- Shorr AF, Zilberberg MD, Sun X, et al. Severe acute hypertension among inpatients admitted from the emergency department. J Hosp Med. 2012;7(3):203–210.
- Zampaglione B, Pascale C, Marchisio M, et al. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27(1):144–147.
- Hannemann A, Friedrich N, Lüdemann J, et al. Reference intervals for aldosterone, renin, and the aldosterone-to-renin ratio in the population-based study of health in pomerania (SHIP-1). Horm Metab Res. 2010;42(06):392–399.
- Janke A, McNaughton C, Brody A, et al. Trends in the incidence of hypertensive emergencies in US Emergency Departments From 2006 to 2013. J Am Heart Assoc. 2016;5(12):e004511.
- Martin J, Higashiama E, Garcia E, et al. Hypertensive crisis profile. Prevalence and clinical presentation. Arq Bras Cardiol. 2004;83(2):131–136.
- Polgreen LA, Suneja M, Tang F, et al. Increasing trend in admissions for malignant hypertension and hypertensive encephalopathy in the United States. Hypertension. 2015;65(5):1002–1007.
- Salvetti M, Paini A, Colonetti E, et al. Hypertensive emergencies and urgencies: a single-centre experience in Northern Italy 2008–2015. J Hypertens. 2020;38(1):52–58.
- Armitage LC, Whelan ME, Watkinson PJ, et al. Screening for hypertension using emergency department blood pressure measurements can identify patients with undiagnosed hypertension: a systematic review with meta-analysis. J Clin Hypertens. 2019;21(9):1415–1425.
- Kotruchin P, Mitsungnern T, Ruangsaisong R, et al. Hypertensive urgency treatment and outcomes in a Northeast Thai population: the results from the hypertension registry program. High Blood Press Cardiovasc Prev. 2018;25(3):309–315.
- Shao P, Sawe H, Murray B, et al. Profile of patients with hypertensive urgency and emergency presenting to an urban emergency department of a tertiary referral hospital in Tanzania. BMC Cardiovasc Disord. 2018;18(1):158.
- Kumar N, Simek S, Garg N, et al. Thirty-day readmissions after hospitalization for hypertensive emergency. Hypertension. 2019;73(1):60–67.
- Rossi GP, Seccia TM, Pessina AC. Clinical use of laboratory tests for the identification of secondary forms of arterial hypertension. Crit Rev Clin Lab Sci. 2007;44(1):1–85.
- Rossitto G, Cesari M, Ceolotto G, et al. Effects of mineralocorticoid and AT-1 receptor antagonism on the aldosterone-renin ratio (ARR) in primary aldosteronism patients (EMIRA Study): rationale and design. J Hum Hypertens. 2019;33(2):167–171.
- Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957–1965.
- Lip GY, Beevers M, Beevers G. The failure of malignant hypertension to decline: a survey of 24 years’ experience in a multiracial population in England. J Hypertens. 1994;12(11):1297–1305.
- Van den Born B-JH, Koopmans RP, Groeneveld JO, et al. Ethnic disparities in the incidence, presentation and complications of malignant hypertension. J Hypertens. 2006;24(11):2299–2304.
- Rossi GP, Seccia TM, Barton M, et al. Endothelial factors in the pathogenesis and treatment of chronic kidney disease Part I: general mechanisms: a joint consensus statement from the European Society of Hypertension Working Group on Endothelin and Endothelial Factors and The Japanese Society. J Hypertens. 2018;36 (3):451–461.
- Rossi G, Seccia Y, Barton M, et al. Endothelial factors in the pathogenesis and treatment of chronic kidney disease part II: role in disease conditions: a joint consensus statement from the european society of hypertension working group on endothelin and endothelial factors and the Japanese. J Hypertens. 2018;36(3):426–471.
- Blumenfeld JD, Laragh JH. Management of hypertensive crises: the scientific basis for treatment decisions. Am J Hypertens. 2001;14(11 Pt 1):1154–1167.
- Palatini P. ECG changes during minoxidil therapy. Arch Intern Med. 1981;141(6):817.
- Ahmed N, NäSman P, Wahlgren NG. Outcome after acute stroke. Stroke. 2000;31(6):1250–1255.
- Sandset EC, Bath PMW, Boysen G, et al . The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377(9767):741–750.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
- ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–1938.
- Gierthmühlen J, Allardt A, Sawade M, et al. Role of sympathetic nervous system in activity-induced cerebral perfusion. J Neurol. 2010;257(11):1798–1805.
- Bartynski WS. Posterior reversible encephalopathy syndrome, Part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–1042.
- Mishima E, Funayama Y, Suzuki T, et al. Concurrent analogous organ damage in the brain, eyes, and kidneys in malignant hypertension: reversible encephalopathy, serous retinal detachment, and proteinuria. Hypertens Res. 2021;44(1):88–97.
- Miller J, Suchdev K, Jayaprakash N, et al. New developments in hypertensive encephalopathy. Curr Hypertens Rep. 2018;20(2):13.
- Narbone MC, Musolino R, Granata F, et al. PRES: posterior or potentially reversible encephalopathy syndrome? Neurol Sci. 2006;27(3):187–189.
- Legriel S, Schraub O, Azoulay E, et al. Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS One. 2012;7(9):e44534.
- Rossi GP, Ceolotto G, Rossitto G, et al. Prospective validation of an automated chemiluminescence-based assay of renin and aldosterone for the work-up of arterial hypertension. Clin Chem Lab Med. 2016:54(9):1441–1450.
- Bruce BB. Nonmydriatic ocular fundus photography in the emergency department: how it can benefit neurologists. Semin Neurol. 2015;35(5):491–495.
- Fox L, Cohney S, Kausman J, et al. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Intern Med J. 2018;48(6):624–636.
- Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy. Eur Heart J. 2011;32(24):3147–3197.
- Webster K, Fishburn S, Maresh M, et al. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366(September):1–8.
- Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376(9741):631–644.
- Acog Committee On Obstetric Practice. ACOG Practice bulletin: diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;77(1):67–75.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39(33):3021–3104.
- Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–2618.
- Howard DPJ, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127(20):2031–2037.
- Clouse WD, Hallett JW, Schaff H V, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–180.
- Collet J-P, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;42(14):1289–1367.
- Dal Palu C, Pessina AC, Semplicini A, et al. Intravenous labetalol in severe hypertension. Br J Clin Pharmacol. 1982;13(1 Suppl):97S–99S.
- Bravo EL, Fouad-Tarazi F, Rossi G, et al. A reevaluation of the hemodynamics of pheochromocytoma. Hypertension. 1990;15(2 Suppl):I128–I131.
- Grossman E, Nadler M, Sharabi Y, et al. Antianxiety treatment in patients with excessive hypertension. Am J Hypertens. 2005;18(9 Pt):1174–1177.
- Wilson DJ, Wallin JD, Vlachakis ND, et al. Intravenous labetalol in the treatment of severe hypertension and hypertensive emergencies. Am J Med. 1983;75(4A):95–102.
- Fischberg GM, Lozano E, Rajamani K, et al. Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;19(4):339–346.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association/American Stroke. Stroke. 2019;50(12):e344–e418.
- Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46(7):2032–2060.
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Socie. Eur Heart J. 2018;39(2):119–177.
- Singh A, Khaja A, Alpert MA. Cocaine and aortic dissection. Vasc Med. 2010;15(2):127–133.
- Papadopoulos DP, Sanidas EA, Viniou NA, et al. Cardiovascular hypertensive emergencies. Curr Hypertens Rep. 2015;17(2):5.
- Padilha WSC, Annes M, Massant CG, et al. Cocaine-induced renal artery dissection as a cause of secondary hypertension: a rare presentation. Am J Case Rep. 2020;21:e921565.
- Torresan F, Rossitto G, Bisogni V, et al. Resolution of drug-resistant hypertension by adrenal vein sampling-guided adrenalectomy: a proof-of-concept study. Clin Sci. 2020;134(11):1265–1278.