Abstract
Purpose
We investigated continuous positive airway pressure (CPAP) adherence and its association with the blood pressure (BP) and pulse rate changes in patients with obstructive sleep apnoea syndrome (OSAS) and hypertension.
Materials and methods
In a single-blind trial, patients were randomly assigned to CPAP or sham CPAP treatment for 3 months. We performed clinic, ambulatory and home BP measurements at baseline and during follow-up. CPAP adherence was assessed as the CPAP frequency per week and time per night. Non-adherence was defined as a CPAP use for <5 days/week or <4 h/night.
Results
In the CPAP (n = 26) and sham CPAP groups (n = 21), the CPAP frequency was 5.5 and 4.8 days/week (p = 0.17), respectively, and the CPAP time was 5.0 and 4.1 h/night (p = 0.03), respectively. The corresponding prevalence of non-adherence was 46.2% and 66.7% (p = 0.16), respectively. The CPAP frequency but not time tended to be associated with the changes in BP and pulse rate at 3 months of follow-up, especially home systolic/diastolic BP in the CPAP group (3.2/1.3 mmHg greater reductions per 1 day increment, p ≤ 0.01). Adherent, compared with non-adherent patients, had greater reductions in BP or pulse rate at 3 months of follow-up. In the CPAP and sham CPAP groups combined, statistical significance was achieved for the adjusted between adherence and non-adherence differences in home systolic/diastolic BP (–5.0/–3.8 mmHg) and 24-h, daytime and night-time ambulatory pulse rate (–6.2, −7.8 and −4.4 beats/min, respectively, p ≤ 0.04).
Conclusion
CPAP adherence was associated with the BP lowering and pulse rate slowing effects, especially the CPAP frequency.
Introduction
Obstructive sleep apnoea syndrome (OSAS) is a common secondary cause of hypertension [Citation1–3]. However, continuous positive airway pressure (CPAP) only shows modest blood pressure lowering effect [Citation4], although it is effective in the relieve of daytime sleepiness and other symptoms of OSAS [Citation5]. The low antihypertensive efficacy might have accounted for the lack of efficacy in the prevention of cardiovascular events [Citation6]. Indeed, previous individual studies of relatively large size [Citation7] and meta-analyses [Citation8,Citation9] consistently demonstrated that CPAP treatment for a few months only had a 2–3 mmHg of systolic and diastolic blood pressure lowering efficacy [Citation7–9]. The antihypertensive effect of the CPAP treatment seemed to be slightly greater in patients with OSAS and resistant hypertension, but again was not sufficiently large, being in a range from 4 to 7 mmHg of systolic and diastolic blood pressure reductions [Citation10,Citation11].
One of the possible reasons for the low antihypertensive efficacy might be the low adherence to CPAP therapy [Citation12]. Several previous studies have shown that the blood pressure lowering efficacy is positively associated with the number of hours per night of the CPAP use [Citation11,Citation13,Citation14]. When the CPAP use is longer than 4 h, the blood pressure lowering effect can be up to 5/4 mmHg in 24-h ambulatory systolic/diastolic blood pressure [Citation13]. Nonetheless, few studiessystematically investigated CPAP adherence with regard to its prediction and clinical relevance. In the present analysis of a randomised controlled trial in patients with moderate-to-severe OSAS and nocturnal hypertension, we investigated predictors of CPAP adherence as assessed by both the number of days per week and the number of hours per night, and the association between the CPAP adherence and blood pressure and pulse rate changes as assessed by clinic, ambulatory and home monitoring techniques.
Materials and methods
Study population
The study participants were patients enrolled in a single-blind, parallel group, randomised, controlled trial conducted from November 2014 to October 2016 in Ruijin Hospital in Shanghai, China. The study complied with the International Conference on Harmonisation Guidelines for Good Clinical Practice local regulations and the ethical principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
The study protocol was described in detail previously [Citation15]. Briefly, after a written informed consent was given, male and female (18–75 years of age) patients with moderate-to-severe OSAS (apnoea-hypopnoea index [AHI] ≥ 15 events/h) and nocturnal hypertension (night-time systolic blood pressure ≥120 mmHg or diastolic blood pressure ≥70 mmHg) and tolerance to the CPAP treatment in a one week run-in period (treatment time with CPAP ≥2 h/night and ≥3 nights/week) were randomly assigned to the CPAP treatment or sham CPAP control group. In the CPAP treatment group, an auto-CPAP (REMstar Auto A-Flex 557 P, Philips Respironics, Pittsburgh, PA, US) was used for the titration of CPAP treatment and the titrated pressure was maintained throughout the study. In the sham CPAP group, a fixed pressure of 4 cm H2O was used throughout the study. All patients were followed-up at 1, 2 and 3 months of treatment for efficacy and compliance. Unless clinically indicated, patients were instructed not to start antihypertensive drug treatment if previously untreated, and not to change the use of antihypertensive medication if previously treated in the whole study period from baseline to the end of follow-up.
We excluded from the present analysis patients with secondary hypertension, severe hypertension (clinic systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥110 mmHg), previous or current use of CPAP or other treatments for OSAS, any contraindication to CPAP, insomnia, and other severe sleep disorders, being unable to give informed consent due to cognitive impairment, and having a plan of pregnancy or surgery.
CPAP treatment adherence
CPAP or sham CPAP treatment adherence was assessed using the internal clock of the device. Data on both the average CPAP frequency and time were collected at each of the follow-up visits. CPAP non-adherence was defined as a persistent use of CPAP for <5 days per week or <4 h per night according to the Centre for Medicare and Medicaid Services (CMS) [Citation16].
Blood pressure measurement
Clinic blood pressure was measured three times consecutively at baseline and at each of the monthly follow-up visits using a validated automated oscillometric blood pressure monitor (HEM-7011-C1, Omron Healthcare, Kyoto, Japan) with an appropriately sized cuff, after at least 5 min rest in the sitting position. These three blood pressure readings were averaged for analysis.
Ambulatory blood pressure monitoring was performed at baseline and after three months of treatment. We programmed validated oscillometric SpaceLabs 90217 monitors (SpaceLabs, Redmond, Washington, US) to obtain ambulatory blood pressure readings at 20-minute intervals from 06:00 to 22:00 and 30-minute intervals from 22:00 to 06:00. Patients were instructed to complete a diary card, and recorded the sleep and wake up time for the definition of daytime and night-time. Within individual subjects, we weighted the means of the ambulatory blood pressure by the time interval between successive readings. Ambulatory pulse rate was derived from the same ambulatory blood pressure recording.
Home blood pressure monitoring was performed at baseline and at each of the follow-up visits using the Omron HEM-7080-C1 monitor (Omron Healthcare, Kyoto, Japan). At the study entry, patients were instructed for the procedure of home blood pressure measurement. The patients were advised to measure their blood pressure at home three times consecutively every morning and evening, respectively, for seven consecutive days after the first run-in visit and before every clinic visit during follow-up, after at least 5 min rest in the sitting position. A pause of 30–60 s was allowed between two successive measurements. The patients were also instructed to place an appropriately sized cuff directly on their non-dominant arm and keep the position of the cuff at the level of the heart. Morning blood pressure was measured before breakfast and drug intake (if treated) and within one hour from waking up, and evening blood pressure at least two hours after dinner, after shower or bath and drug intake (if treated) and before going to bed. The blood pressure and pulse rate readings in the morning and evening sessions were averaged for analysis.
Statistical analysis
The SAS software (version 9.4, SAS Institute, Cary, NC, US) was used for database management and statistical analyses. Means and proportions were compared by the unpaired Student’s t-test and the χ2 test, respectively. The multivariable linear regression analysis via a stepwise selection was used to evaluate possible predictors of the CPAP frequency per week and time per night. The logistic stepwise regression analysis was used to compute adjusted odds ratios (OR) for non-adherence of CPAP therapy. An analysis of variance with the repeated measures method was used to compare adherence between groups. An analysis of covariance was performed to compare the changes in blood pressure and pulse rate from baseline, while controlling for covariables. Pearson correlation analysis was performed to investigate the associations of interest. The missing home blood pressure and pulse rate values at the end of follow-up was imputed by the last follow-up values (n = 4). All tests were two-sided and a p value <0.05 was considered to be statistically significant.
Results
CPAP treatment adherence
26 patients in the CPAP group and 21 in the sham CPAP group completed the study and were included in the present analysis. The CPAP frequency per week during the 3 months of follow-up (mean ± SD) was 5.5 ± 2.3 and 4.8 ± 2.5 days in the CPAP and sham CPAP groups, respectively, with a between-group difference of 0.7 days (95% CI −0.3 to 1.7 days, p = 0.17). The CPAP time per night during the 3 months of follow-up was 5.0 ± 1.9 and 4.1 ± 2.1 h in the CPAP and sham CPAP groups, respectively, with a significant between-group difference of 0.9 h (95% CI 0.1–1.7 h, p = 0.03, ). The prevalence of non-adherence during the 3 months of follow-up was 46.2% and 66.7% in the CPAP and sham CPAP groups, respectively (OR = 0.43, 95% CI 0.13–1.41, p = 0.16, ).
Figure 1. CPAP frequency and time during follow-up by randomisation group. Symbols represent individual values. Mean values (middle horizontal line) and standard deviations (upper and lower horizontal lines) at each of the follow-up visits are given for the two groups alongside the symbols. p values for the between-group comparison are given by the analysis of variance with the repeated measures method. The number of patients is given for the CPAP (dots) and sham CPAP groups (circles) separately.
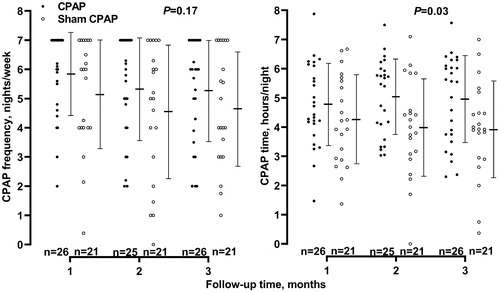
Table 1. Comparison between adherent and non-adherent patients in baseline characteristics by randomisation group.
In multiple stepwise regression with the randomisation group, sex, age and body mass index forced in the model, the CPAP frequency was significantly (p ≤ 0.007) associated with 24-h diastolic blood pressure (–0.4 days per 5 mmHg increment) and the use of antihypertensive medication (+1.6 days) at baseline, and the CPAP time per night was significantly (p ≤ 0.04) associated with the randomisation group (+1.0 h in the CPAP group) and 24-h pulse rate (–0.3 h per 5 beats/min increment, ) at baseline. In multiple stepwise logistic regression also with the randomisation group, sex, age and body mass index forced in the model, the OR for non-adherence was statistically significant (p ≤ 0.04) for body mass index (OR 1.23, per 1 kg/m2 increment) and 24-h diastolic blood pressure at baseline (OR 2.55, per 5 mmHg increment, Supplementary Table S1).
Table 2. Multivariable liner stepwise regression analyses on the predictors of CPAP adherence.
CPAP adherence as predictors of blood pressure and pulse rate changes
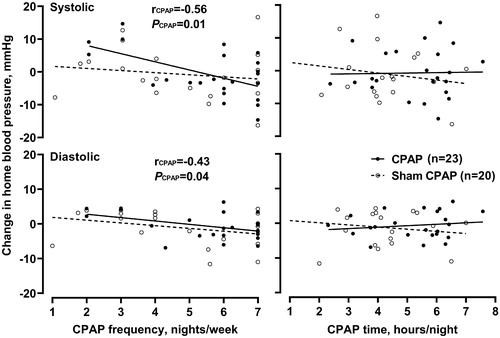
In further analyses, we investigated the association of CPAP adherence with the clinic, ambulatory and home blood pressure and pulse rate changes at 3 months of follow-up. In the CPAP group, both before () and after adjustment for sex, age, body mass index, use of antihypertensive medication and baseline blood pressure as appropriate, the CPAP frequency, but not the CPAP time (p ≥ 0.49), tended to be associated with the changes in some of the blood pressure measurements, such as home systolic/diastolic blood pressure with 3.2/1.3 mmHg greater reductions for 1 day per week increment in the CPAP frequency (p ≤ 0.01). In the sham CPAP group, none of the associations were statistically significant for blood pressure nor pulse rate changes (p ≥ 0.10).
Figure 2. Correlation between the CPAP frequency (left panel) and time (right panel) and the change from baseline in home systolic (top) and diastolic blood pressure (bottom). Regression lines are drawn for the CPAP (dots with solid line) and sham CPAP groups (circles with dashed line) separately. Pearson correlation coefficient (r) and the p value are given for the regression line.
In categorical analyses, adherent, but not non-adherent, patients had greater reductions from baseline to 3 months of follow-up in blood pressure or pulse rate regardless of the randomisation group (). In the two groups combined, both before () and after adjustment for the confounding factors as listed above (), adherent, compared with non-adherent, patients tended to have greater clinic, ambulatory and home blood pressure lowering and pulse rate slowing effects. Statistical significance was achieved for the adjusted between adherence and non-adherence differences (p ≤ 0.04) in home systolic (–5.0 mmHg, 95% CI −9.8 to −0.2 mmHg) and diastolic blood pressure (–3.8 mmHg, 95% CI −6.7 to −0.8 mmHg) and ambulatory 24-h (–6.2 beats/min, 95% CI −10.0 to −2.5 beats/min), daytime (–7.8 beats/min, 95% CI −12.6 to −3.0 beats/min) and night-time pulse rate (–4.4 beats/min, 95% CI −7.7 to −1.0 beats/min).
Figure 3. Comparison between adherent (dots) and non-adherent patients (circles) in home systolic (left panel) and diastolic (right panel) blood pressure changes from baseline. Symbols represent individual values. Mean values (middle horizontal line) and standard deviations (upper and lower horizontal lines) at each of the follow-up visits are given for the two groups alongside the symbols. Negative values indicate decrease from baseline. p values for the between-group comparison at 3 months of follow-up are given by the unpaired Student’s t-test. The number of patients is given for the adherent and non-adherent patients separately.
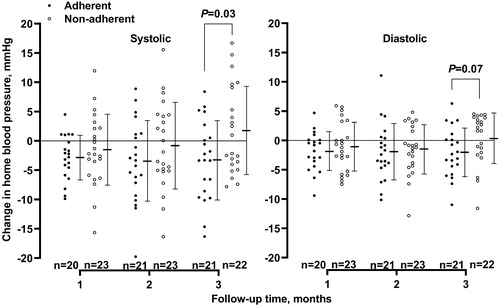
Table 3. Comparison between adherent and non-adherent patients in the least square mean changes from baseline in clinic, 24-h, daytime and night-time ambulatory and home blood pressure and pulse rate.
Discussion
Our study had two main findings. First, the CPAP treatment with an appropriately titrated pressure had a better adherence than the sham CPAP with a fixed low pressure. Second, CPAP adherence, especially the CPAP frequency per week, was associated with the blood pressure lowering and pulse rate slowing effects. An immediate implication is that future studies in this field may have to focus on improvement in CPAP adherence and its clinical relevance for cardiovascular protection and prevention.
Treatment adherence in the present study was low, as in most of previous studies. Indeed, in a recent systematic review of 82 randomised controlled trials on CPAP therapy, the mean CPAP time was 4.5 h per night, the CPAP frequency ranged from 4.2 to 6.3 days per week, and the prevalence of non-adherence ranged from 25% to 50% [Citation17]. It is therefore imperative to investigate in future studies how the treatment adherence to CPAP therapy can be improved.
The observation on a better adherence in the CPAP than sham CPAP group is in keeping with the results of several previous randomised controlled trials [Citation18,Citation19]. In a 6-month multicenter randomised controlled trial (n = 558), the CPAP time per night was significantly (p < 0.001) longer in the CPAP than sham CPAP group at both 2 (4.87 ± 2.04 h vs. 4.07 ± 2.14 h) and 6 months of follow-up (4.70 ± 2.08 h vs. 3.41 ± 2.19 h) [Citation19]. The better adherence in the CPAP group might be a positive response to the efficacy of the CPAP therapy in relieving symptoms of OSAS such as snoring, daytime sleepiness and cognitive dysfunction, and in improving blood pressure control and quality of life [Citation5].
Despite moderate sample size and short duration of follow-up, our study provided further evidence on the association between CPAP adherence and blood pressure lowering and pulse rate slowing effects of CPAP therapy. Several previous studies showed similar results [Citation13,Citation20]. In a multicenter, open-label, randomised trial in patients with resistant hypertension and moderate-to-severe OSAS (n = 194), only patients with a mean CPAP use of at least 4 h per night, but not those with a shorter use, showed a statistically significant (p ≤ 0.01) decrease in 24-hour systolic/diastolic blood pressure (–4.9/–4.1 mmHg) [Citation13]. Similar results were observed in several other studies with regard to the CPAP time per night [Citation21,Citation22]. 4 h per night seemed to be a nadir for the CPAP use to be sufficiently effective in lowering blood pressure and slowing heart rate in patients with OSAS [Citation23]. There is little evidence on the clinical relevance of the CPAP frequency for the CPAP therapy to be effective. The CPAP frequency might be an even better target of intervention for the improvement in the CPAP therapy and its efficacy.
Our study had several limitations. First, the sample size of our study was moderate. Chance findings are possible. In fact, sample size estimation was based on the assumptions of a between-group mean 24-h systolic blood pressure difference of 10 mmHg in favour of the CPAP treatment, an α level of 5%, and a power of 80%. The number of patients per group was estimated to be 28. Assuming a drop-out rate of 10%, approximately 30 patients per group would be required [Citation15]. Second, the follow-up time of our study was short, which might be a reason for the relatively small changes in blood pressure and pulse rate. Third, though patients were instructed not to change the use of antihypertensive treatment, and only three patients reported changes in the use of antihypertensive treatment (2 in the CPAP treatment group and 1 in the sham CPAP group), drug treatment could still be a confounding factor for the blood pressure lowering effects. Those adherent patients in the sham CPAP group had significant changes in blood pressure and pulse rate. These changes might be attributable not only to lifestyle modifications, but possibly also a better antihypertensive treatment adherence. Finally, the use of sham CPAP may also be a confounding factor. The Hawthorne effect is possible and induces positive changes in blood pressure and pulse rate. However, in the meantime, wearing a mask with suboptimal pressure potentially increases sympathetic activity and in turn induces negative changes.
In conclusion, CPAP adherence was low, and associated with the blood pressure lowering and pulse rate slowing effects, especially the CPAP frequency. Future studies may have to address whether and how the CPAP frequency can be improved and the improvement, if any, improves clinical outcomes in patients with OSAS and hypertension.
iblo_a_1922267_sm9312.docx
Download MS Word (15.5 KB)Acknowledgements
The authors gratefully acknowledge the voluntary participation of all study participants and the expert technical help of Yi-Ni Zhou (The Shanghai Institute of Hypertension, China).
Disclosure statement
Dr Wang reports having received lecture and consulting fees from Novartis, Omron, Servier and Takeda. The other authors declare no conflict of interest.
Additional information
Funding
References
- Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16:182–241.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115.
- Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8:010405.
- Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22:6.
- Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019;380:1442–1449.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931.
- Duran-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415–433.
- Hu X, Fan J, Chen S, et al. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens. 2015;17:215–222.
- Lei Q, Lv Y, Li K, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol. 2017;43:373–379.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens. 2014;32:2341–2350.
- Bakker JP, Weaver TE, Parthasarathy S, et al. Adherence to CPAP: What should we be aiming for, and how can we get there? Chest. 2019;155:1272–1287.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: The HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415.
- Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–726.
- Chen Q, Cheng YB, Shen M, et al. A randomized controlled trial on ambulatory blood pressure lowering effect of CPAP in patients with obstructive sleep apnea and nocturnal hypertension. Blood Press. 2020;29:21–30.
- Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400.
- Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43.
- May AM, Gharibeh T, Wang L, et al. CPAP adherence predictors in a randomized trial of moderate-to-severe OSA enriched with women and minorities. Chest. 2018;154:567–578.
- Budhiraja R, Kushida CA, Nichols DA, et al. Impact of randomization, clinic visits, and medical and psychiatric cormorbidities on continuous positive airway pressure adherence in obstructive sleep apnea. J Clin Sleep Med. 2016;12:333–341.
- Pamidi S, Chapotot F, Wroblewski K, et al. Optimal continuous positive airway pressure treatment of obstructive sleep apnea reduces daytime resting heart rate in prediabetes: a randomized controlled study. J Am Heart Assoc. 2020;9:e016871.
- Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280–2293.
- Wang X, Qiu J, Wang Y, et al. Beneficial response of blood pressure to short-term continuous positive airway pressure in Chinese patients with obstructive sleep apnea-hypopnea syndrome. Blood Press Monit. 2018;23:175–184.
- Bazzano LA, Khan Z, Reynolds K, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423.
