Abstract
Purposes
Central blood pressure is a stronger predictor of cardiovascular prognosis rather than brachial blood pressure. The reflection wave reaches the abdominal aorta sooner than ascending aorta. Thus, the contribution of central pulse pressure (cPP) to renal events may differ from that of cardiovascular events.
Methods
The subanalysis of the ABC-J II study was performed. Subjects were 3434 treated hypertensive patients with a mean follow-up of 4.7 years. Left ventricular hypertrophy, an index of cardiovascular risk, correlated with cPP better than central systolic blood pressure in this cohort. The contribution of brachial pulse pressure (bPP) and cPP to cardiovascular and renal events was analysed.
Results
Cox proportional-hazard analysis revealed that sex (p < 0.001), height (p < 0.05), history of cardiovascular diseases (p < 0.001), number of antihypertensive drugs (p < 0.05), and cPP (p < 0.05) contributed to cardiovascular events. However, Cox proportional-hazard analysis disclosed that baseline serum creatinine (p < 0.001) and bPP (p < 0.05) predicted renal events. After adjusting for the history of cardiovascular diseases, Cox regression demonstrated only sex as a significant predictor of cardiovascular events. After adjusting for baseline serum creatinine, no parameters were shown to predict renal events.
Conclusions
The present findings support our previous data that the absence of cardiovascular or renal diseases is an important determinant for event-free survival, and suggest that cPP and bPP contribute to cardiovascular and renal events in treated hypertensive patients.
Introduction
Central systolic blood pressure (SBP) is a direct pressure load on the heart and a stronger predictor of cardiovascular events than brachial systolic blood pressure [Citation1,Citation2]. Williams et al. examined the impact of two different blood pressure-lowering (amlodipine-based vs. atenolol-based) regimens on central pressure. They reported that despite similar brachial blood pressure between treatments, there were substantial reductions of central pressure in the amlodipine-based group and that central pulse pressure (cPP) was associated with composite outcome of cardiovascular events [Citation3]. Roman et al. examined the relationship of central blood pressure to incident cardiovascular events and concluded that cPP is more strongly related to cardiovascular events than brachial pressure [Citation4]. However, findings from the Framingham Heart Study suggested that central blood pressure was not predictive of cardiovascular disease events [Citation5].
The coronary and cerebral arteries originate from the thoracic aorta, while the renal arteries derive from the abdominal aorta. Furthermore, the arterial stiffness gradient and pulse amplification are inverted during the progression of chronic kidney disease (CKD) [Citation6,Citation7]. Because the reflection wave reaches the abdominal aorta sooner than the ascending aorta [Citation8], it is conceivable that the impact of cPP is greater for cardiovascular (cardiac and brain) events than for renal events. To examine this possibility, a sub-analysis of the Antihypertensives and Blood Pressure of Central Artery in Japan (ABC-J) II study was performed.
Methods
The ABC-J II study is an expanded version of the original ABC-J study [Citation9]. As detailed previously [Citation1], the ABC-J II study is an observational prospective study designed to evaluate the predictive value of central blood pressure on cardiovascular outcomes in hypertensive Japanese patients. Hypertension was defined as office blood pressure of >140/90 mmHg. The study was approved by each participating institutional ethical board and was officially registered (UMIN000002966). From January 2007 to March 2011, the central blood pressure data were recorded from 4310 subjects enrolled throughout Japan. The chart review was performed until May 2013, and the event information was collected from each institution. The main results have been published previously [Citation1].
The inclusion criteria for the study were as follows [Citation1]: 1) the subjects had been taking a stable dose of antihypertensive medication for at least 3 months, 2) radial tonometry data including radial argumentation index (rAI) and central blood pressure were available. All clinical data were obtained from medical records. The exclusion criteria were as follows: 1) subjects with extremely abnormal blood pressure (SBP by 40 mmHg or diastolic blood pressure [DBP] by 20 mmHg higher or lower than usual office blood pressure or home blood pressure, to ascertain the accurate measurement of blood pressure at the time of tonometry measurement, 2) arrhythmia or heart failure history, to ensure accurate tonometry measurement; 3) age <35 y/o—as some younger subjects may show exaggerated pulse amplification [Citation10]; 4) no baseline serum creatinine data or estimated glomerular filtration rate <15 mL/min/1.73 m2; 5) no follow-up data or follow-up period less than 100 days; and 6) rAI ≤50%.
Diabetes mellitus was defined as follows: antidiabetic medication use, fasting plasma glucose ≥7 mmol/L, or haemoglobin A1c ≥6.5%. Dyslipidaemia was defined as total cholesterol ≥6.2 mmol/L, triglyceride ≥1.7 mmol/L, high-density lipoprotein cholesterol ≤1.0 mmol/L, or lipid-lowering drug treatment. Heart failure was diagnosed using the Framingham criteria [Citation1].
Brachial blood pressure was measured using an automated device for blood pressure and tonometry measurements (HEM-9000AI, Omron Healthcare) after ≥5 min of rest in the sitting position just before the radial artery waveform measurement. The arm circumference was measured, and an appropriate cuff size was selected. This device calculates rAI as (P2-DBP)/(P1-DBP), by taking P1 and P2 as the first and second inflection points on the radial pulse waveform [Citation11], and the brachial blood pressure was measured simultaneously. The central SBP was estimated from the late SBP in the radial artery waveform and was calibrated using brachial SBP and DBP. The mean blood pressure (MBP) was calculated using the radial pulse waveform. The central SBP estimated with HEM-9000AI was identical to that estimated by the SphygmoCor system [Citation12]. However, as stated earlier, we carefully excluded subjects with ≤ rAI 50% [Citation13], as the HEM-9000AI-based method, may underestimate the central SBP in patients with lower augmentation, especially in Murgo type C [Citation14].
All cardiovascular and serious adverse events were reported by the participating investigators and adjudicated by the Endpoint Committee, which was blinded to any knowledge of individual participants (Appendix). Cardiovascular events were a composite of the occurrence of any of the following, whatever comes first: myocardial infarction (MI), stroke, sudden death, or acute aortic dissection. Renal events were a composite of starting renal replacement therapy and doubling serum creatinine levels, which were listed as serious adverse events. All the events that were to be analysed had been determined before the patients’ participation in the study [Citation1].
We performed sub-analyses of data from 3434 treated hypertensive patients who met the above criteria, and the mean follow-up period was 4.7 years. The contribution of the cPP and the brachial pulse pressure (bPP) in cardiovascular (myocardial infarction [MI], stroke, sudden death, or acute aortic dissection) and renal events (renal replacement therapy, doubling serum creatinine) were assessed using the Cox proportional hazards analysis. The factors associated with central SBP were used as independent variables for Cox proportional hazards analysis, similar to the original study [Citation1]. These factors were sex, age, height, weight, diabetes, dyslipidaemia, history of MI and/or stroke at the time of study entry, serum creatinine level, pulse rate, and the number of antihypertensive medications taken. Since left ventricular hypertrophy, an index of cardiovascular risk, correlated with cPP better than central SBP in this cohort, both cPP and bPP were used as independent variables in the sub-analysis [Citation7,Citation15]. The Kaplan–Meier method was applied to examine the contribution of a single factor to the events. The median cPP or bPP value was used to divide the patients into two groups. All statistical analyses were performed using the IBM SPSS software (version 25). Additional multivariate Cox regressions were performed by restricting independent variables according to the number of events [Citation16]. The probability values (P) <0.05 were considered statistically significant.
Results
Patient backgrounds are detailed in . During the observation period, 63 cardiovascular events occurred: 11 MIs (7 with higher cPP), 45 strokes (28 with higher cPP), 4 sudden deaths (all with higher cPP), and 3 acute aortic dissections (2 with higher cPP). In addition, a total of 34 renal events occurred: 29 cases of initiation of renal replacement therapy (13 with higher bPP) and 5 patients with double serum creatinine levels (all with higher bPP).
Table 1. Patient backgrounds.
Univariate Cox regression analysis revealed that the sex, age, history of MI and/or stroke, cPP, bPP, and the number of antihypertensive drugs taken predicted cardiovascular disease events. Multivariate Cox proportional hazards analysis disclosed that the sex, height, history of MI and/or stroke, cPP, and the number of antihypertensive drugs prescribed for each patient substantially predicted the cardiovascular events (). The results remained unchanged after adjusting for MBP. In addition, the Kaplan–Meier method revealed that the patients with higher cPP displayed more cardiovascular events according to the log-rank test (χ2 = 7.9, p < 0.005) (). On the other hand, the univariate Cox regression showed that age, diabetes, baseline serum creatinine level, bPP and the number of prescribed antihypertensive drugs predicted renal events. Moreover, multivariate Cox proportional hazards analysis detected baseline serum creatinine level and bPP as significant predictors of renal events (). Adjustment to MBP did not alter the results. Furthermore, our data using the Kaplan–Meier method with a log-rank test demonstrated that the patients with higher bPP showed an increase in the instances of doubling serum creatinine levels (χ2 = 5.8, p < 0.05). Notably, the variance inflation factor between cPP and bPP was 5.02.
Figure 1. Kaplan–Meier analysis for cardiovascular events above and below the median of central pulse pressure (cPP, left panel) and brachial pulse pressure (bPP, right panel). Log-rank test demonstrated significant differences between the two groups.
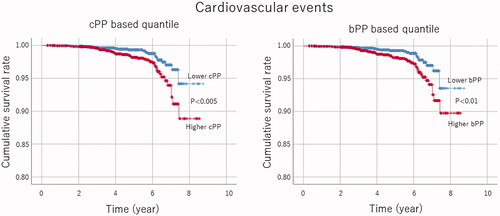
Table 2. Summary of Cox regression for cardiovascular events.
Table 3. Summary of Cox regression for renal events.
The Kaplan–Meier method also suggested that bPP showed a weak tendency to predict cardiovascular events (, χ2 = 6.8, p < 0.01) and that cPP exhibited a delicate tendency to anticipate the incidence of doubling serum creatinine level (χ2 = 5.1, p < 0.05). In addition, the Kaplan–Meier method with log-rank test demonstrated that neither bPP (χ2 = 0.1, p = 0.9) nor cPP (χ2 = 0.8, p = 0.3) significantly predicted renal events. Similarly, the Kaplan–Meier method using the log-rank test displayed that neither bPP (χ2 = 1.1, p = 0.3) nor cPP (χ2 = 3.8, p = 0.1) significantly predicted the incidence of renal replacement therapy.
Multivariate proportional hazards Cox regression analysis showed that the sex, height, and history of MI and/or stroke were related to the cardiovascular events, after adjustment for bPP (Supplemental Table 1). Multivariate Cox regression demonstrated that only sex was associated with cardiovascular events, after adjustment for the history of MI or stroke. However, multivariate Cox regression for the subjects without a history of MI or stroke (n = 3049) revealed that sex, height, and cPP anticipated the cardiovascular events. (Supplemental Table 2). Additional Cox regression using 6 independent variables (sex, age, history of MI or stroke, cPP, bPP, and the number of antihypertensive drugs) for cardiovascular events detected with sufficient statistical power that sex, the history of MI or stroke, cPP and number of antihypertensive drugs predicted cardiovascular events (Supplemental Table 3).
In addition, multivariate proportional hazards Cox regression indicated that baseline serum creatinine was related to renal events, after adjustment for cPP (Supplemental Table 4). Multivariate Cox regression found that any parameters were not associated with renal events, after adjustment for baseline serum creatinine. Of note, multivariate Cox regression for renal events in the subjects without high serum creatinine (more than 177 umol/L) was hindered (13 renal events in 3389 patients), due to insufficient statistical power [Citation16]. However, additional Cox regression enrolling 3 independent variables (baseline serum creatinine, cPP, and bPP) for renal events demonstrated with adequate statistical power that baseline serum creatinine and bPP predicted renal events (Supplemental Table 3).
Discussion
The present study demonstrated that sex, height, history of cardiovascular disease, number of antihypertensive drugs and cPP contributed to cardiovascular events in treated hypertensive patients, supporting the importance of central blood pressure in cardiovascular health. Our results for the first time revealed that baseline serum creatinine and bPP predicted renal events. However, with adjustment for history of cardiovascular diseases, Cox regression showed only sex as a predictor of cardiovascular events. Similarly, with adjustment for baseline serum creatinine, Cox regression did not show any parameters significantly contributed to renal events. These data support our previous findings indicating that the absence of cardiovascular or renal diseases is important determinant for event-free survival [Citation17], and further suggest that cPP and bPP are associated with cardiovascular and renal events in treated hypertensive patients.
Our results indicate that male sex and a history of cardiovascular disease are strong predictors of cardiovascular events, which is consistent with previous reports, indicating that both are risk factors [Citation18]. The present findings are comparable to those of Lee et al., who showed that short height was a significant cardiovascular risk factor in the Asia-Pacific region [Citation19]. We observed that patients treated with a greater number of antihypertensive drugs suffer more cardiovascular events, being compatible with the poor prognosis of patients with resistant hypertension [Citation20]. The Cox proportional hazards analysis showed that cPP was a significant contributor to cardiovascular events. These results were validated by the Kaplan–Meier method. Moreover, multivariate Cox regression for the subjects without a history of MI or stroke indicated that cPP predicted cardiovascular events, providing further support for designing and conducting a prospective and randomised clinical trial to investigate whether treatment can be based on cPP.
Our findings constitute novel demonstrations that a higher bPP has a small but significant contribution to renal events. The Kaplan–Meier method demonstrated that the patients with higher bPP showed more doubling serum creatinine levels, suggesting the importance of blood pressure control in the management of renal dysfunction. The renal artery originating from the abdominal, and not the thoracic aorta, may account for the different contributions of cPP and bPP to cardiovascular and renal events, respectively. A recent study showed that pulse amplification between the ascending aorta and the abdominal aorta at the renal artery level ranged between 8 and 9 mmHg, which was similar to that from the ascending aorta to the brachial artery [Citation21]. Our study also demonstrated that the difference between cPP and bPP was 10 mmHg. Although it is difficult to estimate abdominal aortic blood pressure non-invasively, the values appear to be closer to brachial blood pressure rather than central blood pressure.
The presence of CKD considerably worsens cardiovascular prognosis [Citation22], with nephrosclerosis being an important complication of hypertension. In univariate Cox regression, diabetes predicted renal events, supporting that diabetic nephropathy is the leading cause of initiating renal replacement therapy among various CKDs [Citation23]. The arterial stiffness gradient is inverted in CKD progression [Citation6,Citation24], and the pulse amplification is elevated in advanced kidney disease [Citation7], suggesting a nonlinear relationship between central haemodynamics and CKD progression [Citation1]. The Framingham Heart Study did not account for the kidneys, which may be the reason for their inability to predict cardiovascular events by central blood pressure [Citation5]. The diverse results regarding the predictive role of cPP and bPP in cardiovascular and renal events may be attributable to the anatomy that the abdominal aorta feeds renal arteries, whereas the thoracic aorta feeds cerebral and coronary arteries.
This study has several limitations. First, there was a significant correlation between bPP and cPP, presumably accounting for the significant contributions of cPP to cardiovascular events and of bPP to renal events were missing in Cox regression after adjustment for either bPP or cPP. Hence there might be considerable multicollinearity between the two variables. However, the variance inflation factor between cPP and bPP was less than 10. Notably, multivariate Cox regression with good statistical power also detected that cPP and bPP anticipated cardiovascular and renal events. Furthermore, Kaplan–Meier tests showed similar trends to Cox proportional hazards analyses in that cPP and bPP preferentially predicted cardiovascular and renal events, respectively. Second, it was surprising that age was not a risk factor for either cardiovascular or renal events in multivariate Cox regressions. Although the specific reasons are not readily apparent, they might involve that age considerably related to bPP and cPP in this cohort [Citation7]. Finally, more than half of the patients who required renal replacement therapy belonged to the lower bPP group. The baseline serum creatinine levels were substantially related to the number of antihypertensive drugs taken, indicating that the blood pressure was strictly controlled in patients with CKD.
In summary, for cardiovascular risk assessment, the central blood pressure measurement appears to be an essential and useful index for hypertensive patients without CKD. In addition, the present study indicates that underlying kidney dysfunction strongly predicts renal events, suggesting that early detection and intervention for CKD are important for the prevention of renal replacement therapy. Finally, our data also suggest that brachial blood pressure measurement is a necessary and sufficient diagnostic marker in hypertensive patients with CKD for both cardiovascular and renal risk management.
Supplemental Material
Download MS Word (22.4 KB)Acknowledgements
The part of data in this manuscript was presented at 9th Clinical Hypertension Forum in Osaka Japan, May 2021, and published as an abstract. We thank Editage (www.editage.com) for English language editing. TT: acquired and interpreted the data, drafted the manuscript, YO: analysed the data, drafted the manuscript, KE: acquired the data, revised the manuscript, HM: acquired the data, revised the manuscript, HS: designed the study and interpreted the data, drafted the manuscript, KS: designed the study, revised the manuscript. All the authors approved the submission. The data are available within the article, supplemental material, and the following website (https://doi.org/10.1038/s41440-018-0075-8).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Eguchi K, Miyashita H, Takenaka T, et al. High central blood pressure is associated with incident cardiovascular events in treated hypertensives: the ABC-J II study. Hypertens Res. 2018;41(11):947–956.
- Terentes-Printzios D, Gardikioti V, Vlachopoulos C. Central over peripheral blood pressure: an emerging issue in hypertension research. Heart Lung Circ. 2021;30(11):1667–1674.
- Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the conduit artery function evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension. 2007;50(1):197–203.
- Mitchell GF, Hwang SJ, Larson MG, et al. Transfer function-derived central pressure and cardiovascular disease events: the Framingham Heart Study. J Hypertens. 2016;34(8):1528–1534.
- London GM, Safar ME, Pannier B. Aortic aging in ESRD: structural, hemodynamic, and mortality implications. JASN. 2016;27(6):1837–1846.
- Takenaka T, Suzuki H, Eguchi K, et al. Elevated pulse amplification in hypertensive patients with advanced kidney disease. Hypertens Res. 2018;41(4):299–307.
- Yamashita S, Dohi Y, Takase H, et al. Central blood pressure reflects left ventricular load, while brachial blood pressure reflects arterial damage. Blood Press. 2014;23(6):356–362.
- Miyashita H, Aizawa A, Hashimoto J, et al. Cross-sectional characterization of all classes of antihypertensives in terms of central blood pressure in Japanese Hypertensive Patients. Am J Hypertens. 2010;23(3):260–268.
- O'Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth. Vasc Med. 2000;5(3):141–145.
- Takazawa K, Kobayashi H, Shindo N, et al. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens Res. 2007;30(3):219–228.
- Herbert A, Cruickshank JK, Laurent S, et al. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35(44):3122–3133.
- Millasseau SC, Patel SJ, Redwood SR, et al. Pressure wave reflection assessed from the peripheral pulse: is a transfer function necessary?. Hypertension. 2003;41(5):1016–1020.
- Lin MM, Cheng HM, Sung SH, et al. Estimation of central aortic systolic pressure from the second systolic peak of the peripheral upper limb pulse depends on central aortic pressure waveform morphology. J Hypertens. 2012;30(3):581–586.
- Kannel WB, Cobb J. Left ventricular hypertrophy and mortality-results from the Framingham Study. Cardiology. 1992;81(4–5):291–298.
- Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503.
- Suzuki H, Kanno Y. Efficacy of candesartan on outcome in Saitama trial (E-COST) group. Effects of candesartan on cardiovascular outcomes in Japanese Hypertensive Patients. Hypertens Res. 2005;28(4):307–314.
- Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the management of Hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–1481.
- Lee CM, Barzi F, Woodward M, et al. Adult height and the risks of cardiovascular disease and major causes of death in the Asia-Pacific region: 21,000 deaths in 510,000 men and women. Int J Epidemiol. 2009;38(4):1060–1071.
- Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens. 2014;28(8):463–468.
- Temmar M, Jankowski P, Peltier M, et al. Intraaortic pulse pressure amplification in subjects at high coronary risk. Hypertension. 2010;55(2):327–332.
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108(17):2154–2169.
- Nakai S, Hanafusa N, Masakane I, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2012). Ther Apher Dial. 2014;18(6):535–602.
- Briet M, Boutouyrie P, Laurent S, et al. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82(4):388–400.
Appendix. Members of ABC-J II study group
Collaboration and related facilities
Kazuyuki Shimada, Sadayoshi Ito, Yutaka Imai, Kazuo Eguchi, Yuko Ota, Mari Odaira, Kazuomi Kario, Yuhei Kawano, Mineko Kino, Katsuhiko Kohara, Hiromichi Suzuki, Kenji Sunagawa, Kenji Takazawa, Tsuneo Takenaka, Yasuharu Tabara, Yasuaki Dohi, Hirofumi Tomiyama, Junichiro Hashimoto, Yoshitaka Hirooka, Hiroshi Miyashita, Akira Yamashina, Joji Ishikawa, Hideaki Takata, Motoki Fukutomi, Yoshio Matsui, Nobuyuki Shiba, Takahiro Komori
Cooperation facilities
Toshiro Iketani, Mitsutoshi Kato, Toru Awaya, Yoshikazu Aoka, Tsuguhisa Hatano, Naoto Yagi, Ken Oyama, Masaaki Miyakawa, Hiro Yamakawa, Hareaki Yamamoto, Hisao Mori, Kiyoshi Uchiba, Takeshi Takami
Independent members for event evaluation and data analysis
Yasuhisa Kitagawa, Junichi Yamazaki, Koichi Hayashi, Uichi Ikeda, Hirohisa Okuma, Kentaro Tokuoka, Shinji Hisatake, Shu Wakino, Hirofumi Tokuyama, and Takayoshi Ohkubo
