Abstract
Purpose
While poor drug adherence is frequent in patients with resistant hypertension, detailed analyses of the impact of drug adherence on the success of renal denervation are scarce. We report drug adherence at baseline, changes in drug adherence, and the influence of these parameters on blood pressure changes at 6 and 12 months in patients treated with alcohol-mediated renal denervation as part of the Peregrine study.
Materials and methods
Urinary detection of antihypertensive drugs was performed using high-performance liquid chromatography-tandem mass spectrometry. Full adherence, partial adherence, and complete non-adherence were defined as 0, 1, or ≥2 drugs not detected, respectively.
Results
Renal denervation was performed in 45 patients with uncontrolled hypertension on ≥3 antihypertensive medications (62% men, age 55 ± 10 years). At baseline, the proportion of fully, partially, and non-adherent patients was 62% (n = 28), 16% (n = 7), and 22% (n = 10), respectively. At 6 months, adherence improved by 21% (n = 9), remained unchanged at 49% (n = 21), and worsened by 30% (n = 13). Mean 24-h systolic blood pressure decreased by 10 ± 13, 10 ± 4, and 14 ± 19 mmHg in fully, partially, and non-adherent patients (p = 0.77), and by 14 ± 14, 8 ± 11, and 14 ± 18 mmHg in patients who improved, maintained, or decreased adherence, respectively (p = 0.35). The results at 12 months were similar.
Conclusion
About 40% of patients with apparently treatment-resistant hypertension were not fully adherent at baseline, and adherence decreased further in 30%. Nevertheless, mean blood pressure changes after renal denervation were similar irrespective of drug adherence. Our results suggest that such patients may benefit from alcohol-mediated renal denervation, irrespective of drug adherence. These findings are hypothesis-generating and need to be confirmed in ongoing sham-controlled trials.
Introduction
Hypertension is the most common preventable risk factor for premature death and disability worldwide [Citation1,Citation2], and lowering systolic and diastolic blood pressure (BP) to recommended limits is associated with a substantial reduction in cardiovascular complications [Citation3,Citation4]. The renal sympathetic nervous system contributes to the pathophysiology of hypertension [Citation5–8]. Catheter-based renal denervation (RDN) has been investigated as a potential treatment for hypertension using radiofrequency ablation [Citation9], ultrasound ablation [Citation10], and perivascular alcohol injection (chemical denervation) [Citation11]. Long-term safety data from RDN studies do not indicate the existence of long-term safety signals [Citation12].
The Peregrine Catheter (Ablative Solutions, Inc., San Jose, CA) delivers micro doses of dehydrated alcohol, as a neurolytic agent, locally into the peri-adventitial space [Citation13] of the renal artery to perform perivascular ablation of sympathetic nerve bundles [Citation14,Citation15]. Recently published data from this current study, using the Peregrine Catheter to perform bilateral alcohol-mediated RDN in hypertensive patients with a single dose of 0.6 mL of alcohol/artery, significantly lowered ambulatory and office BP in patients with severe uncontrolled hypertension, with an acceptable safety profile [Citation11].
Non-adherence to prescribed antihypertensive medication is a major challenge in the treatment of hypertension [Citation16], particularly in patients with apparently treatment-resistant hypertension (ATRH) [Citation17,Citation18]. Hypertensive patients with high drug adherence have been shown to be 45% more likely to achieve BP control than those with medium or low adherence after controlling for age, gender, and comorbidities [Citation19]. However, improving adherence is complex and time-consuming [Citation20]. An analysis of data from nine studies including a total of 747 patients with ATRH revealed rates of poor adherence and full non-adherence to drug regimens ranging from 13 to 46% and 2 to 35%, respectively [Citation21]. Furthermore, a substantial proportion of patients with ATRH are thought to display intentional non-adherence [Citation22] and may have a particular psychological profile or a history of traumatic life experience [Citation22]. This makes improvement of drug adherence even more difficult in these patients, leaving the door open to alternative treatment strategies, such as RDN. However, the BP-lowering efficacy of RDN in poorly adherent patients and, more generally, the impact of adherence level on BP changes after RDN needs to be further studied.
The current sub-analysis of drug adherence derives from the Peregrine Study (ClinicalTrials.gov number NCT02570113) conducted in patients with uncontrolled hypertension on ≥3 antihypertensive medications who underwent bilateral alcohol-mediated RDN using the Peregrine Catheter. The primary aim of the present study was to ascertain whether adherence to antihypertensive medications has an impact on BP response to renal nerve ablation [Citation11].
Methods
Participants and protocol
This was a prospective, single-arm, open-label, multicentre trial intended to collect safety and efficacy data of the three-needle-based delivery device, the Peregrine Catheter, to perform bilateral alcohol-mediated RDN in hypertensive patients with a single dose of 0.6 mL of dehydrated alcohol per artery as the neurolytic agent. All patients had uncontrolled hypertension, defined as a mean office BP of ≥150/≥85 mmHg, with a 24-h mean ambulatory systolic BP of ≥135 mmHg while receiving a stable medication regimen of ≥3 antihypertensive medications of different classes (including a diuretic) for at least 4 consecutive weeks. As in most third-generation RDN trials, non- or poor drug adherence was not an exclusion criterion, but drug adherence was assessed at repeated time points by LC-MS/MS (see below). Patients were included if the renal artery diameter was ≥4 and ≤7 mm, with a renal artery length of ≥5 mm. Accessory renal arteries with similar anatomy were also eligible. The primary efficacy endpoint was a reduction of 24-h mean ambulatory systolic BP at 6 months vs. baseline. The trial was reviewed by the Ethics Committees of the participating research centres, and if applicable, by the relevant Competent Authorities. This trial was conducted in conformity with the ethical principles stated in the Declaration of Helsinki, and all patients provided written informed consent. The safety was monitored by a Data Safety Monitoring Board [Citation11].
Baseline assessments included a collection of medical history, antihypertensive medication use, physical examination, electrocardiogram results, blood and urine tests, office and 24-h ambulatory BP measurements. Clinical and laboratory follow-up data were obtained from patients in hospital, 7 days, and at 1, 3, 6, and 12 months after the RDN procedure. The office systolic and diastolic BP at each visit were reported as the mean of three consecutive BP readings, taken seated and 1–3 min apart, and performed by a registered nurse or physician with an OMRON BP monitor (OMRON 705IT with printer). The 24-h ambulatory BP measurement devices (Spacelabs Healthcare Monitor) recorded BP every 30 min for 24 h, and a core laboratory (ERT, St. Louis, MO, USA) collected, validated, and calculated the 24-h mean ambulatory systolic and diastolic BP. Based on the patients’ recorded sleep and waking times, the mean daytime and night-time ambulatory systolic and diastolic BP were calculated by the core laboratory.
Measurement of adherence
Details regarding antihypertensive medication use and dose were recorded at baseline, and at 1, 3, 6, and 12 months post-procedure. Urine samples from baseline (pre-procedure) and follow-up were tested in a specialised central core laboratory (SYNLAB, Switzerland) to monitor adherence to the antihypertensive medication regimen by assessment of antihypertensive drugs or their metabolites. Samples were assessed using high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS). Non-adherence to antihypertensive medication was not an exclusion criterion, and investigators were blinded to the results of LC-MS/MS to prevent bias [Citation17,Citation18].
Overall adherence was calculated as the number of detected medications divided by the number of analysable medications according to the core laboratory. A patient was classified as fully adherent if all drugs that were potentially analysable were detected, partially adherent if 1 potentially analysable drug was not detected, and non-adherent if ≥2 potentially analysable drugs were not detected. Additionally, change in adherence was classified as improved from baseline if the percent adherence was greater at follow-up than at baseline, unchanged if the percent adherence was unchanged and worse if the percent adherence was lower at follow-up than at baseline.
Statistical analysis
Continuous variables (including BP) are presented as mean and standard deviation (SD) and binary variables are reported as frequencies and percentages. A paired t-test was used to compare BP changes between baseline and follow-up. Between-group changes in BP between non-adherent, partially adherent, and fully adherent patients were tested using analysis of variance (ANOVA). Percent adherence over time was tested using Friedman’s non-parametric repeated measures test and responder analyses were conducted using the chi-square test. A p-value <0.05 was considered statistically significant. All analyses were conducted using SAS Version 9.4.
Results
Patient and procedural characteristics
Data pertaining to patient and procedural characteristics have been published elsewhere [Citation11]. A summary of baseline characteristics is presented in . Treated patients (n = 45) included 62% men and had a mean age of 55 ± 10 years, a mean body mass index of 30.7 ± 5.8 kg/m2, and a mean estimated Glomerular Filtration Rate of 85 ± 16 mL/min/1.73m2. Baseline office BP was 169/99 ± 15/13 mmHg with a 24-h mean ambulatory BP of 151/89 ± 14/12 mmHg. Patients were prescribed an average of 5.1 ± 1.5 antihypertensive drugs at baseline, with the majority of patients taking diuretics (44/45, 98%), calcium channel blockers (38/45, 84%), beta-blockers (34/45, 76%), and angiotensin receptor blockers (33/45, 73%).
Table 1. Baseline characteristics.
Efficacy
Changes in 24 h-ambulatory and office BP over time are presented in . The mean 24-h ambulatory systolic/diastolic BP was significantly reduced by −11/−7 mmHg at 6 months as compared to baseline (p < 0.001). Mean systolic and diastolic office BP was reduced by −18/−10 mmHg at 6 months as compared to baseline (p < 0.001).
Figure 1. Systolic ambulatory (a) and office (b) blood pressure 1, 3, 6, and 12 months after renal denervation. Mean reductions in 24-h systolic ambulatory (ASBP) (a) and office (OSBP) (b) blood pressure over time.
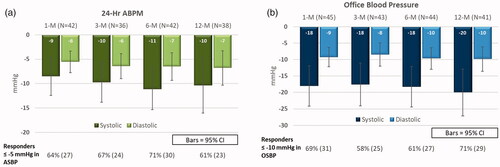
The clinically significant reductions in 24-h ambulatory and office systolic/diastolic BP persisted at 12 months. The changes from baseline were −10 ± 17/−7 ± 11 mmHg for 24-h ambulatory systolic/diastolic BP (p = 0.001) and −20 ± 23/−10 ± 12 mmHg for office systolic/diastolic BP (p < 0.001).
Adherence
At baseline, the proportion of fully adherent, partially adherent and non-adherent patients was 62% (n = 28), 16% (n = 7), and 22% (n = 10), respectively (). At 6 and 12 months post-procedure, respectively, 21% (n = 9) and 22% (n = 9) of patients had improved adherence, 49% (n = 21) and 49% (n = 20) had no change in drug adherence and 30% (n = 13) and 29% (n = 12) had worse adherence compared with baseline ().
Table 2. Adherence to antihypertensive medication during the study.
Table 3. Change in antihypertensive medication adherence during the study.
Efficacy and relationship to adherence
At 6 months post-procedure, the mean decrease in 24-h systolic ambulatory BP was similar in patients classified as fully adherent, partially adherent, and non-adherent (10 ± 13, 10 ± 4, and 14 ± 19 mmHg; p = 0.77). Along the same lines, no significant difference in mean 24-h systolic ambulatory BP decrease was found in patients who improved, maintained, or decreased adherence (14 ± 14, 8 ± 11, and 14 ± 18 mmHg, respectively; p = 0.35), or in patients who remained adherent through 6 months vs. patients whose adherence was <80% on at least one follow-up visit (10 ± 11 vs. 12 ± 16 mmHg, respectively, p = 0.68) ().
Figure 2. Change in 24-h systolic ambulatory blood pressure at 6 and 12 months vs. adherence. (a) Change in 24-h systolic ambulatory blood pressure in fully, partially, and non-adherent subjects. (b) Change in 24-h systolic ambulatory blood pressure presented by subjects who were ≥80 and <80% adherent to antihypertensive treatment and by worse, no change, and improved adherence.
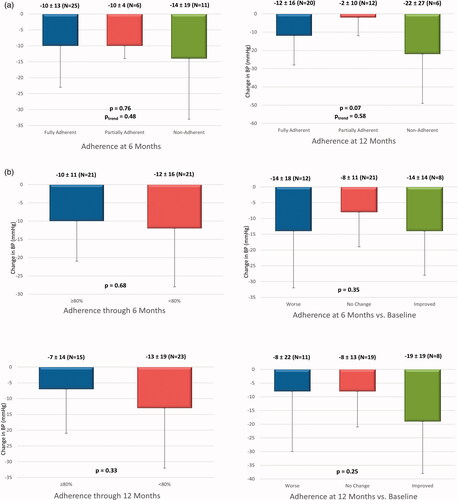
Similarly, the mean decrease in 24-h systolic ambulatory BP from baseline to 12 months post-procedure did not differ significantly between patients classified as fully adherent, partially adherent, and non-adherent (12 ± 16, 2 ± 10, and 22 ± 27 mmHg, respectively; p = 0.07). Furthermore, no significant difference in mean 24-h systolic ambulatory BP decrease was found in patients who improved, maintained, or decreased adherence (19 ± 19, 8 ± 13, and 8 ± 22 mmHg, respectively; p = 0.25), or in patients who remained adherent up to 6 months post-procedure vs. patients whose adherence was <80% on at least one follow-up visit (7 ± 14 vs. 13 ± 19 mmHg; p = 0.33) ().
Finally, the proportion of responders (patients with a reduction in systolic 24-h ambulatory BP of ≥5 mmHg) was not significantly different in patients who had improved adherence at 6 and 12 months post-procedure (24 and 26% of patients) compared with patients in whom adherence was maintained (45 and 52% of patients) or worsened (31 and 22% of patients) ().
Table 4. Responder analysis of change in antihypertensive medication adherence during the study.
These results are consistent with an examination of individual patient data. At 6 and 12 months post-procedure, 12 (30%) and 9 (23%) patients who were non- and partially adherent, respectively, had ≥5 mmHg decreases in mean 24-h systolic ambulatory BP, suggesting that the RDN procedure has a blood-pressure-lowering effect in poorly/non-adherent patients (Supplementary Figure S1). In addition, at 6 months post-procedure, most patients (9/12 [75%]) with decreased drug adherence had ≥5 mmHg decreases in mean 24-h systolic ambulatory BP (Supplementary Figure S2), which further suggests that RDN can lower BP despite decreasing antihypertensive medication use.
At 12 months post-procedure, there was no statistically significant relationship between weight change and drug adherence, change in drug adherence, and magnitude of change in blood pressure (see Supplementary Figures S3–S5).
Discussion
In this cohort of 45 patients with uncontrolled hypertension on ≥3 antihypertensive medications treated with alcohol-mediated RDN, evaluation of baseline drug adherence by LC-MS/MS disclosed poor adherence in 38%. Furthermore, during follow-up, adherence changes, with either improvement or worsening, were observed in ∼50% of patients. Such a high proportion of poor drug adherence [Citation21] and erratic changes during follow-up [Citation23] are consistent with existing literature and characteristics of ATRH.
Differences in drug adherence both at baseline and throughout follow-up may influence the results of RDN. Firstly, while patients with true resistant hypertension are characterised by markedly increased sympathetic activity, this may not be the case for non-adherent patients with ATRH [Citation24]. Therefore, the latter group of patients may respond less well to RDN. Secondly, improvement in drug adherence after RDN as part of the so-called Hawthorne effect [Citation25] may lead to BP decreases which could be falsely attributed to RDN, while worsening of drug adherence may obscure truly RDN-related BP decreases.
While changes in drug adherence may have an impact on BP, also in patients with ATRH [Citation25] and there is no reason to think this would be different in the context of RDN, our findings at least suggest that such changes did not lead to a gross under- or overestimation of the impact of alcohol-mediated RDN on BP, as shown by similar BP changes in the subgroup of patients with maintained adherence throughout the study compared to BP changes in the whole cohort. Still, the final demonstration of the efficacy and extent of BP decrease after alcohol-mediated RDN rests on ongoing sham-controlled randomised studies.
More importantly, BP changes after RDN were similar irrespective of baseline drug adherence. In particular, poorly or non-adherent patients appeared to derive BP benefits from RDN similar to patients with truly resistant hypertension, which is also supported by the results of the most recent trials in RDN [Citation9,Citation10,Citation26,Citation27]. Indeed, from a pathophysiological point of view, patients prescribed 3 or more drugs and taking 0, 1, or 2 drugs are supposedly similar to patients prescribed and taking 0, 1, or 2 drugs, but their previous life experience, psychology, and exposure to drug adverse events may be quite different [Citation28,Citation29]. Furthermore, some of these patients may have become secondarily non-adherent because their BP remained uncontrolled despite intake of 4 or 5 antihypertensive drugs. Such patients may prove truly difficult to control even in case of improved drug adherence. As a consequence, while adherent patients with mild to moderate hypertension may be managed by drugs, achieving BP control in patients with severe, ATRH may prove as if not more difficult than in patients with truly resistant hypertension. It is thus reassuring that RDN can lower BP in these patients, and therefore in the near future may prove to be a reasonable treatment option for them. As reported in the main article [Citation11], not all patients respond to RDN, but the overall complication rate was low with a favourable safety profile.
The impact of drug adherence assessed by LC-MS/MS on BP outcome of RDN has been evaluated in a comparable way, though less in-depth, in some other studies [Citation30–32], all using the first-generation unipolar SIMPLICITY catheter in patients with ATRH. In the DENERHTN open-label randomised trial [Citation30], the baseline-adjusted difference in the change in daytime ambulatory systolic BP in denervated patients vs. controls was similar in fully adherent (−6.7 mmHg, 95% CI: −13.3 to −0.1 mmHg) vs. non-adherent (−7.8 mmHg, −17.1 to 1.5 mmHg) patients, as well as between partly and totally non-adherent patients. In two other observational studies including 52 [Citation31] and 80 [Citation32] patients, neither adherence rates at baseline [Citation31] nor changes in drug adherence [Citation32] were associated with ambulatory BP changes at 6 months. Along the same lines, no signal in favour of a lesser BP effect of RDN in poorly adherent patients was found in the randomised INSPIReD pilot trial [Citation33].
Our study further expands on previous results, for the first time at 12 months post-RDN, and for the first time in patients treated by alcohol-mediated RDN. In line with most recent RDN studies, this study had a pragmatic design, including patients with uncontrolled blood pressure on ≥3 anti-HTN drugs, irrespective of drug adherence. We believe that this makes sense as both patients with truly resistant hypertension and patients with ATRH and poor drug adherence typically have severe, difficult-to-treat hypertension, and a given patient may shift from one category to the other during their follow-up [Citation29]. Other strengths of the current study include the use of LC-MS/MS, a direct method of evaluation of adherence [Citation21], which is particularly important when dealing with ATRH, in view of a higher expected proportion of patients with intentional non-adherence [Citation22] who may otherwise escape detection [Citation34,Citation35]. Limitations of the current study include the open-label design and the absence of sham control, which makes the study vulnerable to patient and physician-related bias, Hawthorne, and placebo effects. Furthermore, the sample size was small, especially in some subgroups, resulting in the possibility of type 1 or 2 errors. In addition, it should be noted that changes in antihypertensive medication regimen increased over time in the observed patient population, with 28 and 39% of patients having changes in antihypertensive medications at 6 and 12 months, respectively. Changing antihypertensive medication confounds the assessment of RDN BP-lowering effects. Additional, well controlled, randomised, blinded, studies that account for antihypertensive medication changes are necessary to account for this effect. The effect of prescribed antihypertensive medication at baseline and changes in medication post-procedure on drug adherence is multifactorial, difficult to assess, and beyond the scope of this manuscript. Therefore, data should be interpreted with caution and regarded as hypothesis-generating. However, it should be noted that the use of ambulatory BP as a main primary endpoint and blinded drug monitoring is expected to substantially limit patient- and physician-related biases inherent to the observational nature of the study. Also, the results cannot be extrapolated to other patient populations, e.g. leaner patients. Furthermore, while detection of drugs by LC-MS/MS in urine is increasingly considered the gold standard for assessment of drug adherence, it is mostly a qualitative method, and pharmacokinetic individual characteristics, renal and liver function as well as drug half-lives may to some extent influence the results [Citation21]. Also, drug adherence varies over time, a complex phenomenon that cannot be entirely captured even by repeated drug measurements [Citation33]. Finally, one must take into account the possibility of white-coat drug adherence [Citation36], i.e. patients resuming medication intake just before/the day of the consultation. However, it should be noted that even though patients were aware of the fact that adherence would be checked, they did not know when, and both physicians and patients were blinded to the results of drug adherence determination during the trial. Therefore, the influence of these analyses on patient and physician behaviour was likely limited. Finally, as in most current studies in RDN, direct evidence of the technical success of renal nerve ablation was not available. Therefore, we cannot formally exclude that differences in completeness of RDN in the different subgroups may have influenced the results.
Conclusion and perspectives
While poor drug adherence can be frequently found in patients with ATRH, few studies have focussed on the impact of drug adherence on the BP-lowering effects of RDN. The results from this sub-analysis of the Peregrine study may suggest a beneficial effect of alcohol-mediated RDN on blood pressure also in patients with sub-optimal drug adherence. While our results are strengthened by the use of 24-h ambulatory BP measurements and direct evaluation of adherence by LC-MS/MS, further confirmation is needed from the ongoing larger, randomised sham-controlled trials.
Supplemental Material
Download MS Word (172.3 KB)Acknowledgments
Alexandre Persu wishes to acknowledge Prof. Jean Renkin (Cardiology Department, Cliniques Universitaires Saint-Luc, Brussels, Belgium) for his important contribution to the Peregrine trial and Dr. Jean-Philippe Lengelé (Nephrology Department, GHDC, Charleroi, Belgium and Cardiology Department, Cliniques Universitaires Saint-Luc, Brussels, Belgium) for his contribution to the recruitment of the study and for stimulating discussions. In addition, Prof. Persu would like to thank Dr. Debbie Brix Reynolds for her assistance with drafting the publication.
Disclosure statement
Felix Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK) and Deutsche Forschungsgemeinschaft (SFB TRR219) and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic and ReCor Medical. AP has received honoraria for consultancy, grant support and travel grants from Ablative Solutions, Quantum Genomics, Servier and Recor Medical. NH, TAF, and HP are employees of Ablative Solutions, Inc. The other authors have nothing to declare.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Vital signs: awareness and treatment of uncontrolled hypertension among adults – United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2012;61:109–709.
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1923–1994.
- Mancia G, Fagard F, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–1357.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104.
- DiBona GF. Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R633–R641.
- Böhm M, Linz D, Ukena C, et al. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond. Circ Res. 2014;115(3):400–409.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;62(22):2031–2045.
- Weber MA, Kirtane A, Mauri L, et al. Renal denervation for the treatment of hypertension: making a new start, getting it right (editorial). J Clin Hypertens. 2015;17(10):743–454.
- Bohm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–1451.
- Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335–2345.
- Mahfoud F, Renkin J, Sievert H, et al. Alcohol-mediated renal denervation using the peregrine system infusion catheter for treatment of hypertension. JACC Cardiovasc Interv. 2020;13(4):471–484.
- Schmieder RE, Mahfoud F, Mancia G, et al. European society of hypertension position paper on renal denervation 2021. J Hypertens. 2021;39(9):1733–1741.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv. 2016;9(6):589–598.
- Fischell TA, Fischell DR, Ghazarossian VE, et al. Next generation renal denervation: chemical “perivascular” renal denervation with alcohol using a novel drug infusion catheter. Cardiovasc Revasc Med. 2015;16(4):221–227.
- Fischell TA, Vega F, Raju N, et al. Ethanol-mediated perivascular renal sympathetic denervation: preclinical validation of safety and efficacy in a porcine model. EuroIntervention. 2013;9(1):140–147.
- Poulter NR, Borghi C, Parati G, et al. Medication adherence in hypertension. J Hypertens. 2020;38(4):579–587.
- Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31(4):766–774.
- Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100(11):855–861.
- Bramley TJ, Gerbino PP, Nightengale BS, et al. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. JMCP. 2006;12(3):239–245.
- Parthan A, Vincze G, Morisky DE, et al. Strategies to improve adherence with medications in chronic, ‘silent’ diseases representing high cardiovascular risk. Expert Rev Pharmacoecon Outcomes Res. 2006;6(3):325–336.
- Berra E, Azizi M, Capron A, et al. Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension. 2016;68(2):297–306.
- Ruzicka M, Hiremath S. Can drugs work in patients who do not take them? The problem of non-adherence in resistant hypertension. Curr Hypertens Rep. 2015;17(9):579.
- Wunder C, Persu A, Lengelé J-P, et al. Adherence to antihypertensive drug treatment in patients with apparently treatment-resistant hypertension in the INSPiRED pilot study. Blood Press. 2019;28(3):168–172.
- Dell’Oro R, Quarti-Trevano F, Seravalle G, et al. Sympathetic nerve traffic and arterial baroreflex function in apparent drug-resistant hypertension. Hypertension. 2019;74(4):903–909.
- Persu A, Kjeldsen S, Staessen JA, et al. Renal denervation for treatment of hypertension: a second start and new challenges. Curr Hypertens Rep. 2016;18(1):6.
- Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346–2355.
- Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476–2486.
- Petit G, Elena Berra E, Georges CMG, et al. Impact of psychological profile on drug adherence and drug resistance in patients with apparently treatment-resistant hypertension. Blood Press. 2018;27(6):358–367.
- Pappaccogli M, Di Monaco S, Georges C, et al. Predictors of blood pressure control in patients with resistant hypertension after intensive management in two expert centres: the Brussels-Torino experience. Blood Press. 2019;28(5):336–344.
- Azizi M, Pereira H, Hamdidouche I, et al. Adherence to antihypertensive treatment and the blood pressure-lowering effects of renal denervation in the renal denervation for hypertension (DENERHTN) trial. Circulation. 2016;134(12):847–857.
- Ewen S, Meyer MR, Cremers B, et al. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol. 2015;104(12):1097–1105.
- Schmieder RE, Ott C, Schmid A, et al. Adherence antihypertensive medication in Treatment-Resistant hypertension undergoing renal denervation. J Am Heart Assoc. 2016;5(2):e002343.
- Jacobs L, Persu A, Huang QF, et al. European network coordinating research on renal denervation. Results of a randomized controlled pilot trial of intravascular renal denervation for management of treatment-resistant hypertension. Blood Press. 2017;26(6):321–331.
- Durand H, Hayes P, Morrissey EC, et al. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens. 2017;35(12):2346–2357.
- Pandey A, Raza F, Velasco A, et al. Comparison of Morisky medication adherence scale with therapeutic drug monitoring in apparent treatment-resistant hypertension. J Am Soc Hypertension. 2015;9(6):420–426.e2.
- Burnier M, Wuerzner G, Struijker-Boudier H, et al. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62(2):218–225.
