Abstract
Purpose: Takayasu arteritis (TA) is a rare disease, which is frequently misdiagnosed or its diagnosis can be missed. This study aimed to analyse the characteristics of four-limb blood pressure (4LBP) and brachial-ankle pulse wave velocity (baPWV) in patients with TA, which could be useful in disease detection.
Materials and Methods: We consecutively enrolled 182 patients with TA at Fuwai Hospital between January 2013 and January 2016. Pulse pressure (PP), pulsatile index (PI), inter-arm systolic blood pressure (SBP) difference (IASBPD), inter-leg SBP difference (ILSBPD), ankle–brachial index (ABI), baPWV, and inter-side baPWV difference (ΔbaPWV) were analysed and compared with those of age-, sex-, and SBP-matched participants without cardiovascular diseases.
Results: In the TA group, the diastolic blood pressure was lower (67.4 ± 23.7 vs 84.1 ± 15.0 mmHg), PP was larger (69.7 ± 23.6 vs 53.7 ± 10.6 mmHg), PI was higher (1.3 ± 2.1 vs. 0.6 ± 0.1 mmHg), IASBPD was larger (18.2 ± 24.1 vs 4.2 ± 3.3 mmHg), and ILSBPD was larger (10.7 ± 15.0 vs 5.3 ± 4.1 mmHg) than those of the controls (all p < 0.01). Moreover, the proportions of PP >70 mmHg (36.8% vs 4.4%), PI > 1.0 (40.1% vs 2.2%), IASBPD >15 mmHg (34.6% vs. 0%), highest ABI >1.4 (17.6% vs. 0%), ILSBPD >15 mmHg (14.8% vs. 3.3%), lowest ABI < 0.9 (24.7% vs 2.2%), and ΔbaPWV > 185 cm/s (28.6% vs. 1.1%) were significantly greater in the TA group than in the control group (all p < 0.01). Approximately 80.8% of patients with TA (vs. 10.4% of controls) presented with at least one of these seven parameters (p = 0.000).
Conclusion: The characteristics of 4LBP and baPWV in most patients with TA were abnormal, which helped us perform non-invasive primary screening and comprehensive evaluation of vascular lesions in such patients.
PLAIN LANGUAGE SUMMARY
In daily life, many people measure the blood pressure of the arm but measuring the blood pressure of a single arm is inadequate because some hypertension and vascular diseases cannot be detected this way. Synchronous limb blood pressure measurements may be used to close this gap. Measuring synchronous limb blood pressure is very convenient and helps patients understand the value of limb blood pressure and examine many other useful parameters, such as the blood pressure differences between the two arms and two legs, as well as the ankle arm index. These values and derived parameters can also help detect many vascular diseases.
Takayasu arteritis is a rare disease in young women. However, the aorta and branches of these patients are narrow or occluded. Patients often experience vague and ambiguous symptoms, such as hypertension or dizziness, so they are likely to be overlooked or misdiagnosed.
Our study summarises the results of synchronous limb blood pressure measurements in patients with Takayasu arteritis and compares their results with those of a control population. Synchronous limb blood pressure measurements are easy and convenient and can detect vascular problems, which may improve the ability to diagnose Takayasu arteritis.
Introduction
Takayasu arteritis (TA) is a chronic, non-specific inflammation involving the aorta and its primary branches [Citation1,Citation2], which is frequently misdiagnosed or can be missed in the diagnosis. The American College of Rheumatology Classification Criteria for TA were proposed in 1990 [Citation1]; the last five criteria are related to the subclavian arteries and other arteries, such as the aorta and lower extremity arteries, which would inevitably lead to corresponding haemodynamic changes, resulting in a difference in blood pressure (BP) between the limbs [Citation3–5]. The four-limb BP (4LBP) and brachial–ankle pulse wave velocity (baPWV) are simple and non-invasive methods for the diagnosis of peripheral arterial diseases [Citation6,Citation7]. However, few studies have investigated the characteristics of 4LBP and baPWV in patients with TA. Therefore, to explore the characteristics of 4LBP and baPWV and their clinical value in TA, data of 4LBP and baPWV measurements were collected and analysed in participants with and without TA.
Materials and methods
Patient population
A total of 182 patients with TA were hospitalised in the Fuwai Hospital from January 2013 to January 2016. Patients with TA who completed the 4LBP and baPWV measurement as required and those with complete baseline information who underwent peripheral vascular examination were included. Patients who were <18 years of age, had unclear or questionable TA diagnosis, in whom comparison of the 4LBP and baPWV measurement could not be performed as required, had obvious errors or missing 4LBP and baPWV measurements, and did not have computed tomography or angiography to assess peripheral vascular disease were excluded. TA diagnostic criteria were based on Ishikawa’s criteria (1988) [Citation8], Ishikawa’s criteria modified (1996) [Citation9] for the diagnosis of TA, and the 1990 American College of Rheumatology Classification Criteria for TA [Citation1]. Another 182 participants, including patients with primary hypertension or healthy volunteers, matched by age, sex, body mass index (BMI) (±5%), and the highest systolic BP (SBP) of the brachial artery (±5%), served as the control group. The latest European hypertension guideline (2013) [Citation10] was used to diagnose primary hypertension. For inclusion, the conditions of patients with primary hypertension were as follows: (1) no history of other peripheral vascular diseases; (2) no previous peripheral vascular interventional therapy; (3) no severe chronic liver and lung diseases, malignant tumours, or acute infectious diseases; and (4) no other cardiac diseases, such as primary heart disease, myopathy, myocarditis, congenital heart disease, or valvular heart disease.
Written consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards and with the Declaration of Helsinki and our Institutional Review Board approval (2018-993).
Simultaneous measurements of 4LBP and baPWV
Room temperature was maintained at 22–25 °C, and all patients laid down for 5 min. A Boso ABI-system 100 (BOSCH & SOHN, Germany) or Omron VP1000 (Omron, Kyoto, Japan), an oscillometric device, was used to automatically record the 4LBP and baPWV measurements simultaneously [Citation11]. The patients were placed in a supine position with their hands and palms on both sides of the body-facing upward. The BP cuff of the limbs was tied to the upper arm and ankles of the lower extremities. The upper-arm cuff airbag mark was aligned with the brachial artery, and the lower edge of the cuff was 2–3 cm away from the cubital fossa. The lower extremity cuff airbag mark was located in the medial part of the lower extremity, and the lower edge of the cuff was 1–2 cm away from the malleolus medialis. The device automatically repeated the measurements of 4LBP and baPWV twice for each patient and recorded the second data as the final result if no obvious interference was observed in the pulse waveform. Measurements and verification were performed by skilled nurses.
Definition of parameters for 4LBP and baPWV measurements
The side with high SBP of the brachial artery was selected as the SBP, and the difference between the SBP and the corresponding diastolic BP (DBP) of the brachial artery was selected as the pulse pressure (PP). The pulsatile index (PI) was defined as the ratio of the PP to the DBP. Inter-arm SBP difference (IASBPD) and inter-leg SBP difference (ILSBPD) are the absolute values of the SBP difference between the brachial and ankle arteries, respectively. The unilateral ankle–brachial index (ABI) was defined as the ratio of the SBP in the ankle artery to that in the brachial artery. The baPWV in the high SBP group was selected as the baPWV, and the ΔbaPWV was defined as the absolute value of the difference between the bilateral baPWVs (). IASBPD >10 mmHg [Citation12], IASBPD >15 mmHg [Citation13], IASBPD >20 mmHg [Citation14], ILSBPD >10 mmHg [Citation7], ILSBPD >15 mmHg [Citation12], PP >60 mmHg [Citation15], PP > 70 mmHg [Citation16], ABI <0.9 [Citation11], ABI >1.4 [Citation11], PI [Citation17], ΔbaPWV ≥88 cm/s [Citation18], and ΔbaPWV ≥185 cm/s [Citation19] were selected as the investigated parameters of 4LBP and baPWV measurements in this study.
Figure 1. A scheme of investigated parameters of 4LBP and baPWV measurements. 4LBP: four-limb blood pressure; baPWV: brachial–ankle pulse wave velocity; SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: pulse pressure; PI: pulsatile index; IASBPD: inter-arm systolic blood pressure difference; ILSBPD: inter-leg systolic blood pressure difference; ABI: ankle–brachial index; ΔbaPWV: inter-side baPWV difference.
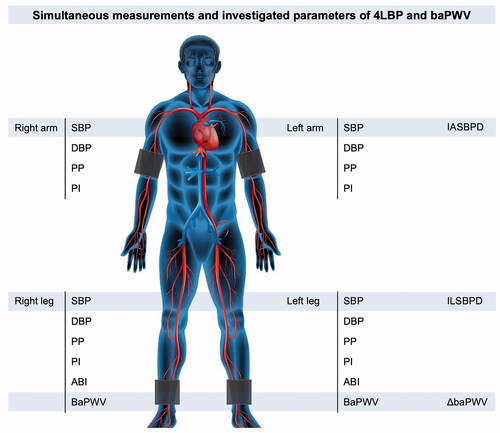
An IASBPD >10, 15, or 20 mmHg or ILSBPD >10 or 15 mmHg indicates arterial stenosis of the upper or lower extremities [Citation20]. A unilateral ABI <0.9 indicates lower extremity artery stenosis [Citation21], and symmetry of bilateral ABI reduction suggests aortic stenosis [Citation4]. In addition, ABI >1.4 suggests brachial artery stenosis [Citation22] and increased aortic stiffness [Citation23]. Increased PP is associated with hypertension [Citation24], aortic stiffness [Citation25], and aortic valve regurgitation [Citation26]. The increase in PIs [Citation17] and baPWV [Citation27] are also associated with arterial stiffness. Finally, an increased ΔbaPWV was significantly correlated with ABI <0.9 and high baPWV [Citation18].
Definition of vascular stenosis
All patients with TA underwent peripheral angiography (PAG), and most underwent computed tomography angiography (CTA); the severity of arterial lesions was recorded. The arteries of the superior arch, aorta, and lower extremities were segmented to determine the location, number, and degree of stenosis. According to PAG and CTA, the following Hata types [Citation28] of TA were analysed: Type I (involvement of primarily the branches from the aortic arch), Type IIa (involvement of the ascending aorta, aortic arch), Type III (involvement of the thoracic descending aorta, abdominal aorta, and/or renal arteries), Type IV (involvement of only the abdominal aorta and/or renal arteries), and Type V (involvement of the ascending aorta, aortic arch with its branches and thoracic descending aorta combined with the abdominal aorta and/or renal arteries). Arterial stenosis was defined as a reduction in the diameter of the artery >50%. Stenosis of the subclavian artery (including the brachiocephalic trunk), axillary artery, and brachial artery was defined as upper limb artery stenosis. Stenosis of the artery from the bifurcation of the abdominal aorta to the left and right iliac arteries, femoral artery, and inferior genicular artery was defined as stenosis of the lower limb artery.
Statistical analysis
IBM SPSS Statistics, version 24.0 (IBM Corp., Armonk, NY, USA) was used to analyse the data. The measurement data were expressed as means ± standard deviations. The t-test or Mann–Whitney test was used to compare the measurement data between the two groups according to whether the normal distribution was satisfied. Data are expressed in frequency (percentage) form, and the X2 test was used to compare the counting data between the groups. Sensitivity and specificity define how effectively a test discriminates individuals with disease from those without disease. Sensitivity is the percentage of individuals with a disease who have abnormal test results. Specificity is the percentage of those without disease who have normal test results. The sensitivity and specificity of the test were calculated using the following formulas: Sensitivity = true positive/(true positive + false negative) × 100. Specificity = True negative/(True negative + False positive) × 100. Statistical significance was set at p < 0.05, and p < 0.01 was considered highly significant. The p-values were both bilateral.
Results
Clinical characteristics of patients with TA
Among 182 patients with TA, 154 (84.6%) were female, and the mean age was 35.3 ± 13.3 years. The lesions included 66 upper-limb arteries (36.3%), 57 renal arteries (31.3%), 48 carotid arteries (26.4%), 45 aortas (24.7%), 19 coronary arteries (10.4%), 21 pulmonary arteries (11.5%), and 17 lower-limb arteries (9.3%). The main types of TA were Type II, I, and IV in 91 (50.0%), 46 (25.3%), and 39 (16.5%) cases, respectively. The secondary symptoms included hypertension, aortic valve regurgitation, and heart failure in 101 (55.5%), 44 (24.2%), and 37 (20.3%, ) cases, respectively.
Table 1. Basic characteristics of the patients with TA.
Characteristics of 4LBP and baPWV in the TA group vs the control group
No differences were observed in sex, age, BMI, and highest SBP of the brachial artery among the 182 patients with TA and control participants (p > 0.05). Compared with the control group, the DBP was lower (67.4 ± 23.7 vs 84.1 ± 15.0 mmHg), the PP was higher (69.7 ± 23.6 vs 53.7 ± 10.6 mmHg), the PI was higher (1.3 ± 2.1 vs 0.6 ± 0.1), the IASBPD was larger (18.2 ± 24.1 vs 4.2 ± 3.3 mmHg), the ILSBPD was larger (10.7 ± 15.0 vs 5.3 ± 4.1 mmHg) (all p < 0.01), and the baPWV of the highest side was larger in the TA group (1370.8 ± 1136.2 vs 1320.1 ± 255.9 cm/s, p < 0.05). However, no difference was found in the ABI of the highest side (1.2 ± 0.4 vs 1.1 ± 0.1), ABI of the lowest side (1.1 ± 0.4 vs 1.1 ± 0.1), BaPWV (1367.3 ± 1117.4 vs 1314.5 ± 252.7 cm/s), and baPWV of the lowest side (1262.8 ± 754.0 vs 1140.1 ± 222.6 cm/s) compared with those of the control group (all p > 0.05, ).
Table 2. The characteristics of 4LBP and baPWV in TA group versus control group.
The proportion of PP >60 mmHg (58.2% vs 24.7%), PP >70 mmHg (36.8% vs 4.4%), PI >0.9 (50.0% vs 5.5%), PI >1.0 (40.1% vs 2.2%), IASBPD > 10 mmHg (43.4% vs 5.5%), IASBPD >15 mmHg (34.6% vs 0%), IASBPD >20 mmHg (28.0% vs 0%), ILSBPD >10 mmHg (28.0% vs 9.9%), ILSBPD >15 mmHg (14.8% vs 3.3%), highest ABI <0.9 (18.7% vs 0.5%), lowest ABI <0.9 (24.7% vs 2.2%), highest side ABI >1.4 (17.6% vs 0%), ΔbaPWV ≥185 cm/s (28.6% vs 1.1%), and ΔbaPWV ≥88 cm/s (44.5% vs 14.3%) were all significantly higher in the TA than in the control group (all p < 0.01, ).
Comprehensive comparison of abnormal 4LBP and baPWV measurements indices between the TA and control groups
Comprehensive analysis of abnormal indices of 4LBP and baPWV measurements showed sensitivities of 100%, 100%, 89.3%, 94.8%, 81.8%, 91.8%, and 96.3% and specificities of 60.5%, 54.8%, 60.2%, 62.0%, 53.2%, 56.5%, and 58.1% when IASBPD >15 mmHg, highest ABI >1.4, PP >70 mmHg, PI >1.0, ILSBPD >15 mmHg, lowest ABI <0.9, and ΔbaPWV ≥185 cm/s were satisfactory, respectively. The sensitivities were 73.1%, 91.2%, 100%, 100%, 100%, and 100%, and the specificities were 53.8%, 57.6%, 54.3%, 52.0%, 51.4%, and 50.5% when two, three, four, five, and six () indices were satisfied simultaneously, respectively. Compared with the control group, the proportion of the TA group (80.8% vs 10.4%) was significantly higher (p = 0.000) when one or more of the aforementioned items were satisfied; the sensitivity and specificity were 88.6% and 82.3%, respectively. The sensitivities were 95.6%, 100%, 100%, and 100%, and the specificities were 70.8%, 59.3%, 54.2%, and 52.0% when ≥2, ≥3, ≥4, and ≥5 items were satisfied, respectively ().
Figure 2. A 48-year-old female TA patient with many arteries involved had six abnormal investigated parameters of 4LBP and baPWV measurements. A: Boso 4LBP and baPWV measurements with the IASBPD > 15 mmHg, ILSBPD > 15 mmHg, bilateral ABI < 0.9, PP > 70 mmHg, PI > 1.0, and ΔbaPWV > 185 cm/s. B: CTA showed severe stenosis of the proximal left subclavian artery (white arrow), aneurysmal dilatation of aortic root (yellow arrow), thoracic aortic dilatation with wall calcification (red arrow), severe stenosis of the proximal abdominal aorta (blue arrow), occlusion of left renal artery (green arrow). TA: Takayasu arteritis; 4LBP: four-limb blood pressure; baPWV: brachial–ankle pulse wave velocity; IASBPD: inter-arm systolic blood pressure difference; ILSBPD: inter-leg systolic blood pressure difference; ABI: ankle-brachial index; PP: pulse pressure; PI: pulsatile index; ΔbaPWV: inter-side baPWV difference; CTA: computed tomography angiography.
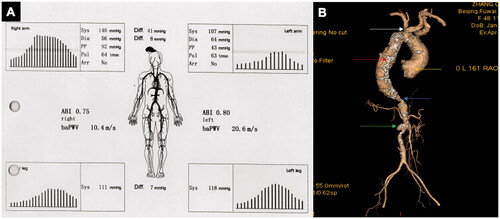
Table 3. Comprehensive comparison of 4LBP and baPWV between TA group and control group.
Discussion
TA is a non-specific chronic vasculitis, often involving the aorta and its main branches, resulting in vascular stenosis and/or occlusion [Citation1,Citation2,Citation9]. As a result, there is a difference in BP between the limbs. The measurement of bilateral brachial artery BP is not sufficient to reflect systemic vascular lesions in patients with TA. In contrast, 4LBP and baPWV measurements can provide comprehensive information on vascular lesions.
To our knowledge, this is the first large-scale cohort study to characterise 4LBP and baPWV measurements in patients with TA. IASBPD >15 mmHg, highest ABI >1.4, PP > 70 mmHg, PI >1.0, ILSBPD >15 mmHg, lowest ABI <0.9, and ΔbaPWV >185 cm/s were used as indices of 4LBP and baPWV measurements, which were helpful for non-invasive primary screening and comprehensive evaluation of vascular lesions in patients with TA. The sensitivity was 88.6% and the specificity was 82.3% when one or more items were observed.
IASBPD is associated with subclavian artery stenosis [Citation29,Citation30]. IASBPD >10 mmHg, one of the TA diagnostic criteria (1990), is of great significance for TA screening and in the evaluation of upper extremity artery stenosis [Citation1]. The proportions of IASBPD >10, 15, and 20 mmHg in the TA group were 43.3%, 34.6%, and 28.0%, respectively. However, the PAG confirmed that 36.3% of the upper extremity arteries were involved. The IASBPD was mainly screened for asymmetric stenosis of the upper extremities. Therefore, excessive length and stenosis of TA vascular lesions <50% may also lead to arm pressure differences.
Simultaneous stenosis of the bilateral upper extremity arteries may lead to IASBPD <10 mmHg, which is an important cause of the missed diagnosis of upper extremity arterial involvement and hypertension screening in patients with TA. Bilateral upper-extremity arterial involvement is common in such patients. This study showed that 14.3% of patients with TA had bilateral upper extremity arterial stenosis, which may be difficult to detect using IASBPD >10 mmHg alone. A model analysis of ABI and vascular stenosis in China showed that the degree of brachial artery stenosis was closely related to ABI elevation [Citation22]. Therefore, ABI >1.4 represented a greater probability of upper extremity artery stenosis. In this study, the highest ABI rate (ABI > 1.4) in the TA group was 17.6%, which was slightly higher than the proportion of bilateral upper extremity artery stenosis.
An ABI <0.9 is the diagnostic index for lower extremity artery stenosis [Citation21]. In addition, ILSBPD >10 or 15 mmHg is also an index for evaluating lower-extremity artery stenosis [Citation31]. This study showed that the ratio of the lowest ABI <0.9 and ILSBPD >15 mmHg was higher in the TA than in the control group, which could be used as a non-invasive and objective index for evaluating lower extremity vascular stenosis in patients with TA.
This study found that 24.7% of patients with TA had thoracoabdominal aortic stenosis, which was similar to a previous report from our hospital [Citation32]. However, the 1990 TA diagnostic criteria [Citation1] and the 2008 European TA diagnostic criteria [Citation2] lack non-invasive and objective indicators for assessing thoracoabdominal aortic and lower extremity artery stenosis. ABI reduction has been reported symmetrically in patients with TA with aortic stenosis [Citation4]. In this study, 18.7% of patients with TA had a bilateral ABI < 0.9; however, only 4.4% of the patients had bilateral lower extremity involvement, which confirmed that bilateral ABI < 0.9 suggests the existence of thoracoabdominal aortic stenosis ().
TA can increase the aortic stiffness [Citation33]. PP is an important indicator of arterial stiffness, and an increased PP often indicates an increase in vascular stiffness [Citation34]. This study showed that the proportion of patients in the control group with PP > 60 mmHg was very high, indicating that hypertension itself could result in an increased PP [Citation24]. The proportions of PP > 60 and 70 mmHg in the TA group were significantly higher than those in the control group, suggesting an increase in vascular stiffness in patients with TA. The aforementioned findings were consistent with those of previous reports of typical cases, in which a TA-like aorta leads to an extremely high PP [Citation35]. Some patients with TA had aortic valve regurgitation, which could lead to increased PP. Previous studies have reported that an increase in PP is a manifestation of aortic valve regurgitation [Citation36]. This study found that the DBP in the TA group was lower, whereas the PP was higher; therefore, the PI was significantly larger. The proportion of PI > 1.0% was significantly higher in the TA than in the control group and was more sensitive than PP > 70 mmHg.
TA may present with a higher baPWV because of prolonged arterial stiffness caused by inflammation [Citation37]. This study found that the highest baPWV was observed in patients with TA. Both ΔbaPWV and the proportion of patients with ΔbaPWV > 185 cm/s were greater in the TA group. We speculated that precise baPWV measurement may be affected by multiple peripheral artery stenoses caused by TA.
In this study, (1) IASBPD > 15 mmHg, (2) highest ABI > 1.4, (3) PP > 70 mmHg, (4) PI > 1.0, (5) ILSBPD > 15 mmHg, (6) lowest ABI < 0.9, and (7) ΔbaPWV > 185 cm/s were used as parameters for 4LBP and baPWV measurements. The proportion of the TA group (80.8% vs 10.4%) was significantly higher compared with that of the control group (p = 0.000), while the sensitivity was 88.6% and the specificity was 82.3%, when one or more items were observed. Thirty-five cases (19.2%) did not meet any investigated parameters in the TA group, which may be related to the classification of TA and location of the lesions. When the carotid, renal, coronary, and pulmonary arteries are involved alone or simultaneously, 4LBP and baPWV measurements may be indifferent. When the upper and lower limbs are involved simultaneously with the aorta or only the lower limbs, the BP of the limbs may also be normal.
This study has some limitations. First, the characteristics of DBP, PP, and pulse volume recordings and its quantitative index similar to mean artery pressure and upstroke time had not been analysed; these results may also reflect the vascular lesions in patients with TA. Second, because of the different lengths and shapes of vascular lesions caused by TA, only defining TA as >50% stenosis of the artery may not fully represent the vascular stenosis, and dilatation and aneurysms were not analysed. Third, patients with other vascular diseases, such as fibromuscular dysplasia, thromboangitis obliterans, Behcet’s disease, and neurofibromatosis were not analysed as controls. Future studies should focus on designing multicentre cohort studies to validate the value of 4LBP and baPWV measurements and whether these methods can be incorporated into the guidelines of TA diagnosis, prognosis, and management.
In conclusion, the characteristics of the 4LBP and baPWV measurements in most patients with TA were abnormal, which were helpful for non-invasive primary screening and comprehensive evaluation of vascular lesions in patients with TA.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
All data generated or used during the study appear in the submitted article.
Additional information
Funding
References
- Arend WP, Michel BA, Bloch DA, et al. The American college of rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–1134.
- Ozen S, Pistorio A, Iusan SM, for the Paediatric Rheumatology International Trials Organisation (PRINTO), et al. EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798–806.
- Dong H, Che W, Jiang X, et al. An unrecognised presentation of Takayasu arteritis: superficial femoral artery involvement. Clin Exp Rheumatol. 2017;35 Suppl 103(1):83–87.
- Che W, Xiong H, Jiang X, et al. Stenting for middle aortic syndrome caused by Takayasu arteritis-immediate and long-term outcomes. Catheter Cardiovasc Interv. 2018;91(S1):623–631.
- Dong H, Chen Y, Xiong HL, et al. Endovascular treatment of iliac artery stenosis caused by Takayasu arteritis: a 10-Year experience. J Endovasc Ther. 2019;26(6):810–815.
- Clark CE. Four-limb blood pressure measurement: a research tool looking for clinical use. Hypertension. 2013;61(6):1146–1147.
- Herraiz-Adillo A, Soriano-Cano A, Martinez-Hortelano JA, et al. Simultaneous inter-arm and inter-leg systolic blood pressure differences to diagnose peripheral artery disease: a diagnostic accuracy study. Blood Press. 2018;27(2):112–119.
- Ishikawa K. Diagnostic approach and proposed criteria for the clinical diagnosis of Takayasu's arteriopathy. J Am Coll Cardiol. 1988;12(4):964–972.
- Sharma BK, Jain S, Suri S, et al. Diagnostic criteria for Takayasu arteritis. Int J Cardiol. 1996;54 Suppl:S141–S147.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC)[J]. Eur Heart J. 2013;34(28):2159–2219.
- Asbeutah AM, AlMajran AA, Asfar SK. Diastolic versus systolic ankle-brachial pressure index using ultrasound imaging & automated oscillometric measurement in diabetic patients with calcified and non-calcified lower limb arteries. BMC Cardiovasc Disord. 2016;16(1):202.
- Singh S, Sethi A, Singh M, et al. Simultaneously measured inter-arm and inter-leg systolic blood pressure differences and cardiovascular risk stratification: a systemic review and meta-analysis. J Am Soc Hypertens. 2015;9(8):640–650. e612.
- Aboyans V, Criqui MH, Abraham P, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American heart association. Circulation. 2012;126(24):2890–2909.
- Mehlsen J, Wiinberg N. Interarm difference in blood pressure: reproducibility and association with peripheral vascular disease. Int J Vasc Med. 2014;2014:1–4.
- Zhang L, Wang B, Wang C, et al. High pulse pressure is related to risk of type 2 diabetes mellitus in Chinese middle-aged females. Int J Cardiol. 2016;220:467–471.
- Assmann G, Cullen P, Evers T, et al. Importance of arterial pulse pressure as a predictor of coronary heart disease risk in PROCAM[J]. Eur Heart J. 2005;26(20):2120–2126.
- Kim HL, Seo JB, Chung WY, et al. Association between invasively measured central aortic pressure and left ventricular diastolic function in patients undergoing coronary angiography. Am J Hypertens. 2015;28(3):393–400.
- Wei SY, Huang JC, Chen SC, et al. Unequal arterial stiffness with overall and cardiovascular mortality in patients receiving hemodialysis. Am J Med Sci. 2016;351(2):187–193.
- Su HM, Lin TH, Hsu PC, et al. Association of bilateral brachial-ankle pulse wave velocity difference with peripheral vascular disease and left ventricular mass index. PLoS One. 2014;9(2):e88331.
- Osborn LA, Vernon SM, Reynolds B, et al. Screening for subclavian artery stenosis in patients who are candidates for coronary bypass surgery. Catheter Cardiovasc Interv. 2002;56(2):162–165.
- Xu D, Zou L, Xing Y, et al. Diagnostic value of ankle-brachial index in peripheral arterial disease: a meta-analysis. Can J Cardiol. 2013;29(4):492–498.
- Li X, Wang L, Zhang C, et al. Why is ABI effective in detecting vascular stenosis? Investigation based on multibranch hemodynamic model. ScientificWorldJ. 2013;2013:185691.
- Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the strong heart study. Circulation. 2004;109(6):733–739.
- Safar ME. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. 2018;15(2):97–105.
- Su TC, Chien KL, Jeng JS, et al. Pulse pressure, aortic regurgitation and carotid atherosclerosis: a comparison between hypertensives and normotensives. Int J Clin Pract. 2006;60(2):134–140.
- Lee DW, Clark A, Stouffer GA. Hemodynamic findings of severe subacute aortic regurgitation. J Invasive Cardiol. 2017;29(6):E74.
- Munakata M. Brachial-Ankle pulse wave velocity: background, method, and clinical evidence. Pulse (Basel). 2016;3(3-4):195–204.
- Hata A, Noda M, Moriwaki R, et al. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. 1996;54:S155–S163.
- English JA, Carell ES, Guidera SA, et al. Angiographic prevalence and clinical predictors of left subclavian stenosis in patients undergoing diagnostic cardiac catheterization. Cathet. Cardiovasc. Intervent. 2001;54(1):8–11.
- Aboyans V, Criqui MH, McDermott MM, et al. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007;49(14):1540–1545.
- Zhang Z, Ma J, Tao X, et al. The prevalence and influence factors of inter-ankle systolic blood pressure difference in community population. PLoS One. 2013;8(8):e70777.
- Yang L, Zhang H, Jiang X, et al. Clinical manifestations and longterm outcome for patients with Takayasu arteritis in China. J Rheumatol. 2014;41(12):2439–2446.
- Salles Rosa Neto N, Levy-Neto M, Tolezani EC, et al. Determinants of arterial stiffness in female patients with Takayasu arteritis. J Rheumatol. 2014;41(7):1374–1378.
- Yannoutsos A, Ahouah M, Dreyfuss Tubiana C, et al. Aortic stiffness improves the prediction of both diagnosis and severity of coronary artery disease. Hypertens Res. 2018;41(2):118–125.
- Mishima E, Suzuki T, Hashimoto J, et al. Lead pipe'-like stiff aorta with grossly widened pulse pressure in burned-out Takayasu arteritis. Eur Heart J Cardiovasc Imaging. 2017;18(7):819.
- Shimada S, Matsuura M, Yamaguchi T, et al. Analyzing the association between aortic regurgitation and atherosclerosis: is pulse pressure a cause of atherosclerosis?. Clin Exp Hypertens. 2018;40(8):796–802.
- Yang Y, Wang Z, Yuan LJ, et al. Aortic stiffness evaluated by echocardiography in female patients with Takayasu's arteritis. Clin Exp Rheumatol. 2017;35 Suppl 103(1):134–138.
