Abstract
Beta-blockers have solid documentation in preventing cardiovascular complications in the treatment of hypertension; atenolol, metoprolol, oxprenolol and propranolol demonstrate proven cardiovascular prevention in hypertension mega-trials. Hypertension is characterised by activation of the sympathetic nervous system from early to late phases, which makes beta-blockers an appropriate treatment seen from a pathophysiological viewpoint, especially in patients with an elevated heart rate. Beta-blockers represent a heterogenous class of drugs with regard to both pharmacodynamic and pharmacokinetic properties. This position is manifest by reference to another clinical context, beta-blocker treatment of heart failure, where unequivocally there is no class effect (no similar benefit from all beta-blockers); there are good and less good beta-blockers for heart failure. Analogous differences in beta-blocker efficacy is also likely in hypertension. Beta-blockers are widely used for the treatment of diseases comorbid with hypertension, in approximately 50 different concomitant medical conditions that are frequent in patients with hypertension, leading to many de facto beta-blocker first choices in clinical practice. Thus, beta-blockers should be regarded as relevant first choices for hypertension in clinical practice, particularly if characterised by a long half-life, highly selective beta-1 blocking activity and no intrinsic agonist properties.
Beta-blockers have solid documentation in preventing cardiovascular complications in the treatment of hypertension; atenolol, metoprolol, oxprenolol and propranolol demonstrate proven cardiovascular prevention in hypertension mega-trials
Hypertension is characterised by activation of the sympathetic nervous system from early to late phases, which makes beta-blockers an appropriate treatment seen from a pathophysiological viewpoint, especially in patients with an elevated heart rate
Beta-blockers represent a heterogenous class of drugs with regard to both pharmacodynamic and pharmacokinetic properties
This position is manifest by reference to another clinical context, beta-blocker treatment of heart failure, where unequivocally there is no class effect (no similar benefit from all beta-blockers); there are good and less good beta-blockers for heart failure
Analogous differences in beta-blocker efficacy is also likely in hypertension
Beta-blockers are widely used for the treatment of diseases comorbid with hypertension, in approximately 50 different concomitant medical conditions that are frequent in patients with hypertension, leading to many de facto beta-blockers first choices in clinical practice
These observations, in totality, inform our opinion that beta-blockers are relevant first choices for hypertension in clinical practice and this fact needs highlighting
Further, these arguments suggest European hypertension guideline downgrading of beta-blockers is not justified
SUMMARY
The position of beta-blockers and trial evidence in support of beta-blocker use in hypertension
Several hypertension and cardiovascular prevention guidelines [Citation1–4] have removed beta-blockers from a position of the first-choice drug for the treatment of hypertension, recommending their use only in the presence of some specific comorbid clinical conditions or, as an add-on, when there is inadequate blood pressure (BP) response to initially preferred, first-line agents. This downgrading may be unjustified:
Beta-blockers lower BP as effectively as other major antihypertensive drugs thereby fully exploiting the documented protective effect of BP lowering “per se.” Some beta-blockers (e.g. atenolol) are less effective on central BP, which, however, has never been used as a target in outcome trials on cardiovascular protection.
Beta-blockers prevent complications in hypertension compared to placebo in randomised controlled trials (RCTs), and with two exceptions [Citation5,Citation6], their effects have been found to be similar to that of other classes of antihypertensive drugs [Citation7–12], with equal or only marginally less evident overall benefit in meta-analyses of large numbers of trials [Citation13–15].
Specific criticism of beta-blockers in the 2018 guidelines of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) was that beta-blockers are accompanied by a greater number of side effects or contraindications, as well as they are less protective against stroke than other drug classes in various meta-analyses [Citation16]. These points of criticism need to be considered with caution for various reasons:
The difference may have originated from small differences in achieved BP between trials, to which stroke is especially sensitive [Citation6,Citation17,Citation18], which can hardly be neutralised by statistical adjustment procedures.
No study has ever shown a damaging effect of beta-blockers on cerebral blood flow autoregulation or brain tissue.
Beta-blockers substantially reduce the risk of stroke in hypertension when compared to placebo in RCTs [Citation16].
Some earlier specific concerns of beta-blockers appeared overrated, including an increased risk of depression [Citation19] or erectile dysfunction [Citation20], while beta-blockers are now safe and protective in peripheral artery disease [Citation21] and chronic obstructive pulmonary disease [Citation22].
Beyond this clinical trial evidence and in support of beta-blocker prescribing in hypertension, there are other important considerations:
Beta-blockers represent a heterogenous class of drugs with regard to both pharmacodynamic and pharmacokinetic properties. Hypertension guidelines do not discriminate sufficiently between different beta-blockers in their use as antihypertensive drugs, despite these diverse properties. Although direct evidence from the head-to-head comparison in RCTs is absent, it seems likely that there will be no class effect (no similar benefit for all beta-blockers).
Hypertension is characterised by activation of the sympathetic nervous system (SNS) from early to late phases, which makes beta-blockers an appropriate treatment seen from a pathophysiological viewpoint. This applies in particular to patients with elevated heart rate, which is driven by ongoing SNS activation [Citation23,Citation24].
Beta-blockers are widely used for the treatment of diseases comorbid with hypertension, and their use is advisable in approximately 50 different concomitant medical conditions, which are frequent in patients with a chronic BP elevation. Comorbidity-directed prescribing of a beta-blocker in hypertension moves the drug class to first-line prescribing, possibly replacing one or more of the antihypertensive drugs currently given priority in the guidelines.
In the present narrative review, we aim to analyse these additional considerations for beta-blocker prescribing in hypertension. It appears that analysis of hypertension RCT results, consideration of comorbidity prescribing, acknowledgement of hypertension neural pathophysiology and review of their diverse pharmacological properties suggest that beta-blockers continue to play a key role in the treatment of hypertension.
It is probable that there is no class effect for beta-blockers in hypertension
Despite their diverse properties (], recent hypertension guidelines do not discriminate sufficiently or do not discriminate at all between different beta-blockers in their use as antihypertensive drugs [Citation1–4]. In the earlier years beta-blocker RCTs in hypertension investigated primarily propranolol and subsequently, with one exception for metoprolol [Citation9,Citation25,Citation26] and one for oxprenolol [Citation27], atenolol in all trials. However, neither propranolol nor atenolol is “representative” of this entire drug class. Propranolol is a non-selective beta-blocker, competitively antagonising β-1 and β-2 adrenoceptors. Unlike many other beta-blockers, atenolol is a hydrophilic β-1 selective agent with a relatively short half-life that is suboptimal for once-daily dosing. It would be a mistake to hold that the two primary shortcomings cited for beta-blocker use in hypertension, less reduction in stroke incidence and adverse metabolic effects, e.g. induced insulin resistance and lowered HDL cholesterol, necessarily apply to beta-blockers other than propranolol, compounds with agonist activity [Citation28] and atenolol.
Figure 1. Chronology of the development of beta-blockers. The illustration depicts the pharmacological properties characterising the three “generations” of this development. BP indicates blood pressure and HR indicates heart rate.
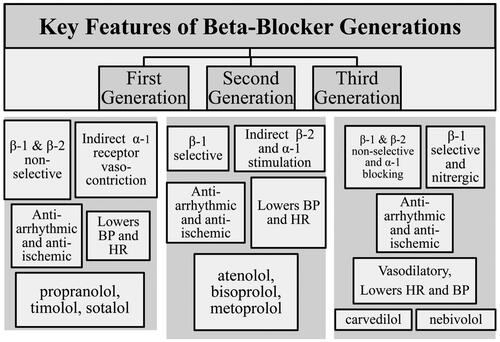
Figure 2. Chemical formulas for the beta-blocker. For some beta-blockers that exist in more than one formula, only one of these is included in this overview. For the function of a beta-blocker, the compound contains an aromatic ring and a β-ethanolamine. The aromatic ring can be either benzo-heterocyclic (such as indole) or heterocyclic (such as thiadiazole). Moving the acyl-amino group to meta- or ortho-positions, on the benzene ring, causes a loss of selectivity but not loss of the β-blockade. This pinpoints the significance of para-substitution for beta-1 selectivity of beta-blockers. Side chains can either be directly linked to the aromatic ring or linked through a —OCH2— group and change the properties of the molecule explaining the intrinsic sympathomimetic (agonist) activity or changing the lipophilic properties. Concomitant alpha-blocking or nitrergic activities and beta-2 agonist activities contribute to vasodilation. Upper left box: Beta-1 selective atenolol (At), betaxolol (Be), bisoprolol (Bi), esmolol (Es), metoprolol (Me), and nebivolol (Ne), which has also nitrergic effects. Lower left box: Beta-1 selective with intrinsic sympatho-mimetic (agonist) activity – celiprolol (Ce, beta-2 agonist), acebutolol (Ac), and xamoterol (Xa). Upper right box: Beta unselective with intrinsic-sympatho-mimetic (agonist) activity – carteolol (Cart), bucindolol (Bu), alprenolol (Al), oxprenolol (O), penbutolol (Pe), and pindolol (Pi). Centre box: alpha and beta-unselective carvedilol (Carv) and labetalol (La). Lower right box: non-selective nadolol (Na), propranolol (Pr), sotalol (So) and timolol (Ti).
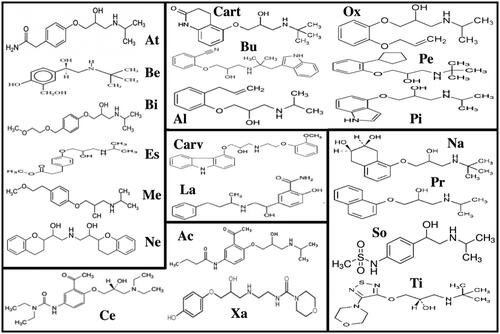
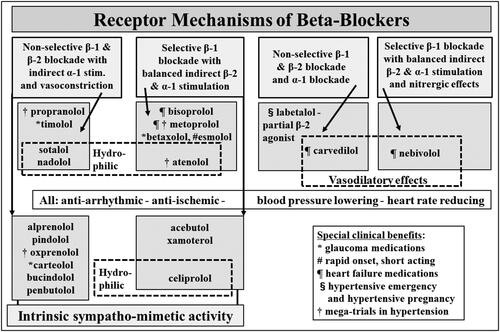
Figure 3. Diversity of the beta-blockers. Beta-adrenoceptor blocker diversity is represented in differences in beta-receptor selectivity of the blockade, the presence or absence of intrinsic sympathomimetic activity, the existence of dual alpha-beta blockade in some cases, differing lipophilicity, and a vascular vasodilatory action possessed by some beta blockers.
This position is manifest by reference to another clinical context, beta-blocker treatment of heart failure, where unequivocally there is no class effect [Citation29]. In the 1990s there was a strong theoretical foundation for evaluating beta-blockers in patients with heart failure. Noradrenaline release from the cardiac sympathetic nerves in heart failure is largely increased, downregulating myocardial β-1 adrenoceptors [Citation29]. The activation of the cardiac sympathetic outflow was a demonstrated mortality factor [Citation30]. Bisoprolol, carvedilol, metoprolol, and nebivolol investigated in large RCTs also appeared to reduce hospitalisation and prolong survival [Citation29]. However, this was not a class effect holding for all beta-blockers. When evaluated in RCTs, bucindolol and xamoterol were not beneficial [Citation29], the explanation apparently being that these drugs possess intrinsic sympatho-mimetic (agonist) activity, which causes cardiac stimulation and accounts for the unfavourable outcome. Analogous differences in beta-blocker efficacy are likely in hypertension also, with major within drug class differences in the beta-blockade selectivity, the presence or absence of ancillary properties (such as combined α-blockade and intrinsic agonist activity) and diverse pharmacokinetic properties. Regarding commonly used beta-blockers in hypertension such as propranolol and atenolol, preliminary studies were done in heart failure but mortality benefits have not been shown in heart failure patients with propranolol and atenolol.
Properties of beta-blockers
As a drug class beta-blockers show remarkable heterogeneity ( and Citation3, ), beyond their common facility to competitively antagonise β-adrenoceptors [Citation31,Citation32]. The adrenoceptor blockade can be selective or non-selective, it can co-exist with intrinsic adrenoceptor stimulation (agonist activity), be combined with α-adrenergic blockade, and be associated with nitric oxide (NO) release [Citation31–33]. These ancillary properties confer a direct vasodilator action with some beta-blockers [Citation34]. The drug molecule can be lipophilic or hydrophilic, and have short or long half-life. This contrasts with the much more homogeneous pharmacological characteristics of the angiotensin converting enzyme (ACE) inhibitors, the angiotensin receptor blockers (ARB) and the dihydropyridine calcium channel blocking (CCB) drug classes.
Table 1. Overview of beta-blockers and properties with the various compounds.
Blood pressure lowering and vasodilator properties of beta-blockers
The mechanisms by which beta-blockers lower BP are partly unknown but several modes of action are likely to be involved [Citation35]. Beta-blockers reduce cardiac output by their negative chronotropic and inotropic effects. This is, however, accompanied by a reflex increase of peripheral resistance, which minimises acute BP reductions. Long-term lowering of BP occurs because of late reduction of peripheral vascular tone that may occur due to the resetting of the baroreflex mechanism. This implies that all beta-blockers may have a direct or indirect vascular effect when administered chronically. A further mechanism includes inhibition of renin secretion from the kidneys with a subsequent decrease in plasma angiotensin II; and perhaps a central reduction in sympathetic outflow leading to reduction of vasomotor tone. In addition, some beta-blockers exhibit unique direct systemic vasodilator actions [Citation34] which leads to a reduction of systemic vascular resistance and afterload to the heart, preventing a reduction of cardiac output and almost entirely accounting for the BP fall [Citation36]. The underlying mechanism of the vasodilator property of the third generation of beta-blockers can be a consequence of intrinsic ß-agonist activity, α-adrenergic blockade, and nitric oxide bioavailability via nitric oxide synthase activation, and/or reduction in oxidative stress [Citation35,Citation36]. An important common favourable consequence, among others, of the vasodilator actions of these beta-blockers is an increase of skeletal muscle blood flow and improved microcirculation. This facilitates distribution of glucose and insulin to skeletal muscle cells, preventing the increase in insulin resistance seen with conventional beta-blockers, in particular, with β-2 adrenergic blockade [Citation31,Citation32,Citation34].
Beta-1 selectivity
Acebutolol, atenolol, bisoprolol, esmolol, metoprolol and nebivolol are relatively β1-selective (cardio-selective) beta-blockers [Citation31,Citation32], which minimises increase in airways resistance and arteriolar resistance in skeletal muscle. Consequently, tolerability in patients with chronic obstructive pulmonary disease is improved and adverse metabolic changes are limited or even absent. The beta-1 selectivity of atenolol is rather weak and atenolol may appear more like the unselective propranolol regarding some of these effects.
Beta-blockers with intrinsic agonist activity (sympathomimetic) properties
Some beta-blockers (acebutolol, celiprolol, labetalol, oxprenolol and pindolol) cause beta-adrenoceptor stimulation (variously β-1 or β-2), in parallel with β-receptor blockade. Beta-blockers with intrinsic agonist activity lower heart rate less than other beta-blockers and exhibit direct vasodilator actions [Citation31,Citation32,Citation37].
Beta-blockers with additional alpha-beta adrenergic blockade
Carvedilol and labetalol are combined alpha-beta adrenergic blockers [Citation32,Citation34]. The α-adrenergic blocking activity underlies their direct vasodilator action and at higher doses can cause postural hypotension, not seen with other beta-blockers. While the use of labetalol, due to its complex pharmacology and short half-life, is restricted to hypertensive emergencies and urgencies [Citation1,Citation2], carvedilol is used for chronic treatment of hypertension and exhibits additional beneficial effects by decreasing oxidative stress, which contributes to its vasodilator action [Citation33].
Beta-blocker with activation of nitric oxide
Nebivolol is a unique beta-blocker due to its potential to activate endothelial nitric oxide synthase (eNOS) [Citation33,Citation38], which contributes to its direct vasodilator effect. It seems now established that the activation of eNOS by nebivolol is caused by the activation of β-3 adreno-receptors [Citation39].
Pharmacokinetics of beta-blockers
Atenolol and metoprolol have a relatively short half-life and duration of action, which limits their use with once-daily dosing [Citation31,Citation32]. However, it has been customary to prescribe atenolol as a once-daily dose, and this inappropriate use has extended to RCTs. Unlike atenolol, metoprolol developed as extended-release succinate formulation, which overcomes this disadvantage and provides the rationale for once-daily use [Citation9,Citation25,Citation26].
Solubility characteristics
Beta-blockers differ in lipid solubility. Hydrophilicity does not appear to have an impact on efficacy, but it reduces penetration in the brain, and reduces the likelihood of disturbed dreaming as an adverse effect.
Prediction of how these differences in beta-blocker properties might impact efficacy and safety
These described differences in beta-blockers influence their BP lowering efficacy, most likely impact cardiovascular protection during the treatment of hypertension, and determine the tolerability profile of individual agents.
Blood pressure lowering
Short-acting beta-blockers inappropriately prescribed with once-daily dosing may not provide adequate 24-hour BP control. The differences in BP control appear to be subtle and difficult to be detectable in clinical practice or even in trials. This is not the case for the reduction in central BP [Citation40]. Thus, there is strong evidence of a greater reduction in central arterial BP with direct vasodilator beta-blockers than with other conventional beta-blockers perhaps due to a lesser amplification of the pulse pressure wave [Citation34,Citation40]. This is probably important, and the lesser reduction in stroke incidence observed in atenolol trials [Citation41] has been attributed to the lack of a direct vasodilator action and a smaller reduction in central BP that characterises the use of the drug.
Cardiovascular protection in hypertension with elevated heart rate
The choice of beta-blocker in hypertension with elevated heart rate, namely heart rate >80 beats/min [Citation1] and not necessarily tachycardia, may be critically important. As discussed, heart rate reduction in those hypertensive patients with an elevated heart rate has become a therapeutic target [Citation1]. The heart rate increase in hypertension is driven by chronic activation of the cardiac sympathetic outflow [Citation23,Citation24], which is adverse in other conditions, e.g. chronic kidney disease [Citation42] and a post-stroke state, and has been linked with reduced survival in heart failure patients with reduced ejection faction [Citation43] and in heart failure patients with preserved ejection fraction [Citation44]. Given the negative experience from heart failure RCTs, beta-blockers with intrinsic agonist activity certainly should be avoided in patients with a heart rate >80 beats/min, as they lower heart rate less than other beta-blockers [Citation37], and cause some cardiac β-adrenoceptor stimulation which maybe also harmful in patients without heart failure.
Adverse effects
The side effect profile of the various beta-blockers relates to individual drug properties. β-1 selective agents minimise risk of bronchospasm. Non-selective beta-blockers reduce skeletal muscle blood flow, increase insulin resistance and can have other adverse metabolic effects like lowering of HDL cholesterol and increasing triglycerides. Vasodilator beta-blockers minimise these adverse effects. Combined alpha-beta blockers can cause postural hypotension at higher dose. Risk of excessive heart rate slowing is limited with sympathomimetic beta-blockers; i.e. pindolol is frequently used in Australia if beta-blockade causes bradycardia or Raynaud’s phenomenon.
Contrasting state of knowledge for beta-blocker treatment of hypertension, and heart failure
There is no class effect for beta-blockers in heart failure [Citation29]. Which are good beta-blockers for heart failure and which are not so good is evident because of RCTs with several individual drugs. The differences between individual beta-blocking drugs are such that there will probably be good and not so good beta-blockers also for hypertension, but this is not known with certainty because RCTs have not been performed for all the different drugs or drugs sub-classes [Citation45].
CCB prescribing in hypertension in the 1990s had a parallel when there were statements concerning imagined dihydropyridine CCB risks. These damaging speculations became quiet when the RCT results showed benefits. The lesson from this example is not to draw conclusions without RCTs while for beta-blockers is not to over-interpret the data from trials based on some drugs only.
Similarly, for beta-blocker use in hypertension, there is a need for a new starting point, which acknowledges that it is unknown whether bisoprolol, carvedilol, labetalol (not available in Germany due to potential hepatotoxicity) and nebivolol will prove to be “good” drugs in hypertension, superior to propranolol and atenolol, and equally good as other commonly used and RCT documented antihypertensive drugs. Individual RCTs are ideally required to establish this point. Meanwhile, we hypothesise that because their pharmacological properties differ from those of propranolol and atenolol in a favourable way, they are most likely better antihypertensive drugs. This said, a recent large-scale observational study [Citation46] indicated no differences in the effectiveness and safety between atenolol and third-generation β-blockers though an observational study does not provide a definitive last word on this important matter.
Beta-blocker treatment was associated with decreased risk for stroke compared with placebo [Citation45]. However, in RCTs beta-blockers proved to be less effective in this outcome than other major antihypertensive agents, especially calcium channel blockers. This large observational study [Citation46] also showed that all beta-blockers had negative effects on stroke irrespective of their characteristics, with the well-known limitations of an observational study.
No class effect with beta-blocker? Insight into new pharmacological concept revisiting old beliefs
The current belief is that an endogenous ligand (adrenaline) binds to β-adrenoceptor in order to induce expected cardiovascular effects on both the heart and vessels. This dogma may falter with the following concepts:
Receptors can be activated independently from the ligand, the so-called ligand-independent activation. This has until now been known as inverse agonism. By simply stretching the cardiomyocyte or vascular wall, β-adrenoceptors can be activated, and this effect can be modulated by beta-blockers. Moreover binding of beta-blockers on the receptor, independent of ligands, can activate the transduction pathway, thus inducing a pharmacodynamic effect. This leads to the revolutionary concept that beta-blockers can have actions independent of a ligand-β-adrenoceptor mediated effect. Beta-blockers can counteract the effect of the endogenous ligand by inhibiting its binding to a β-adrenoceptor but can also per se induce an effect. This effect could be different from one beta-blocker to another, again showing the absence of a class effect. β-Arrestins are ubiquitously expressed proteins that mediate G-protein coupled receptor desensitisation, internalisation, and ubiquitination. β-Arrestins are also important intracellular scaffold proteins that function as signal transducers to initiate signalling cascades independent of, or collaboratively with G proteins downstream of G-protein coupled receptor activation [Citation47,Citation48]. Carvedilol is a commonly used beta-blocker displaying biased signalling properties as a functional antagonist for G protein-mediated signalling while simultaneously functioning as an agonist for β-Arrestins-mediated signalling. Other β-blockers, such as metoprolol and nadolol, function as classical antagonists for both G protein and β-Arrestins-dependent signalling [Citation49,Citation50].
G-protein coupled receptors exist within the membrane as the monomer (classical concept) but receptors can bind to other receptors from the same classes building up homodimer (i.e. beta-1/beta-2 – adrenoceptor) or from different classes leading to the formation of the heterodimer (i.e. alpha-2 – adrenoceptor/angiotensin-1 – receptor). These receptors can be activated through their native ligands but also independently from ligands through the mechanisms described above. The same ligand will not display the same effect when binding to the native monomer or to a homo/heterodimer. Hence, a beta-blocker agent could activate a monomer, a homodimer or a heterodimer and again this varies between beta-blockers. This could lead to signal alteration or modification.
Heterodimer can lead to a completely different response from the one expected when the natural ligands bind to the “classical” receptor. Hence, according to the presence of one or another ligand or both ligands the activated transduction pathway could be completely different. Finally, heterodimer like a classical receptor could be activated in the presence or in the absence of native ligands, but simple beta-blocker binding on these receptors could also induce similar or different effects.
These new pharmacological concepts also show that beta-blockers can act in a totally different fashion according to their target and that the concept that beta-blockers only antagonise the effect of a native endogeneous ligand is not anymore valid. Beta-blockers represent a highly heterogenous class of drugs with regard to these new pharmacodynamic properties, beyond classical characteristics such as selectivity, specificity, and intrinsic sympathomimetic effects. In a future perspective this may help to proceed along the personalised therapeutic approach that is now pursued by current medicine, with particular relevance to hypertension and heart failure. Finally, these new pharmacological aspects and mechanisms of action also suggest that there will probably be a revival of drugs acting on SNS.
Targeting the sympathetic neural pathophysiology of hypertension with beta-blockers
Although RCTs provide the foundational basis for determining the efficacy and safety of antihypertensive drugs, knowledge of hypertension pathogenesis and pathophysiology can inform decision-making in therapy. In another context, knowledge of pathophysiology provided the theoretical underpinning for the testing and proof of the efficacy of beta-blockers in heart failure. With this background, the relevance of the sympathetic neural pathophysiology of hypertension to beta-blocker therapy needs subsequent analysis.
The sympathetic neural pathophysiology of essential hypertension
SNS activation is common in essential hypertension, acting to initiate and sustain the BP elevation [Citation23,Citation24,Citation51–56]. The prevalence of sympathetic activation depends on age and the severity of the hypertension, but by the best estimate is present in not less than 50% of patients [Citation57]. With application of state of the art methodology, sympathetic nerve recording by microneurography and the noradrenaline spill-over technique, the sympathetic activation is involved in the sympathetic outflows to the heart, kidneys and skeletal muscle vasculature, but not the sympathetic outflow to the skin [Citation23,Citation24,Citation52–55]. There is evidence that the activation of the renal sympathetic outflow is pivotal in hypertension pathogenesis [Citation58].
The probable contribution of chronic sympathetic activation to cardiovascular risk, and to clinical cardiovascular endpoints in hypertension
Persisting activation of SNS can be markedly adverse. The prime example is cardiac failure but evidence of a negative prognostic impact is also seen in end-stage renal disease and in the post-stroke state [Citation58]. Perhaps the same might apply to essential hypertension, which overall is also characterised by ongoing SNS activation, although of lower grade than in heart failure [Citation23,Citation30,Citation54].
Sympathetic activation can be unbundled into its individual elements:
Which organ-specific sympathetic outflows are stimulated (heart, kidneys?)
What are the firing characteristics of single sympathetic fibres?
Is sympathetic nerve reuptake of released noradrenaline normal?
Endothelial damage
Endothelial damage is a progenitor of atherosclerosis, but it is under debate whether SNS is modulating endothelial function. In free-moving hypertensive rats, the vasodilator and pressor effects of removing endothelial influences by L-MNNA were unaffected by total sympathectomy suggesting no endothelial dependency on sympathetic activity [Citation61]. However, in humans, chronic sympathetic activation can cause damage to the vascular endothelium, as deterioration in endothelial function with ageing runs in parallel with the age-related SNS activation [Citation62]. Furthermore, in young people, multiunit sympathetic nerve firing in the outflow to the skeletal muscle vasculature of the leg quantitatively links to measures of endothelial dysfunction [Citation63]. Conversely, in patients with the metabolic syndrome, inhibition of sympathetic outflow with the imidazoline antihypertensive, moxonidine, reverses endothelial dysfunction [Citation64].
Atherogenesis and arterial distensibility
The most direct evidence that SNS activation contributes to the development of atherosclerosis is provided by experimental studies utilising animal models, often diet-induced, where an inhibitory effect of a regional sympathectomy on atherosclerosis development in a particular arterial territory is demonstrated. There exist several classical studies of this type in rhesus monkeys fed an atherogenic diet [Citation65], in which surgical thoracic sympathectomy prevented atherosclerosis development in the carotid arteries and thoracic aorta. In addition, data in experimental animals and man show that SNS reduces arterial distensibility, a stiffening influence that, by increasing the traumatic effect of intravascular pressure on the vessel wall, favours atherosclerosis [Citation58].
Left ventricular hypertrophy (LVH)
The primary contributor to the development of LVH in essential hypertension is increased left ventricular impedance, or thoracic electrical bio-impedance, measured by electrical impedance plethysmography. An effect additional to this is the trophic action of SNS on myocardial growth. Noradrenaline stimulates cardiac myocyte growth in tissue culture. In patients with essential hypertension, left ventricular mass correlates directly with cardiac sympathetic activity quantified with cardiac noradrenaline spill-over measurements [Citation66]. This appears to represent a causal relation, with high cardiac sympathetic activity contributing to LVH, as left ventricular mass is unrelated to sympathetic outflow to another field, the skeletal muscle vasculature, measured by microneurography [Citation65]. The ARB losartan induced a strong regression of ECG-LVH, but a major regression of ECG-LVH also appeared with atenolol treatment [Citation5].
Ventricular and atrial arrhythmias
Sympathetic neural mechanisms are most likely to generate ventricular arrhythmias when coupled with coronary artery stenosis and myocardial ischaemia, common in hypertensive patients. This can represent a triggered phenomenon, deriving from the accentuated, high level of cardiac sympathetic activation accompanying mental stress or strenuous exercise [Citation67]. Atrial fibrillation commonly develops in the course of essential hypertension. The evidence is strong that atrial fibrillation is often initiated by an episode of SNS activation [Citation68].
An elevated heart rate in hypertension is a biomarker of SNS activation
One deficiency in the human cardiovascular neuroscience advances of the past three decades has been the inability to develop a simple, clinically accessible biomarker of SNS activation. In heart failure, this need is no longer pressing. Ongoing sympathetic activation exists in heart failure and is detrimental, this being foundational for the beta-blocking drug testing which ultimately led to this therapy being included in international guidelines; no sympathetic nervous biomarker is needed in heart failure to guide beta-blocker therapy. The situation is different in essential hypertension, where a need for an easily accessible sympathetic biomarker exists. An elevated heart rate in hypertension, which is common, might serve as such a sympathetic biomarker. Elevated heart rate in essential hypertension is predictive of reduced survival [Citation69,Citation70]. Accordingly, testing the mechanism of an elevated heart rate becomes doubly important, to evaluate heart rate as a sympathetic biomarker, and further, to better understand the mechanism of cardiac risk conveyed by hypertension tachycardia.
An elevated heart rate in hypertension could derive from any or all of these: activation of the cardiac sympathetic outflow, withdrawal of cardiac vagal activity, high intrinsic heart rate, anxiety and adrenaline stimulation of the heart. Of note, the importance of SNS activation in causing hypertension tachycardia has very recently been established [Citation23,Citation24], in two independent studies published concurrently. In one, the sympathetic outflow to the heart was measured directly with the cardiac noradrenaline spill-over method [Citation24]. Heart rate and cardiac noradrenaline spill-over correlated closely, with 67% of the variance in heart rate being attributable to differences in cardiac sympathetic activity [Citation24]. In the second study, heart rate values greater than 80 beats per minute, the cut-off value in the ESC/ESH guidelines [Citation1], were linked with increased sympathetic cardiovascular drive, as assessed by the microneurography method for measuring the sympathetic outflow to the skeletal muscle vasculature [Citation23]. In other words, an elevated heart rate in hypertension is a biomarker for the presence of SNS activation.
Beta-blockers for heart rate reduction in hypertension
Resting heart rate is elevated in a substantial proportion of patients with essential hypertension, particularly young or middle-aged patients with the mild-to-moderately severe disease having so-called hyperkinetic circulation [Citation24,Citation71]. This characteristic is not simply an expression of anxiety [Citation24]. An elevated heart rate in essential hypertension is predictive of reduced survival [Citation69,Citation70]. The 2018 ESC/ESH Guidelines [Citation1] do note that such patients require control of heart rate with beta-blockade. Elevated heart rate in hypertensive patients increases cardiac work and oxygen demand, augments arterial wall stress, decreases arterial distensibility and facilitates plaque disruption [Citation72]. Taken together, these adverse effects at least partly explain why increased heart rate represents an independent risk factor in the hypertensive population, associated with an elevated probability of development of fatal and nonfatal cardiovascular events, as well as hypertension-mediated organ damage.
However, the relation of an increased heart rate in hypertension to cardiovascular risk is likely to be more complex than this direct interpretation, now when assured that an elevated heart rate in hypertension is a biomarker of sympathetic nervous activation [Citation23,Citation24]. Chronic high-level activation of the cardiac sympathetic outflow can be a lethality factor [Citation59]. It is possible that the linking of higher heart rate with survival in hypertension is to some extent an epiphenomenon, deriving from the underlying toxicity of chronic sympathetic activation, the latter flagged by the elevated heart rate [Citation24]. This is an important point, as it determines whether specific pharmacological antagonism of SNS is needed in hypertensive patients with an elevated heart rate, or alternatively, whether heart rate reduction alone is sufficient, using a drug such as the if inhibitor ivabradine? We believe heart rate reduction achieved through beta-adrenergic blockade is required, this drug class additionally provides the accompanying benefit of sympathetic antagonism, in additional to heart rate slowing.
Prescribing of beta-blockers for disorders comorbid with hypertension
Most hypertension guidelines give some recognition to the need to prescribe beta-blockers for illnesses comorbid with hypertension. Blood pressure lowering from the beta-blocker then becomes an ancillary/secondary benefit. For example, the 2018 ESC/ESH Hypertension Guidelines stated the following recommendations regarding the usage of a beta-blocker [Citation1]. “Beta-blockers have been shown to be particularly useful for the treatment of hypertension in specific situations such as symptomatic angina, for heart rate control, post-myocardial infarction, heart failure with reduced ejection fraction (HFrEF) and as an alternative to ACE inhibitors or ARBs in younger hypertensive women planning a pregnancy or of child-bearing potential.” Additionally, the beta-blocker treatment appeared in the text describing the prevention and treatment of atrial fibrillation and in cases of aortic dissection.
The listing of comorbid beta-blocker prescribing in hypertension guidelines is without exception far less than the comorbid prescribing which actually exists in clinical practice, where fully 50 instances are noted [Citation73]. The list encompasses cardiac ischaemic diseases (post-myocardial infarction, angina pectoris, acute coronary syndromes), heart failure with reduced ejection fraction, hypertrophic cardiomyopathy, cardiac arrhythmias (prophylaxis and treatment of atrial fibrillation, long QT syndrome, supraventricular tachycardia, ventricular tachyarrhythmias, idiopathic sinus tachycardia), perioperative dosing (cardiac surgery, major non-cardiac surgery), non-cardiac vascular diseases (postural tachycardia syndrome -POTS, portal hypertension and oesophageal varices, dissecting aneurysm, thoracolumbar aortic aneurysm), general medical (migraine headache, hyperthyroidism, essential tremor, glaucoma), and psychiatry prescribing (panic disorder, post-traumatic stress disorder, performance anxiety, generalised anxiety disorders).
Beta-blocker therapy for HFpEF investigated in RCTs is almost missing, but the vast majority of patients with this form of heart failure get beta-blocker on clinical indications (). The explanation is likely that most of the patients have hypertension and large fractions of the patients have additional coronary heart disease, atrial fibrillation and diabetes mellitus. On May 5th., this year, a press release announced that the RCT with the acronym DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) was positive for the primary endpoint. The sodium glucose cotransporter-inhibitor (SGLT2i), investigated in the study, prevented both cardiovascular death and worsening of heart failure compared to placebo on top of quite extensive therapy with other heart failure medications in 6263 randomised patients with ejection fraction >40%. Of these patients 89% had a history of hypertension, and more than half of them had a history of coronary heart disease and the same for atrial fibrillation. As many as 76% of these patients were on treatment with beta-blocker at baseline [Citation74], the most commonly used BP lowering drug in these heart failure patients [Citation75].
Table 2. Overview of the use of beta-blockers on clinical indications in the randomised clinical trials of patients with heart failure with preserved ejection fraction (HFpEF).
Guidelines should represent current clinical practice, because hypertension comorbidity prescribing of beta-blocker moves the drug to first-line prescribing, and depending on the accompanying BP fall, can mean the beta-blocker comes to replace one or more of the antihypertensive drugs (ACEi, ARB, diuretic, CCB) given priority in the guidelines.
Summary and conclusion
Beta-blockers have multiple applications in medicine. Many of the illnesses for which they are prescribed and of documented value commonly coexist with hypertension. The listing of comorbid beta-blocker prescribing in hypertension guidelines is without exception far less than the comorbid prescribing which actually exists in clinical practice, where fully 50 instances are noted [Citation73]. Guidelines should better represent this current clinical practice, because hypertension comorbidity prescribing of beta-blocker moves the drug to de-facto first-line prescribing, and depending on the accompanying BP fall, can mean that the beta-blocker can come to replace one or more of the antihypertensive drugs given priority in the guidelines. We have illustrated this principle in in which treatment of hypertension with beta-blocker (and of heart failure) defends a corner in the hexagon of first-line drug choices [Citation73].
Figure 4. Hexagon shows suitable first-line drugs in the treatment of hypertension. Solid lines connect drug classes in combinations documented to prevent cardiovascular complications in hypertension or hospitalisation and death in randomised placebo controlled clinical trials in patients with heart failure with predominantly hypertensive aetiology. ACEi: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNi: angiotensin receptor blocker neprilysin inhibitor; MRA: mineralocorticoid receptor antagonist; SGLT2i: sodium glucose cotransporter inhibitor; DH-CCB: dihydropyridin calcium channel blocker (calcium-antagonist)
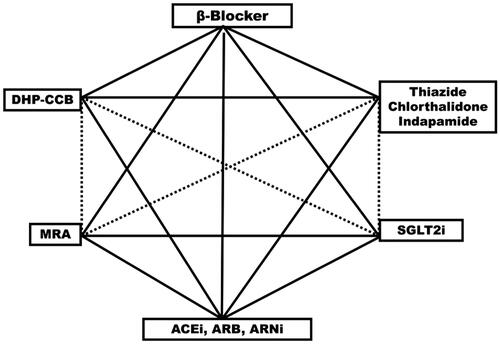
We conclude that these observations, in totality, inform our opinion that beta-blockers as a group are an alternative, first choice for hypertension treatment in clinical practice, and should be recognised as such in clinical guidelines – again, as illustrated in . We consider bisoprololol, sustained release metoprolol and nebivolol to be the beta-blockers with greater selectivity and longer plasma half-life and thus with advantages that are needed in the treatment of hypertension and heart failure.
Acknowledgements
The authors acknowledge that Centre Hospitalier Princesse Grace, Monte Carlo, Monaco generously covers the journal publishing charges.
Disclosure statement
Over the last 3 years, M. Esler reports honoraria for participating in the Renal Denervation advisory boards of Medtronic (USA) and SyMap (China). S.E. Kjeldsen reports lecture honoraria from Getz Pharma, Merck Healthcare KGaA, Sanofi-Aventis and Vector-Intas. A. Pathak has received honoraria from Ablative Solution, Air Liquide, Astra Zeneca, Boehringer Ingelheim, Medtronic, Menarini, Merck, Novartis, Recordati, Recor Medical, Sanofi and Servier. G. Grassi has received honoraria for lectures from Medtronic, Merck Healthcare KGaA and Servier. R. Kreutz reports support for research by Bayer, honoraria for lectures from Bayer, Berlin-Chemie/Menarini, Daiichi Sankyo, Ferrer, Merck, Sanofi and Servier. G. Mancia has received honoraria from Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Menarini, Merck, Novartis, Recordati, Sandoz, Sanofi and Servier.
Additional information
Funding
References
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
- Guideline for the pharmacological treatment of hypertension in adults. Geneva: World Health Organization; 2021.
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357.
- Jones NR, McCormack T, Constanti M, et al. Diagnosis and management of hypertension in adults: NICE guideline update 2019. Br J Gen Pract. 2020;70(691):90–91.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003.
- Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. For the INVEST investigators. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290(21):2805–2816.
- Wilhelmsen L, Berglund G, Elmfeldt D, et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens. 1987;5(5):561–572.
- Wikstrand J, Warnold I, Tuomilehto J, et al. Metoprolol versus thiazide diuretics in hypertension. Morbidity results from the MAPHY study. Hypertension. 1991;17(4):579–588.
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589.
- Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354(9192):1751–1756.
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J. 1985;291:97–104.
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs–overview and meta-analyses. J Hypertens. 2015;33(2):195–211.
- Turnbull F, Neal B, Ninomiya T, et al. For the Blood Pressure Lowering Treatment Trialists' Collaboration (BPLTTC). Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. Br Med J. 2008;336:1121–1123.
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967.
- Thomopoulos C, Bazoukis G, Tsioufis C, et al. Beta-blockers in hypertension: overview and meta-analysis of randomized outcome trials. J Hypertens. 2020;38(9):1669–1681.
- Turnbull F, Neal B, Ninomiya T, et al. For the Blood Pressure Lowering Treatment Trialists' Collaboration (BPLTTC). blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–598.
- Mancia G, Zanchetti A. Choice of antihypertensive drugs in the European Society of Hypertension-European Society of Cardiology guidelines: specific indications rather than ranking for general usage. J Hypertens. 2008;26(2):164–168.
- Riemer TG, Villagomez Fuentes LE, Algharably EAE, et al. Do β-blockers cause depression? Systematic review and meta-analysis of psychiatric adverse events during β-blocker therapy. Hypertension. 2021;77(5):1539–1548.
- Farmakis IT, Pyrgidis N, Doundoulakis I, et al. Effects of major antihypertensive drug classes on erectile function: a network meta-analysis. Cardiovasc Drugs Ther. 2021:1–12.
- Espinola-Klein C, Weisser G, Jagodzinski A, et al. β-Blockers in patients with intermittent claudication and arterial hypertension: results from the nebivolol or metoprolol in arterial occlusive disease trial. Hypertension. 2011;58(2):148–154.
- Yang Y-L, Xiang Z-J, Yang J-H, et al. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2020;41(46):4415–4422.
- Grassi G, Quarti-Trevano F, Seravalle G, et al. Association between the European Society of Cardiology/European Society of Hypertension heart rate thresholds for cardiovascular risk and neuroadrenergic markers. Hypertension. 2020;76(2):577–582.
- Esler M, Lambert G, Esler D, et al. Evaluation of heart rate as a sympathetic nervous system biomarker in essential hypertension. J Hypertens. 2020;38(8):1488–1495.
- Wikstrand J, Warnold I, Olsson G, et al. Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA. 1988;259(13):1976–1982.
- Olsson G, Tuomilehto J, Berglund G, et al. Primary prevention of sudden cardiovascular death in hypertensive patients. Mortality results from the MAPHY study. Am J Hypertens. 1991;4(2 Pt 1):151–158.
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta-blocker oxprenolol: the international prospective primary prevention study in hypertension (IPPPSH). J Hypertens. 1985;3:379–392.
- Kjeldsen SE, Eide I, Leren P, et al. The effect on HDL cholesterol of oxprenolol and atenolol. Scand J Clin Lab Invest. 1982;42(5):449–453.
- Grassi G, Mancia G, Esler M. Central and peripheral sympathetic activation in heart failure. Cardiovasc Res. 2022;118(2):1857–1871.
- Kaye DM, Lefkovits J, Jennings GL, et al. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26(5):1257–1263.
- Frishman WH. β-adrenergic blockade in cardiovascular disease. J Cardiovasc Pharmacol Ther. 2013;18(4):310–319.
- do Vale GT, Ceron CS, Gonzaga NA, et al. Three generations of β-blockers: class differences and clinical applicability. Curr Hypertens Rev. 2019;15(1):22–31.
- Vanhoutte PM, Gao Y. Beta blockers, nitric oxide, and cardiovascular disease. Curr Opin Pharmacol. 2013;13(2):265–273.
- Pucci G, Ranalli MG, Battista F, et al. Effects of β-blockers with and without vasodilating properties on central blood pressure. Systematic review and meta-analysis of randomized trials in hypertension. Hypertension. 2016;67(2):316–324.
- Kreutz R. Algharably EAe-H. In: Offermanns S, and Rosenthal W eds. Encyclopedia of molecular pharmacology. Cham: Springer International Publishing; 2021. p. 165–174.
- Seleme VB, Marques GL, Mendes AEM, et al. Nebivolol for the treatment of essential systemic arterial hypertension: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2021;21(2):165–180.
- Kostis JB, DeFelice EA, Frishman W, et al. Clinical relevance of intrinsic sympathomimetic activity of beta-blockers. Angiology. 1981;32(11):780–787.
- Ritter JM. Nebivolol: endothelium-mediated vasodilating effect. J Cardiovasc Pharmacol. 2001;38(Suppl 3):S13–S16.
- Schnabel P, Maack C, Mies F, et al. Binding properties of beta-blockers at recombinant beta1-, beta2-, and beta3-adrenoceptors. J Cardiovasc Pharmacol. 2000;36(4):466–471.
- McEniery CM, Cockcroft JR, Roman RJ, et al. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35(26):1719–1725.
- Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225.
- Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–1814.
- Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372(9641):817–821.
- Kapoor JR, Heidenreich PA. Heart rate predicts mortality in patients with heart failure and preserved systolic function. J Heart Failure. 2010;16(10):806–811.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.
- Chan You S, Krumholz HM, Suchard MA, et al. Comprehensive comparative effectiveness and safety of first-line β-blocker monotherapy in hypertensive patients: a large-scale multicenter observational study. Hypertension. 2021;77(5):1528–1538.
- Thomsen ARB, Plouffe B, Cahill TJ, et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166(4):907–919.
- Wang J, Hanada K, Staus DP, et al. Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat Commun. 2017;8(1):1706.
- Kim IM, Tilley DG, Chen J, et al. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA. 2008;105(38):14555–14560.
- Wisler JW, DeWire SM, Whalen EJ, et al. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104(42):16657–16662.
- Esler M, Jennings G, Korner P, et al. The assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11(1):3–20.
- Anderson EA, Sinkey CA, Lawton WJ, et al. Elevated sympathetic nerve activity in borderline hypertensive humans: evidence from direct intraneural recordings. Hypertension. 1989;14(2):177–183.
- Yamada Y, Miyajima E, Tochikubo O, et al. Age-related changes in muscle sympathetic nerve activity in essential hypertension. Hypertension. 1989;13(6 Pt 2):870–877.
- Schlaich MP, Lambert E, Kaye DM, et al. Sympathetic nervous augmentation in essential hypertension: role of nerve firing, norepinephrine reuptake and angiotensin neuromodulation. Hypertension. 2004;43(2):169–175.
- Grassi G, Colombo M, Seravalle G, et al. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension. 1998;31(1):64–67.
- Grassi G, Dell’Oro R, Quarti-Trevano F, et al. Neuroadrenergic and reflex abnormalities in patients with the metabolic syndrome. Diabetologia. 2005;48(7):1359–1365.
- Esler M, Lambert E, Schlaich M. Point: counterpoint. Chronic activation of the sympathetic nervous system is the dominant contributor to systemic hypertension. J Appl Physiol. 2010;109(6):1996–1998.
- DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R245–R253.
- Esler M, Lambert G, Schlaich M, et al. Obesity paradox in hypertension. Is this because sympathetic activation in obesity-hypertension takes a benign form? Hypertension. 2018;71(1):22–33.
- Esler M. Sympathetic activation in essential hypertension: understanding the toxic trifecta. Heart Lung Circ. 2018;27(3):271–273.
- Radaelli A, Mircoli L, Mori I, et al. Nitric oxide-dependent vasodilation in young spontaneously hypertensive rats. Hypertension. 1998;32(4):735–739.
- Kaplon RE, Walker AE, Seals DR. Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. J Appl Physiol. 2011;111(5):1416–1421.
- Sverrisdottir YB, Jansson LM, Hagg U, et al. Muscle sympathetic nerve activity is related to a surrogate marker of endothelial function in healthy individuals. PLOS One. 2010;5(2):e9257.
- Topal E, Cikim AS, Cikim K, et al. The effect of moxonidine on endothelial function in metabolic syndrome. Am J Cardiovasc Drugs. 2006;6:343–348.
- Lichtor T, Davis HR, Johns L, et al. The sympathetic nervous system and atherosclerosis. J Neurosurg. 1987;67(6):906–914.
- Schlaich MP, Kaye DM, Lambert E, et al. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108(5):560–565.
- Esler M. Mental stress and human cardiovascular disease. Neurosci Biobehav Rev. 2017;74(Pt B):269–276.
- Shivkumar K, Ajijola OA, Anand I, et al. Clinical neurocardiology – defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016;594(14):3911–3954.
- Okin PM, Kjeldsen SE, Julius S, et al. All-cause and cardiovascular mortality in relation to changing heart rate during treatment of hypertensive patients with electrocardiographic left ventricular hypertrophy. Eur Heart J. 2010;31(18):2271–2279.
- Julius S, Palatini P, Kjeldsen SE, et al. Usefulness of heart rate to predict cardiac events in treated patients with high-risk systemic hypertension. Am J Cardiol. 2012;109(5):685–692.
- Julius S, Pascual AV, London R. Role of parasympathetic inhibition in the hyperkinetic type of borderline hypertension. Circulation. 1971;44(3):413–418.
- Mancia G, Masi S, Palatini P, et al. Elevated heart rate and cardiovascular risk in hypertension. J Hypertens. 2021;39(6):1060–1069.
- Mancia G, Kjeldsen SE, Kreutz R, et al. Individualized beta-blocker treatment for high blood pressure dictated by medical comorbidities: indications beyond the 2018 ESC/ESH Hypertension Guidelines. Hypertension. 2022;79(6):1153–1166.
- Solomon SD, Vaduganathan M, Claggett BL, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction. DELIVER trial. JACC Heart Fail. 2022;10(3):184–197.
- Kjeldsen SE, von Lueder TG, Smiseth OA, et al. Medical therapies for heart failure with preserved ejection fraction. Hypertension. 2020;75(1):23–32.
