Abstract
Purpose. Randomised controlled trials have shown that renal denervation lowers office and ambulatory blood pressure. The aim of the present study was to evaluate whether patients undergoing renal denervation procedure in a real-life setting have a reduction in antihypertensive drug prescription over the subsequent years.
Material and methods. Using the healthcare utilisation database of the Lombardy Region (Italy), the 136 patients who, during the period 2011–2016, were prescribed four or more antihypertensive drugs and underwent renal denervation were included in the study cohort. The number and type of antihypertensive drugs were assessed over the year before and during the three-year period after renal denervation.
Results. The median age of the patients was 67 years and 68% of them were men. Based on a multisource comorbidity score, about 40% of patients showed a poor or very poor clinical status. Before renal denervation, the majority of the patients were prescribed four or five antihypertensive drugs. The number of drugs decreased after the denervation and reached 55% after three years. Over the same period, patients prescribed six drugs decreased from 18% to 2%. All antihypertensive drugs were less prescribed throughout the post denervation period. Compared to the year before the denervation, after three years prescription of diuretics was reduced by 15%, calcium channel blockers by 21%, ACE-inhibitors by 32%, angiotensin receptor blockers by 22%, beta-blockers by 20%, and alfa-blockers by 30%. Use of antihypertensive drugs exhibited a reduction also in an age, sex, and clinically matched control group with no renal denervation to an extent, however, much lower than in denervated patients (p-value = 0.013).
Conclusion. In the real-life setting, patients who underwent renal denervation had a clearcut reduction in antihypertensive drug prescription over the following years.
Patients exhibited a reduction in the prescription of antihypertensive drugs during the three years that followed the denervation procedure
The decrease in the number of antihypertensive drugs was marked, started after a relatively short time (six months), and involved all drugs prescribed before the denervation
The number of hospitalisations for a cardiovascular event was similar before and after renal denervation
Albeit blood pressure values were not recorded in our database, all these findings taken together suggest the renal denervation procedure has a favourable influence on blood pressure control and is not associated with an increase in the risk of major cardiovascular complications
Plain Language Summary
Introduction
After a number of favourable results [Citation1–3], the ability of renal denervation to reduce blood pressure (BP) in resistant hypertension has been challenged by the findings of two randomised controlled trials which showed that in resistant hypertensive patients the office or ambulatory BP reduction associated with renal denervation was not significantly greater than that seen in patients exposed to sham denervation [Citation4,Citation5]. This led hypertension guidelines to deny this procedure any substantial importance for the treatment of individuals in whom BP remains elevated despite use of three or more antihypertensive drugs at adequate doses [Citation6,Citation7]. Despite the appropriate design, however, these negative controlled trials had a number of limitations that made the robustness of the results uncertain [Citation8,Citation9]. This led to further randomised trials which have shown, with rare exceptions [Citation10], that renal denervation lowers office and ambulatory BP more effectively than up-titration of antihypertensive drugs [Citation11] or adoption of a sham intervention, the latter being the case not only in resistant hypertensive patients but also in milder hypertension or with no treatment [Citation12–16]. These findings re-established renal denervation as a potential means to lower an elevated BP when drugs are totally or partially ineffective [Citation17]. This has been also favoured by the results of a study using a large registry in which renal denervation was found to be accompanied by an office and ambulatory BP lowering effect, which persisted unchanged over a three-year observation period with no safety problems for the patients [Citation18].
Randomised trials, and to some extent also certain registries, provide data obtained in clinical environments that are different from those characterising medical practice. In trials, for example, the number and type of antihypertensive drugs is kept under control to minimise confounding variables in the interpretation of the results. This makes real-life data appropriate to provide information on whether, in the real-life setting, renal denervation is followed by a change in the intensity and type of antihypertensive drug use. This was the primary aim of the present study which used the healthcare utilisation database of the Lombardy region for 1) identifying in the general population patients who underwent renal denervation and 2) compare their use of antihypertensive drugs during a three-year period after and a one-year period before the denervation. We also calculated, as secondary aim, the incidence of cardiovascular events during the three post-denervation years, having a three-year pre-denervation period as control. Cardiovascular events were traced from hospitalisations that had a cardiovascular event as the primary diagnosis.
Material and methods
Setting
The target population included Lombardy residents who were beneficiaries of the National Health Service, i.e. virtually all citizens (about ten million people). The data of citizens are included in a health utilisation database that does not contain clinical data (BP, serum cholesterol, blood glucose, etc.) but provides precise information on all reimbursable health services such as outpatient drug prescriptions, medical visits, laboratory tests, instrumental examinations, in-patients and outpatient surgical interventions, primary and secondary diagnoses of hospitalisations and death. Further details of the Lombardy database characteristics are available in other publications [Citation19,Citation20].
Cohort selection
Among Lombardy residents, those who underwent the renal denervation procedure during the period 2011-2016 were identified, and the dates of hospital admission and discharge were respectively defined as the ‘index admission’ and ‘index discharge’. In line with one definition of resistant hypertension [Citation21], patients prescribed at least four antihypertensive drugs in the year before the index admission were included in the final cohort. A random patient was identified from the Lombardy database to be matched for age (±5 years), sex, clinical status (see below), and prescription of antihypertensive drug in the year before the index admission of the corresponding renal denervated patient.
Drug exposure
All prescriptions of antihypertensive drugs dispensed in the year before the index admission and in the three-year period after the index discharge were identified. During the three years following the index discharge, data analysis was carried out at six months, one year, two years and at the end of the third year of follow-up. Cohort members were classified according to the number and type of classes of antihypertensive agents used. Antihypertensive drugs included thiazide and thiazide-like diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, α-blockers, mineralocorticoid receptor antagonists (and separately aldactone) and renin-inhibitors, all dispensed as monotherapy or combination therapy. Use of loop diuretics (and separately of furosemide) was also assessed.
Clinical outcomes
Hospitalisations that occurred during the three years before and during the three years after renal denervation in which a cardiovascular disease (ischaemic heart disease, stroke, heart failure, or other causes) was listed as the primary diagnosis were identified. This was the case also for all-cause death.
Covariates
Baseline characteristics included sex, age, comorbidities (i.e. ischaemic heart disease, heart failure, cerebrovascular disease, diabetes, kidney disease, respiratory disease, and cancer), and co-treatments (digitalis glycosides, organic nitrates, antiarrhythmics, lipid-lowering agents, antiplatelets, anticoagulants agents, antidiabetic drugs, NSAIDs, anti-gout drugs, drugs for respiratory diseases, and antidepressants). In addition, the clinical status of the patients was assessed by the Multisource Comorbidity Score, a prognostic score that has been shown to predict all-cause mortality and hospitalisation of the Italian population better than some widely used conventional scores (i.e. Charlson, Elixhauser, and Chronic Disease scores) [Citation22]. Four categories of clinical status were considered: good (0 ≤score ≤4), intermediate (5 ≤score ≤9), poor (10 ≤score ≤14) and very poor (score ≥ 15).
Data analysis
Summary statistics of the prescribed antihypertensive treatment strategy both before the renal denervation procedure and during follow-up, were expressed as counts and percentages. Mixed models with random intercepts and an unstructured covariance structure were used to test whether the use of antihypertensive drugs changed during follow-up.
The chi-square was used to test differences in antihypertensive drug prescription between groups.
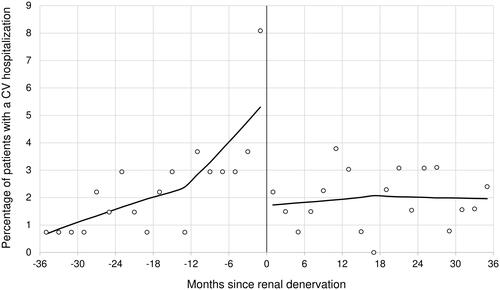
The proportion of patients who experienced a hospitalisation for a cardiovascular event over time were plotted, and the locally estimated scatterplot smoothing (LOESS) regression was fitted. The LOESS is a non-parametric regression method that allows to visually observe the possible trend of one variable [Citation23].
Sensitivity analysis
To verify the robustness of our findings, several additional analyses were performed. First, in line with a more common definition of resistant hypertension [Citation6], we also analysed patients undergoing renal denervation who in the year before the index admission were prescribed ≥3 antihypertensive drugs. A control group was identified also for these patients according to the description given for patients prescribed ≥4 antihypertensive drugs. Second, analysis was extended beyond antihypertensive drugs to drugs used for conditions associated with hypertension such as lipid-lowering, antidiabetic, antiplatelet, anticoagulant, and other agents. Third, to collect information on whether medical attention differed in renal denervated and control patients, calculation was made of the number of outpatient visits over the three years after the index date. Fourth, to verify whether a change in the use of antihypertensive drugs in our cohort can be explained by the fact that some of patients died during follow-up (i.e. informative censoring), we compared the use of antihypertensive drugs of the subjects included in the initial cohort with that of individuals who survived at three years of follow-up. No difference between these groups was assumed to indicate that this bias could be ruled out.
The Statistical Analysis System Software (version 9.4; SAS Institute, Cary, NC, USA) was used for the analyses. For all hypotheses tested, a two-tailed p-value < 0.05 was considered significant.
Results
Patients
During the period from 2011 to 2016, 198 patients underwent the renal denervation procedure. Among these, 136 were prescribed at least four antihypertensive drugs, and thus included in the study cohort. The characteristics of the cohort members are shown in . The median age was 67 years, and two out of three patients were men. About one in ten patients had at least one comorbidity, six in ten patients were co-treated with lipid-lowering and antiplatelets drugs, four in ten patients were under antidiabetic treatment, and almost two in five patients showed a poor or very poor clinical status.
Table 1. Baseline characteristics of cohort members.
Antihypertensive and other drugs
As shown in and , in the year before renal denervation about four in ten patients were prescribed four and five antihypertensive drugs while after renal denervation four or five drugs were prescribed in a significantly smaller number of patients (p-value of the trend: <0.001). Patients prescribed six drugs markedly decreased after the denervation procedure (from 18% to 2%) which was also accompanied by treatment downgrading to three and two drugs in several patients. Following the denervation almost no patient was on monotherapy or without antihypertensive drugs. The reduction in the number of antihypertensive drugs occurred mainly during the first post-denervation year, after which drug prescription remained more stable.
Figure 1. Number of drugs prescribed one year before and during the three years after renal denervation.
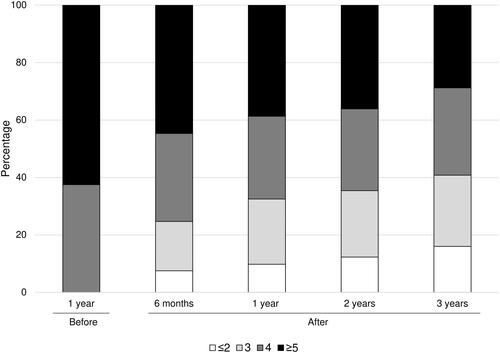
Table 2. Number of antihypertensive drug classes prescribed in the year before the index admission and during the 3 years after the index discharge.
As shown in , compared to the year before renal denervation all antihypertensive drugs were consistently less prescribed during the three-year follow-up after the denervation. At the third year, prescriptions were reduced by 15% for thiazide and thiazide-like diuretics, 21% for calcium channel blockers, 32% for ACE-inhibitors, 22% for angiotensin receptor blockers, 20% for beta-blockers, 30% for alfa-blockers, and 30% for mineralocorticoid receptor antagonists. The exception was the loop diuretics (and individually furosemide) that were prescribed at a similar rate before and after the denervation.
Table 3. Antihypertensive drug classes prescribed during the year before the index admission and during the three years after the index discharge.
Except for an increase in the prescription of antiplatelet drugs at the 6th month after the denervation, non-antihypertensive medicaments did not exhibit any noticeable prescription change during the post-denervation period (Supplementary Table S1).
Controls
Matching between patients under ≥4 antihypertensive drugs who underwent or did not undergo (controls) the renal denervation procedure was achieved in 130 cases (Supplementary Table S2).
As shown in , compared to the year before index admission, prescription of antihypertensive drugs was reduced after the index discharge also in control patients, in whom, however, the magnitude of the reduction was significantly less (p = 0.013) than in patients with renal denervation for almost all drugs.
Table 4. Antihypertensive drug classes prescribed to control patients during the year before the index admission and during the three years after the index discharge of the corresponding patient who underwent the renal denervation.
Similar results were obtained when renal denervated and control patients under 3 or more antihypertensive drugs were considered, which raised their numbers to 163 and 155, respectively (Supplementary Tables S3, S4 and S5).
Clinical outcomes
There were 62 hospitalisations for a cardiovascular event (23 for ischaemic heart disease, 15 for heart failure, 7 for stroke, and 17 for other cardiovascular causes) over the three years before renal denervation, with an apparent trend to an increase near the denervation procedure. The corresponding number after renal denervation was 53 (14 for ischaemic heart, 23 for heart failure, 2 for stroke, and 14 for other cardiovascular causes) similarly distributed during the three years of follow-up (). Ten fatal events were registered in the three-year period after renal denervation.
Sensitivity analysis
During the three years after renal denervation, patients there was on average 2.0 (standard deviation = 2.7) medical visits per patient. This number was similar to that of control patients (1.7 ± 2.2, p-value = 0.302).
Patients who survived at the end of the follow-up did not show a different use of antihypertensive drugs before the index admission compared to the whole cohort members (Supplementary Table S6). This was the case also for use of specific drug classes (Supplementary Table S7), except for angiotensin-converting enzyme inhibitors which were used slightly more frequently in the whole cohort member.
Discussion
The main finding of the present study is that patients using four or more antihypertensive drugs per day before renal denervation exhibited a reduction in the prescription of antihypertensive drugs during the three years that followed the denervation procedure. Other findings are that the reduction in drug prescription was a marked one because (1) about 40% of the patients showed a decrease in the number of prescribed drugs to < 4/day and (2) prescription of an especially high number of drugs, that is, five or more per day also showed a considerable decrease (about 50%) compared to the year before the denervation. It is also important to note that the decrease of drug prescription started after a relatively short time (six months) after the denervation procedure and was maintained throughout the entire observation period. This was the case for almost all drugs prescribed before the denervation, including those more specifically employed for resistant hypertension after failure to achieve BP control with the initial three drugs (usually a thiazide or thiazide-like diuretic, a calcium channel blocker and a blocker of the renin-angiotensin system), i.e. mineralocorticoid receptor antagonists, beta-blockers, and alpha-blockers, although, for some of these drugs, use in a small number of patients did not allow the before-after denervation differences to be statistically significant. This allows to conclude that in a real-life medical environment renal denervation is accompanied by a considerable long-term reduction in the prescription of antihypertensive drugs. It also allows to suggest, albeit with the limitation that BP values were not available, that this was due to a favourable influence of the denervation procedure on BP control due to its direct BP lowering effect and/or a facilitation of the BP-lowering influence of the prescribed antihypertensive drugs, possibly via a decrease of the pressogenic influences that contribute to elevate BP in resistant hypertension [Citation24]. One of these influences is the activity of the sympathetic nervous system which is high in resistant hypertension [Citation25] and reduced by renal denervation [Citation26,Citation27]. It is also possible, albeit untested in the present study, that after renal denervation patients became more adherent to the antihypertensive treatment regimen due to a greater awareness of the need to reduce an elevated BP. An increased adherence would have favoured a greater BP reduction, causing indirectly a reduction of antihypertensive drug prescription by the physician.
Other results of our study deserve mentioning. One, our results are in line with the results of a relatively large ongoing registry which has shown a persistent reduction of BP and use of antihypertensive drugs over a three-year period after renal denervation [Citation17,Citation28]. They add to these findings, however, data that are closer to real-life medicine because in registries the level of medical management is higher than in clinical practice, in part because of their declared scientific scope. Two, we performed our primary analysis in patients under 4 or more antihypertensive agents because our database did not provide information on BP values, and this offered a better guarantee that patients undergoing renal denervation had a resistant hypertensive state [Citation21]. However, the data obtained in renal denervated patients under 4 or more antihypertensive drugs were replicated by the larger number of patients under three antihypertensive drugs, that is, those with a more classical definition of resistant hypertension [Citation6]. Three, treatments with a variety of non-antihypertensive drugs showed no noticeable modification over the three years that followed renal denervation, indicating that drug treatment reduction was specific for antihypertensive treatment. On the assumption that a greater awareness of the benefit of treatment would extend to all cardiovascular risk factors, this finding might speak against an increase of adherence to treatment after renal denervation, although the possibility of a selective increase of adherence to antihypertensive drugs cannot be excluded. Four, over the three-year follow-up period, a reduction in antihypertensive drug prescription was also observed in a referent cohort matched for sex, age, clinical status, and antihypertensive drugs with the renal denervation cohort. We do not have an explanation for this finding except that an increase in adherence due to a closer follow-up is an unlikely explanation because during the 3-year period the number of visits was similar in this and the renal denervation group. At any rate, the magnitude of the drug treatment reduction was clearly less in control compared to renal denervated patients. For example, almost 20% of the patients in the control cohort showed a decrease in the number of prescribed drugs to < 4/day, compared to the 40% reduction observed among patients who underwent renal denervation. Five, in patients undergoing renal denervation treatments for conditions other than hypertension were frequent indicating a high level of comorbidities. This was confirmed by the relatively high number of previous hospitalisations for cardiovascular and other diseases as well as by a clinical status that was defined as poor or very poor (based on a marked reduction of life expectancy) in almost 40% of the recruited individuals. This means that in the real-life conditions of our study patients elected for renal denervation had mostly a high level of cardiovascular risk. Finally, there were 62 hospitalisations for a cardiovascular event during the three-year period before renal denervation, a number which decreased to 53 also evenly distributed events over the 3 years after renal denervation. This does not allow any calculation of the benefit of the procedures on outcomes but it may suggest, in line with the evidence from a worldwide registry from specialised centres [Citation17,Citation28,Citation29], that even in real life renal denervation was not associated with any increase in the risk of major cardiovascular complications.
The present study has some strengths as well as limitations. The strengths are that the study was based on a huge healthcare utilisation database of a very large and unselected population (about 10 million people) which allowed to identify a rare target population (i.e. patients suffering from resistant hypertension who underwent the renal denervation procedure) and investigate its use of antihypertensive drugs in a real-life setting. Furthermore, the therapeutic data made available by the database were accurate because controls by health authorities of the filed prescriptions are frequent and incorrect reports carry legal consequences [Citation30]. Finally, the main finding of our study, i.e. the persistent reduction of drug prescription after the denervation procedure, was confirmed by several sensitivity analyses. The limitations are that the Lombardy database does not include BP values, which prevented information on the BP modifications associated with the denervation procedure and on the number of patients in whom this intervention allowed to achieve BP control. Second, information was limited to reimbursable drugs, that is, it did not include drugs prescribed by doctors in the context of private practice and thus outside the Italian Health Service. However, because antihypertensive and other life-saving cardiovascular drugs are provided free of charge from the Italian Health Service, this represents a very small percentage (about 6% of the entire cardiovascular drug use [Citation31]). Third, our data are limited to drug prescription, which may not precisely reflect patients’ drug assumption, which unfortunately is unmeasurable in long-term studies by currently available approaches. This limitation extends to direct assessment of the drugs prescribed in urine or blood because the few “points” assessments provided by this direct approach cannot take into account the large variations in adherence to treatment over time. Fourth, our inclusion period (i.e. 2011–2016) only allowed patients undergoing renal denervation by first-generation catheters to be studied, which means that our data may not reflect the changes in antihypertensive drug treatment associated with the more advanced denervation procedures available today [Citation32,Citation33]. Finally, despite the extremely large population included in the database, the number of patients undergoing renal denervation was limited. We did not extend recruitment beyond 2016 because use of the renal denervation procedure drastically diminished in Italy after 2016 due to the unfavourable results of trials [Citation4,Citation5] and the negative opinion about this treatment expressed by European guidelines [Citation34]. In addition, we did not want our three-year follow-up to encroach the beginning of the 2020 Covid-19 pandemic, which would have happened by extending recruitment beyond 2016.
In conclusion, our study shows that in the real-life setting 1) the renal denervation procedure is followed by a clearcut reduction in antihypertensive drug prescription and 2) this is the case for all antihypertensive drugs. Future studies are needed to assess in real-life setting the impact of renal denervation on BP control.
Supplemental Material
Download MS Word (30.2 KB)Disclosure statement
Giovanni Corrao received research support from the European Community (EC), the Italian Medicines Agency (AIFA), Italian Ministry of Health, and the Italian Ministry of Education, University and Research (MIUR). He took part to a variety of projects that were funded by pharmaceutical companies (i.e. Novartis, GSK, Roche, AMGEN, BMS and Servier). He also received honoraria as member of Advisory.
Giuseppe Mancia received honoraria for participation as speaker/chairman in national/international meetings from Bayer, Boehringer Ingelheim, CVRx, Daiichi Sankyo, Ferrer, Medtronic, Menarini Int., Merck, Novartis, Recordati and Servier.
Other authors have no disclosures.
Data availability statement
The data that support the findings of this study are available from Lombardy Region, but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Data are however available from the Lombardy Region upon reasonable request.
Additional information
Funding
References
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–1281.
- Esler MD, Krum H, Sobotka PA, Symplicity HTN-2 Investigators, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The symplicity HTN-2 trial): a randomised controlled trial. Lancet. 2010;376:1903–1909.,
- Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128(2):132–140.
- Bhatt DL, Kandzari DE, O'Neill WW, et al. SYMPLICITY HTN-3 investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401.
- Mathiassen ON, Vase H, Bech JN, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24-h blood pressure-based trial. J Hypertens. 2016;34(8):1639–1647.
- Williams B, Mancia G, Spiering W, et al. Authors/task force members:. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. 2018;36:1953–1204. Erratum in: J Hypertens 2019;37:226
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. Erratum in: Hypertension 2018;71:e140–e144
- Esler M. Illusions of truths in the symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8(8):593–598.
- Morganti A, Mancia G. Resistant hypertension: Renal denervation or intensified medical treatment? Eur J Intern Med. 2018;50:6–11.
- Rosa J, Widimský P, Toušek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the prague-15 study. Hypertension. 2015;65(2):407–413.
- Azizi M, Sapoval M, Gosse P, Renal Denervation for Hypertension (DENERHTN) investigators, et al. Renal denervation for hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957–1965.
- Townsend RR, Mahfoud F, Kandzari DE, et al. SPYRAL HTN-OFF MED trial investigators*. catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390(10108):2160–2170.
- Kandzari DE, Böhm M, Mahfoud F, SPYRAL HTN-ON MED Trial Investigators, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346–2355.
- Azizi M, Sanghvi K, Saxena M, RADIANCE-HTN investigators, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476–2486.
- Azizi M, Schmieder RE, Mahfoud F, RADIANCE-HTN Investigators, et al. RADIANCE-HTN investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335–2345. Erratum in: Lancet. 2018;392:820
- Böhm M, Kario K, Kandzari DE, SPYRAL HTN-OFF MED Pivotal Investigators, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–1451.
- Mahfoud F, Mancia G, Schmieder R, et al. Renal denervation in High-Risk patients With hypertension. J Am Coll Cardiol. 2020;75(23):2879–2888.
- Schmieder RE, Mahfoud F, Mancia G, members of the ESH Working Group on Device-Based Treatment of Hypertension, et al. European society of hypertension position paper on renal denervation 2021. J Hypertens. 2021;39(9):1733–1741.
- Corrao G, Mancia G. Research strategies in treatment of hypertension: value of retrospective real-life data. Eur Heart J. 2022;43(35):3312–3322.
- Rea F, Savaré L, Franchi M, et al. Adherence to treatment by initial antihypertensive Mono and combination therapies. Am J Hypertens. 2021;34(10):1083–1091.
- Gupta AK, Nasothimiou EG, Chang CL, ASCOT investigators, et al. Baseline predictors of resistant hypertension in the Anglo-Scandinavian cardiac outcome trial (ASCOT): a risk score to identify those at high-risk. J Hypertens. 2011;29(10):2004–2013.
- Corrao G, Rea F, Di Martino M, et al. Developing and validating a novel multisource comorbidity score from administrative data: a large population-based cohort study from Italy. BMJ Open. 2017;7(12):e019503.
- Jacoby WG. Loess: a nonparametric, graphical tool for depicting relationships between variables. Electoral Studies. 2000;19(4):577–613.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American heart association professional education committee of the council for high blood pressure research. Hypertension. 2008;51(6):1403–1419.
- Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116(6):976–990.
- Schlaich MP, Sobotka PA, Krum H, et al. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361(9):932–934.
- Grassi G, Seravalle G, Brambilla G, et al. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension. 2015;65(6):1209–1216.
- Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the global SYMPLICITY registry. Eur Heart J. 2019;40(42):3474–3482.
- Ott C, Mahfoud F, Mancia G, et al. Renal denervation in patients with versus without chronic kidney disease: results from the global SYMPLICITY registry with follow-up data of 3 years. Nephrol Dial Transplant. 2022;37(2):304–310.
- Corrao G, Mancia G. Generating evidence from computerized healthcare utilization databases. Hypertension. 2015;65(3):490–498.
- Italian Medicines Agency. National Reports on Medicines use in Italy. Year 2019. https://www.aifa.gov.it/documents/20142/241052/OsMed_2019_Eng.pdf. [Accessed 14 September 2022]
- Fengler K, Rommel KP, Blazek S, et al. A Three-Arm randomized trial of different renal denervation devices and techniques in patients With resistant hypertension (RADIOSOUND-HTN). Circulation. 2019;139(5):590–600.
- Yang X, Liu H, Chen S, et al. Intravascular renal denervation reduces ambulatory and office blood pressure in patients with essential hypertension: a Meta-Analysis of randomized Sham-Controlled trials. Kidney Blood Press Res. 2022;47(6):363–374.
- Mancia G, Fagard R, Narkiewicz K, Task Force Members, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the european society of cardiology (ESC). J Hypertens. 2013;31(7):1281–1357.