Abstract
Purpose
The current review is to describe the definition and prevalence of resistant arterial hypertension (RAH), the difference between refractory hypertension, patient characteristics and major risk factors for RAH, how RAH is diagnosed, prognosis and outcomes for patients.
Materials and Methods
According to the WHO, approximately 1.28 billion adults aged 30–79 worldwide have arterial hypertension, and over 80% of them do not have blood pressure (BP) under control. RAH is defined as above-goal elevated BP despite the concurrent use of 3 or more classes of antihypertensive drugs, commonly including a long-acting calcium channel blocker, an inhibitor of the renin-angiotensin system (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker), and a thiazide diuretic administered at maximum or maximally tolerated doses and at appropriate dosing frequency. RAH occurs in nearly 1 of 6 hypertensive patients. It often remains unrecognised mainly because patients are not prescribed ≥3 drugs at maximal doses despite uncontrolled BP.
Conclusion
RAH distinctly increases the risk of developing coronary artery disease, heart failure, stroke and chronic kidney disease and confers higher rates of major adverse cardiovascular events as well as increased all-cause mortality. Timely diagnosis and treatment of RAH may mitigate the associated risks and improve short and long-term prognosis.
PLAIN LANGUAGE SUMMARY
Resistant arterial hypertension is a serious condition that leads to severe cardiovascular complications, such as heart attack, stroke and death.
It is defined as above-goal elevated blood pressure despite the concurrent use of 3 or more classes of antihypertensive medications administered at maximum or maximally tolerated doses and at appropriate dosing frequency.
Non-adherence to antihypertensive medications must be excluded before resistant arterial hypertension is diagnosed.
Blood pressure should be measured appropriately. A person should sit in a comfortable chair with back supported, both feet flat on the ground, and legs uncrossed for at least 5 min before blood pressure measurement. A cuff length is supposed to be at least 80% and a width of at least 40% of the arm circumference. Placing the cuff directly on the skin of the upper arm at the level of the heart. Obtaining 3 readings 1 min apart. Discarding the first reading and taking the mean of the second and third readings
Resistant arterial hypertension should be distinguished from refractory hypertension, when blood pressure remains uncontrolled on maximal or near-maximal therapy of 5 or more antihypertensive agents of different classes.
Definition of resistant arterial hypertension
Resistant arterial hypertension (RAH) is a high risk condition, leading to impaired cardiovascular disease (CVD) outcomes and increased all-cause mortality [Citation1]. It is defined as above-goal elevated blood pressure (BP) despite the concurrent use of 3 or more classes of antihypertensive drug, commonly including a long-acting calcium channel blocker, an inhibitor of the renin-angiotensin system (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker [ARB]), and a thiazide diuretic. All agents should be administered at maximum or maximally tolerated doses and at appropriate dosing frequency. BP should be measured appropriately and the BP threshold for diagnosis and treatment goals should be in line with current clinical practice guidelines [Citation2]. Patients with the white-coat effect should not be included in the definition of RAH as well as those non-adhering to the diagnosis of RAH [Citation3]. Controlled resistant hypertension is said to be present when BP is controlled on ≥4 antihypertensive medications at maximal or maximally tolerated doses [Citation1]. Apparent treatment resistant hypertension (aTRAH) is a term used when medication dose, adherence, or out-of-office BP is not documented or accounted for, and pseudo resistance cannot be excluded in a patient on ≥3 antihypertensive agents [Citation1].
Errors in BP measurement and techniques
Errors in BP measurement can account for the misdiagnosis of RAH. BP results are typically dependent on the patient preparation, environmental conditions, cuff size and measurement techniques, requiring an average of at least two readings obtained on at least two separate occasions [Citation2,Citation4]. Out-of-office BP and self-monitored BP require proper technique for accurate BP results [Citation2,Citation5,Citation6]. A study comparing standard triage BP measurements by clinic staff with an automated device obtaining up to 6 BP measurements 1 min apart, while the patient was unsupervised and seated in a quiet room, showed inaccurately increased BP measurement in 33% of patients referred as RAH [Citation7]. Inappropriately elevated cuff pressure may occur in patients with severe arterial disease and stiff, calcified arteries, a condition called pseudohypertension [Citation8].
Proper BP measurement technique includes [Citation4,Citation9]:
Preparing the individual by emptying a full urinary bladder and then sitting with legs uncrossed and back, arm and feet supported in a quiet room, ideally 5 min before the first BP measurement is obtained;
Choosing a BP cuff with a cuff length of at least 80% and a width of at least 40% of the arm circumference;
Placing the cuff directly on the skin of the upper arm at the level of the heart on the supported arm;
Obtaining three readings 1 min apart. Discarding the first reading and taking the mean of the second and third reading
White-coat effect
The ‘white-coat effect’ is defined as an inappropriate BP response to a clinic visit, but there is no agreement as to exactly how it should be defined. The most widely used definition is the BP difference between the average clinic and daytime ambulatory measurements [Citation4]. In contrast to previous observations Mancia et al. recently showed that the risk of CVD complications in patients with white-coat hypertension without organ damage is higher compared with the risk in hypertensive patients with controlled BP [Citation10]. To rule out true RAH, an out-of-office BP monitoring is generally required. The white-coat effect has been attributed to an alerting reflex triggered by the healthcare provider or the clinic environment that activates the sympathetic nervous system [Citation11]. A clinically significant white-coat effect may be present in 28% up to 39% of individuals with aTRAH identified by office BP measurement [Citation12]. De la Sierra et al. found that among >8200 patients with apparent RAH included in the Spanish Ambulatory Blood Pressure Monitoring Registry, 62.5% were classified as having true RAH, while the remaining 37.5% were identified as having white coat hypertension [Citation13].
Medication non-adherence
Non-adherence to antihypertensive medications must also be excluded before RAH is diagnosed. Medication non-adherence is very common in patients with more severe hypertension [Citation14,Citation15]. Approximately 50–80% of hypertensive patients demonstrate suboptimal adherence to antihypertensive medications [Citation16,Citation17]. This relatively high proportion of non-adhering patients that may mimic RAH is related, at least in part, to the large pill burden, dosing complexity, expense, high frequency of adverse reactions with multidrug antihypertensive regimens, poor patient–clinician relationship and clinician inertia with reduced insistence on adherence despite patients being consistently non-adherent [Citation16]. Several systematic reviews and meta-analyses have assessed the impact of interventions, including modification of antihypertensive therapy, on adherence to antihypertensive medications [Citation18,Citation19]. Thus, in patients with hypertension, fixed-dose combination regimens reduced the risk of medication non-compliance by 24% compared to free-drug combination regimens [Citation20]. In the recently published meta-analysis (15 RCI and 7415 participants) showed that the behavioural self-monitoring interventions combined with tailored advice compared to usual care or minimal intervention resulted in higher and significant changes in both SBP and diastolic blood pressure (DBP) (SBP: −2.92 mmHg, 95% CI −3.94 to −1.90, n = 3102 vs −0.72 mmHg, 95% CI −1.67 to 0.23, n = 4199, χ2 = 9.65, p = .002; DBP: −2.05 mmHg, 95% CI −3.10 to −1.01, n = 968 vs 1.54 mmHg, 95% CI −0.53 to 3.61, n = 400, χ2 = 9.19, p = .002) [Citation21].
RAH vs refractory hypertension
Refractory hypertension is a condition when BP remains uncontrolled on maximal or near-maximal therapy of five or more antihypertensive agents of different classes, including a long-acting thiazide-like diuretic (such as chlorthalidone) and mineralocorticoid antagonists [Citation1]. RAH can ususally brought under control by adjusting treatment. In contrast, refractory hypertension most often cannot be controlled despite skillful attempts to use synergistic antihypertensive drugs.
Prevalence of resistant arterial hypertension worldwide and by demographics
Among the treated adults with hypertension, RAH occurs in approximately in 12–15% of population-based and 15–18% of clinic-based reports [Citation22–26] mostly in those with a long history of hypertension and chronic kidney disease (CKD) [Citation24,Citation27]. In population- and clinic-based studies, some RAH cases may remain unrecognised because patients are not prescribed ≥3 drugs at maximal doses despite uncontrolled BP. In contrast, clinical trials usually include forced titration schemes that unmask RAH by reducing the prevalence of suboptimal treatment [Citation28]. Demographic correlates of RAH include black race, older age and male sex [Citation29]. RAH is characterised by variable clustering of distinct demographics, comorbidities, physiological aberrations and metabolic abnormalities. However, these factors are not mutually exclusive because, in fact, they can be substantially interdependent (e.g. non-dipping or reverse dipping BP and sympathetic nervous system overactivity, visceral obesity and excess aldosterone secretion) [Citation1].
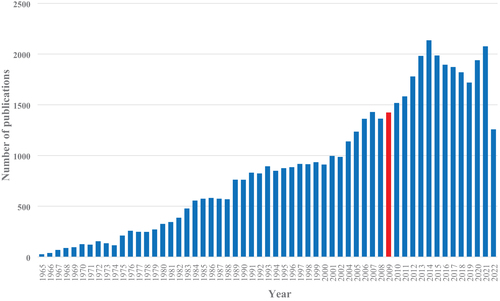
Interestingly, the number of articles on RAH on PubMed per year increased substantially after the advent of renal denervation in 2009. However, it may have reached a peak in 2015 and seems to plateau or even decline since then ().
Patient’s characteristics of RAH
Lifestyle risk factors
Obesity
Findings from the NHANES (National Health and Nutrition Examination Survey) of 13,375 hypertensive adults demonstrated that body mass index (BMI) ≥30 kg/m2 approximately doubles the risk for aTRH [Citation28]. In 14,461 patients with RAH in the Spanish Ambulatory Blood Pressure Monitoring Registry, a BMI ≥30 kg/m2 was also an independent risk factor for RAH (odds ratio, 1.62; 95% CI 1.32–1.99) [Citation30]. Among 3367 hypertensives with chronic kidney disease (CKD), increasing levels of obesity were independently associated with higher risks of aTRH, ranging from an odds ratio of 1.52 (BMI ≥ 30 kg/m2) to 2.26 (BMI ≥ 40 kg/m2) [Citation24]. In the study with over >470,000 patients from the Kaiser Permanente Southern California health system, obesity (BMI ≥ 30 kg/m2) was also found to be an independent risk factor for RAH (odds ratio, 1.62; 95% CI 1.42–1.51) [Citation29]. The Dietary Approaches to Stop Hypertension (DASH) recommended eating pattern consistently reduces BP by 6.7/3.5 mmHg as documented in a recent meta-analysis [Citation31,Citation32]. However, in the REGARDS cohort, a low DASH diet questionnaire score was not independently associated with aTRH [Citation33].
Dietary sodium
Higher dietary sodium intake is independently associated with increases in arterial BP [Citation34–37]. However, relatively large interindividual and racial variations in ‘salt sensitivity’ of BP exist. This leads to excess volume retention, vascular dysfunction, arterial stiffness, sympathetic activation, impaired renin–angiotensin axis suppression, mineralocorticoid receptor activation and immune cell modulation [Citation38–40]. Several studies demonstrated a significant reduction in BP among patients with RAH following a low sodium diet [Citation41,Citation42].
Physical inactivity
Both reduced physical activity and lower physical fitness are independent risk factors for hypertension. [Citation33,Citation43–47]. Self-reported inactivity was not predictive of RAH among patients in the REGARDS cohort [Citation48]. Conversely, a thrice-weekly treadmill walking exercise program for 8–12 weeks significantly lowered daytime ambulatory BP (6 ± 12/3 ± 7 mmHg; p = 0.03) among 50 treated patients with RAH [Citation48].
Alcohol intake
Alcohol intake has been linked to increases in BP and the risk for developing hypertension [Citation49–51]. The dose–response association may differ between men (linear) and women (J shaped) [Citation51] and is modified by metabolic genes [Citation50]. Drinking alcohol >30–50 g/day was an independent risk factor for hypertension [Citation52].
Anxiety and depression
Globally, up to 33.7% of the population are suffering from anxiety disorder during lifetime [Citation53]. In the systematic review and meta-analysis of eight prospective studies [Citation54], baseline anxiety increased risk of incident hypertension, the pooled adjusted HR was 1.55 (95% CI 1.24–1.94), with strong heterogeneity detected (PQ < 0.001; I2 = 84.6%). Recent studies showed a positive association between comorbid anxiety and RAH [Citation55]. In particular, patients with RAH scored higher points in Beck Depression Inventory (≥5 points) and the Hamilton Anxiety Scale (≥3 points)compared with healthy controls and non-resistant hypertensive patients [Citation56]. Depression can negatively affect the course of hypertension, interfere with adherence to medications [Citation3].
Natural disasters, catastrophes, war
Mental and physical stress, insomnia, excess salt intake due to consumption of stored food, and infection (pneumonia) is a leading cause of hypertension during natural disasters, catastrophes and war [Citation57]. Hypertension-related diseases develop immediately after a disaster, and their risk continues until the living environment stabilised and lifestyle habits are improved [Citation57]. Large individual differences in the elevation of BP and duration of elevated BP exist. Thus, SBP 5–25 mmHg average increase has been reported increase for 2–4 weeks after an earthquake [Citation58]. Survey of Health, Ageing and Retirement in Europe (SHARE) demonstrated that exposure to war during childhood is associated with increased lifetime risk of CVD, diabetes, high cholesterol and hypertension [Citation59].
Patient comorbidities
Multiple comorbidities have been associated with RAH. Such as obesity [Citation60–62], left ventricular hypertrophy [Citation63], albuminuria [Citation64,Citation65], diabetes mellitus [Citation29,Citation60,Citation64,Citation66], CKD [Citation29,Citation60,Citation67], higher Framingham 10-year risk score [Citation60], and obstructive sleep apnoea (OSA) [Citation68]. A very high proportion (60 − 84%) of individuals with RAH have sleep apnoea [Citation69–71]. Other sleep abnormalities are also manifest in RAH (relative to those with controlled hypertension or normotensives), including shorter sleep duration, reduced sleep efficiency and less rapid eye movement sleep [Citation72].
Physiological derangements
Physiological aberrations in RAH include vascular disease/dysfunction as evidenced by high rates of peripheral [Citation60] and carotid artery atherosclerosis [Citation63], impaired endothelial function [Citation65,Citation73], reduced arterial compliance and raised systemic vascular resistance [Citation61], all of which appear to be more pronounced in RAH compared with non-RAH. The normal nocturnal decline in BP is also attenuated in a high proportion (43 − 65%) of individuals with RAH [Citation12,Citation74,Citation75].
Metabolic derangements
RAH has also been associated with metabolic derangements, including hyperuricaemia [Citation22] aldosterone excess [Citation76] and suppressed circulating renin levels [Citation77]. In general, RAH is characterised by exquisite salt sensitivity of BP. Reducing dietary sodium intake to levels significantly below the level of usual intake in Western societies (e.g. 50 mmol/day) promptly and impressively lowers BP in many individuals with RAH [Citation41].
Genetic variants
Heritability of RAH was shown in some family-based studies indicating that 50–60% of BP variability can be attributed to genetic factors [Citation78–80]. Common genetic variants influencing BP have been identified at >300 independent loci, but require scores based on hundreds of thousands of individuals for their detection as their individual contribution is minute [Citation81]. The majority of genetic studies of RAH had significant limitations such as non-adequate sample sizes and multiple testing across the many candidate gene studies [Citation82–86].
Prognosis and outcomes of resistant arterial hypertension
In a retrospective study of >200,000 patients with new onset hypertension, those with RAH were 47% more likely to suffer the combined outcomes of death, myocardial infarction, heart failure, stroke, or CKD over the median 3.8 years of follow-up [Citation87]. In a study with over 400,000 participants, patients with RAH had a 32% increased risk of developing end-stage renal disease, a 24% increased risk of an ischaemic heart event, a 46% increased risk of heart failure, a 14% increased risk of stroke and a 6% increased risk of death compared with patients without RAH [Citation88]. Prospective studies using ambulatory BP monitoring showed an almost 2-fold increased risk of CVD events in patients with true RAH compared with those with hypertension responsive to treatment [Citation12,Citation69,Citation89–91].
RAH is associated with worse outcomes among patients with some comorbid conditions. Thus, in patients with coronary artery disease, RAH is associated with higher rates of major adverse cardiovascular events (MACE; i.e. CV death, myocardial infarction and stroke) [Citation28,Citation92,Citation93]. In patients with CKD, RAH is associated with higher risk of myocardial infarction, stroke, peripheral arterial disease, heart failure and all-cause mortality compared with those without RAH [Citation24]. Conversely, RAH is not associated with increased adverse clinical events in patients with heart failure with reduced ejection fraction and may lower the risk for heart failure-related rehospitalization [Citation94].
Further pharmacologic treatment
According to the recent guidelines, a prescription as a fourth-line agent the addition of spironolactone, a mineralocorticoid receptor antagonist, despite common, dose-limiting adverse effects is recommended [Citation3]. Diuretic therapy may be maximised, e.g. loop diuretics in patients with chronic kidney disease and/or patients receiving potent vasodilators (e.g. minoxidil) ().
Figure 2. Diagnosis of resistant arterial hypertension (modified from [Citation3]). *Phase 2 Trial of Baxdrostat for Treatment-Resistant Hypertension. Dose-dependent changes in systolic BP of −20.3, −17.5, −12.1 mmHg were observed in the 2-, 1-, 0.5-mg Baxdrostat in patients with treatment-resistant hypertension [Citation95].
![Figure 2. Diagnosis of resistant arterial hypertension (modified from [Citation3]). *Phase 2 Trial of Baxdrostat for Treatment-Resistant Hypertension. Dose-dependent changes in systolic BP of −20.3, −17.5, −12.1 mmHg were observed in the 2-, 1-, 0.5-mg Baxdrostat in patients with treatment-resistant hypertension [Citation95].](/cms/asset/ab759d7d-e7d6-44d7-9d8c-5cb570696bb6/iblo_a_2185457_f0002_b.jpg)
Recently published Phase 2 Clinical Trial of Baxdrostat for Treatment-Resistant Hypertension showed a dose-dependent changes in systolic BP of −20.3, −17.5, −12.1 mmHg were observed in the 2-, 1-, 0.5-mg Baxdrostat in patients with treatment-resistant hypertension [Citation95]. Baxdrostat, a selective inhibitor of aldosterone synthase, a likely cause of treatment resistance acts by suppressing hormone synthesis rather than by blocking the mineralocorticoid receptor. Preclinical and phase 1 studies have shown that baxdrostat has high selectivity (selectivity ratio, 100:1) for aldosterone synthase as compared with the enzyme required for cortisol synthesis, 11β-hydroxylase, which shares 93% sequence similarity with aldosterone synthase [Citation96].
Patients should be referred to appropriate specialists for known or suspected secondary cause(s) of hypertension ().
Conclusion
RAH is a serious disorder where patients had a substantially increased risk of developing end-stage renal disease, major adverse cardiovascular events and stroke, heart failure and all-cause mortality compared with patients without RAH. Timely diagnosis of RAH will allow prompt preventive and therapeutic interventions to mitigate RAH-associated outcomes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Acelajado MC, Hughes ZH, Oparil S, et al. Treatment of resistant and refractory hypertension. Circ Res. 2019;124(7):1061–1070.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2017;71(6):1269–1324.
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement From the American heart association. Hypertension. 2018;72(5):E53–E90.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American heart association cou. Circulation. 2005;111(5):697–716.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American heart association professional education committee of the council for high blood pressure research. Hypertension. 2008;51(6):1403–1419.
- Parati G, Stergiou GS, Asmar R, et al. European society of hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24(12):779–785.
- Bhatt H, Siddiqui M, Judd E, et al. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016;10(6):493–499.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med. 1985;312(24):1548–1551.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical pr. J Am Coll Cardiol. 2018;71(19):e127–e248.
- Mancia G, Facchetti R, Vanoli J, et al. White-coat hypertension without organ damage: impact on long-term mortality, new hypertension, and new organ damage. Hypertension. 2022;79(5):1057–1066.
- Grassi G, Turri C, Vailati S, et al. Muscle and skin sympathetic nerve traffic during the ‘white-coat’ effect. Circulation. 1999;100(3):222–225.
- Salles GF, Cardoso CRL, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340–2346.
- de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902.
- Hameed MA, Tebbit L, Jacques N, et al. Non-adherence to antihypertensive medication is very common among resistant hypertensives: results of a directly observed therapy clinic. J Hum Hypertens. 2016;30(2):83–89.
- Schulz M, Krueger K, Schuessel K, et al. Medication adherence and persistence according to different antihypertensive drug classes: a retrospective cohort study of 255,500 patients. Int J Cardiol. 2016;220:668–676.
- Elliott WJ. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens (Greenwich). 2008;10(Suppl 1):20–26.
- Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(4):e5641.
- Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;2014(11):CD000011.
- Hyman DJ, Pavlik V. Medication adherence and resistant hypertension. J Hum Hypertens. 2015;29(4):213–218.
- Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713–719.
- Kassavou A, Wang M, Mirzaei V, et al. The association between smartphone app-based self-monitoring of hypertension-related behaviors and reductions in high blood pressure: systematic review and meta-analysis. JMIR mHealth uHealth. 2022;10(7):e34767.
- Borghi C, Tubach F, De Backer G, et al. Lack of control of hypertension in primary cardiovascular disease prevention in Europe: results from the EURIKA study. Int J Cardiol. 2016;218:83–88.
- de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with reviewer disclosures reviewer employment research grant other research support speakers’ Bureau/Honoraria Exp – Search Results – PubMed; 2022. https://pubmed.ncbi.nlm.nih.gov/?term=longqueryec1bd4807d9e18d38fb3
- Thomas G, Xie D, Chen H-Y, CRIC Study Investigators, et al. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report From the chronic renal insufficiency cohort study. Hypertension. 2016;67(2):387–396.
- Shalaeva E, Bano A, Kasimov U, et al. Coronary artery calcium score and coronary computed tomography angiography predict one-year mortality in patients with type 2 diabetes and peripheral artery disease undergoing partial foot amputation. Diabetes Vasc. Dis. Res. 2022;19(5):147916412211251.
- Egan BM, Zhao Y, Li J, et al. Prevalence of optimal treatment regimens in patients with apparent treatment-resistant hypertension based on office blood pressure in a community-based practice network. Hypertension. 2013;62(4):691–697.
- Tanner RM, Calhoun DA, Bell EK, et al. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8(9):1583–1590.
- Smith SM, Huo T, Delia Johnson B, et al. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI-sponsored WISE study. J Am Heart Assoc. 2014;3(1):e000660.
- Sim JJ, Bhandari SK, Shi J, et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88(10):1099–1107.
- Osborn JW, Banek CT. Catheter-based renal nerve ablation as a novel hypertension therapy: lost, and then found, in translation. Hypertension. 2018;71(3):383–388.
- Appel LJ, American Society of Hypertension Writing Group; Giles TD, et al. ASH position paper: dietary approaches to lower blood pressure. J Clin Hypertens (Greenwich). 2009;11(7):358–368.
- Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of dietary approaches to stop hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24(12):1253–1261.
- Shimbo D, Levitan EB, Booth JN, et al. The contributions of unhealthy lifestyle factors to apparent resistant hypertension: findings from the reasons for geographic And racial differences in stroke (REGARDS) study. J Hypertens. 2013;31(2):370–376.
- Kim MT, Hill MN, Bone LR, et al. Development and testing of the Hill-Bone compliance to high blood pressure therapy scale. Prog Cardiovasc Nurs. 2000;15(3):90–96.
- Coppolino G, Pisano A, Rivoli L, et al. Renal denervation for resistant hypertension. Cochrane Database Syst Rev. 2017;2(2):CD011499.
- Whelton PK, Appel LJ, Sacco RL, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American heart association sodium reduction recommendations. Circulation. 2012;126(24):2880–2889.
- Messerli FH, Bavishi C, Brguljan J, et al. Renal denervation in the antihypertensive arsenal – knowns and known unknowns. J Hypertens. 2022;40(10):1859–1875.
- Blaustein MP, Leenen FHH, Chen L, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302(5):H1031–1049.
- Sidikov A, Zaslavsky D, Sadykov A, et al. The new differential diagnostic test for the lichenoid drug eruption. Dermatol Ther. 2020;33(6):e13784.
- Oh YS, Appel LJ, Galis ZS, et al. National heart, lung, and blood institute working group report on salt in human health and sickness: building on the current scientific evidence. Hypertension. 2016;68(2):281–288.
- Pimenta E, Gaddam KK, Oparil S, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54(3):475–481.
- McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24(12):2096–2103.
- Diaz KM, Shimbo D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. 2013;15(6):659–668.
- Carnethon MR, Evans NS, Church TS, et al. Joint associations of physical activity and aerobic fitness on the development of incident hypertension: coronary artery risk development in young adults. Hypertension. 2010;56(1):49–55.
- Chase NL, Sui X, Lee DC, et al. The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am J Hypertens. 2009;22(4):417–424.
- Crump C, Sundquist J, Winkleby MA, et al. Interactive effects of physical fitness and body mass index on the risk of hypertension. JAMA Intern Med. 2016;176(2):210–216.
- Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–411.
- Dimeo F, Pagonas N, Seibert F, et al. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60(3):653–658.
- McFadden CB, Brensinger CM, Berlin JA, et al. Systematic review of the effect of daily alcohol intake on blood pressure. Am J Hypertens. 2005;18(2 Pt 1):276–286.
- Chen L, Smith GD, Harbord RM, et al. Alcohol intake and blood pressure: a systematic review implementing a mendelian randomization approach. PLoS Med. 2008;5(3):0461–0471.
- Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2012;14(11):792–798.
- Husain K, Ansari RA, Ferder L. Alcohol-induced hypertension: mechanism and prevention. World J Cardiol. 2014;6(5):245–252.
- Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17(3):327–335.
- Lim LF, Solmi M, Cortese S. Association between anxiety and hypertension in adults: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;131:96–119.
- Johnson HM. Anxiety and hypertension: is there a link? A literature review of the comorbidity relationship Between anxiety and hypertension. Curr Hypertens Rep. 2019;21(9):1–7.
- Nemcsik-Bencze Z, et al. Depression and anxiety in different hypertension phenotypes: a cross-sectional study. Ann Gen Psychiatry. 2022;21(1):23.
- Narita K, Hoshide S, Tsoi K, et al. Disaster hypertension and cardiovascular events in disaster and COVID‐19 pandemic. J Clin Hypertens (Greenwich). 2021;23(3):575–583.
- Shimokawa H, et al. Guidelines for disaster medicine for patients with cardiovascular diseases (JCS 2014/JSH 2014/JCC 2014) – digest version. Circ. J. 2016;80(1):261–284.
- Haas SA, Ramirez D. Childhood exposure to war and adult onset of cardiometabolic disorders among older europeans. Soc Sci Med. 2022;309:115274.
- Acharya T, Tringali S, Singh M, et al. Resistant hypertension and associated comorbidities in a veterans affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741–745.
- Bakhtar O, Ference BA, Hedquist LA, et al. Relationship of resistant hypertension and treatment outcomes with total arterial compliance in a predominantly african American hypertensive cohort. J Clin Hypertens (Greenwich). 2012;14(9):618–622.
- Holecki M, Duława J, Chudek J. Resistant hypertension in visceral obesity. Eur J Intern Med. 2012;23(7):643–648.
- Cuspidi C, Macca G, Sampieri L, et al. High prevalence of cardiac and extracardiac target organ damage in refractory hypertension. J Hypertens. 2001;19(11):2063–2070.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57(6):1076–1080.
- de La Sierra A, Larrousse M, Oliveras A, et al. Abnormalities of vascular function in resistant hypertension. Blood Press. 2012;21(2):104–109.
- Shalaeva EV, Shadmanov AК, Azizova FL, et al. Is lone hypertension a risk factor for more severe COVID-19 outcomes? Glob Heart. 2022;17(1):17.
- Oliveras A, De La Sierra A. Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertens. 2014;28(4):213–217.
- Demede M, Pandey A, Zizi F, et al. Resistant hypertension and obstructive sleep apnea in the primary-care setting. Int J Hypertens. 2011;2011:340929.
- Muxfeldt ES, Cardoso CRL, Salles GF. Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch Intern Med. 2009;169(9):874–880.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–817.
- Pimenta E, Stowasser M, Gordon RD, et al. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest. 2013;143(4):978–983.
- Friedman O, Bradley TD, Ruttanaumpawan P, et al. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. Am J Hypertens. 2010;23(2):174–179.
- Quinaglia T, Martins LC, Figueiredo VN, et al. Non-dipping pattern relates to endothelial dysfunction in patients with uncontrolled resistant hypertension. J Hum Hypertens. 2011;25(11):656–664.
- Salles GF, Ribeiro FM, Guimarães GM, et al. A reduced heart rate variability is independently associated with a blunted nocturnal blood pressure fall in patients with resistant hypertension. J Hypertens. 2014;32(3):644–651.
- Kansui Y, Matsumura K, Kida H, et al. Clinical characteristics of resistant hypertension evaluated by ambulatory blood pressure monitoring. Clin Exp Hypertens. 2014;36(7):454–458.
- Gonzaga CC, Gaddam KK, Ahmed MI, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6(4):363.
- Eide IK, Torjesen PA, Drolsum A, et al. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22(11):2217–2226.
- Hottenga J-J, Boomsma DI, Kupper N, et al. Heritability and stability of resting blood pressure. Twin Res Hum Genet. 2005;8(5):499–508.
- Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41(6):1196–1201.
- Kupper N, Willemsen G, Riese H, et al. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 2005;45(1):80–85.
- Newton-Cheh C, Johnson T, Gateva V, Wellcome Trust Case Control Consortium, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41(6):666–676.
- Yugar-Toledo JC, Martin JFV, Krieger JE, et al. Gene variation in resistant hypertension: multilocus analysis of the angiotensin 1-converting enzyme, angiotensinogen, and endothelial nitric oxide synthase genes. DNA Cell Biol. 2011;30(8):555–564.
- Oliveira-Paula GH, Lacchini R, Coeli-Lacchini FB, et al. Inducible nitric oxide synthase haplotype associated with hypertension and responsiveness to antihypertensive drug therapy. Gene. 2013;515(2):391–395.
- Lynch AI, Irvin MR, Davis BR, et al. Genetic and adverse health outcome associations with treatment resistant hypertension in GenHAT. Int. J. Hypertens. 2013;2013:1–10.
- Fontana V, McDonough CW, Gong Y, et al. Large-scale gene-centric analysis identifies polymorphisms for resistant hypertension. JAHA. 2014;3(6):e001398.
- Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41(3):348–353.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642.
- Sim JJ, Bhandari SK, Shi J, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88(3):622–632.
- Messerli FH, Shalaeva EV, Rexhaj E. Optimal BP targets to prevent stroke and MI: is there a lesser of 2 evils? J Am Coll Cardiol. 2021;78(17):1679–1681.
- Tsioufis C, Kasiakogias A, Kordalis A, et al. Dynamic resistant hypertension patterns as predictors of cardiovascular morbidity: a 4-year prospective study. J Hypertens. 2014;32(2):415–422.
- Pierdomenico SD, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18(11):1422–1428.
- Bangalore S, Fayyad R, Laskey R, et al. Prevalence, predictors, and outcomes in treatment-resistant hypertension in patients with coronary disease. Am J Med. 2014;127(1):71–81.e1.
- Smith SM, Gong Y, Handberg E, et al. Predictors and outcomes of resistant hypertension among patients with coronary artery disease and hypertension. J Hypertens. 2014;32(3):635–643.
- Jin C-N, Liu M, Sun J-P, et al. The prevalence and prognosis of resistant hypertension in patients with heart failure. PLoS One. 2014;9(12):e114958.
- Freeman MW, Halvorsen Y-D, Marshall W, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388(5):395–405.
- Bogman K, Schwab D, Delporte M-L, et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2). Hypertension. 2017;69(1):189–196.