Abstract
Purpose: Interventional approaches to treat hypertension are an emerging option that may be suitable for patients whose BP control cannot be achieved with lifestyle and/or pharmacotherapy and possibly for those who do not wish to take drug therapy.
Materials and Methods: Interventional strategies include renal denervation with radiofrequency, ultrasound and alcohol-mediated platforms as well as baroreflex activation therapy and cardiac neuromodulation therapy. Presently renal denervation is the most advanced of the therapeutic options and is currently being commercialised in the EU.
Results: It is apparent that RDN is effective in both unmedicated patients and patients with more severe hypertension including those with resistant hypertension.
Conclusion: However, at present there is no evidence for the use of RDN in patients with secondary forms of hypertension and thus evaluation to rule these out is necessary before proceeding with a procedure. Furthermore, there are numerous pitfalls in the diagnosis and management of secondary hypertension which need to be taken into consideration. Finally, prior to performing an intervention it is appropriate to document presence/absence of hypertension-mediated organ damage.
PLAIN LANGUAGE SUMMARY
RDN has emerged as a safe and effective approach to treat hypertension with BP lowering efficacy equivalent to antihypertensive monotherapy albeit with guaranteed ‘adherence’
Presently populations most likely to respond to RDN are not clearly defined but given the costs of the procedure it is likely to be initially made available to those with resistant hypertension and those at highest cardiovascular risk
There is no evidence to support the use of RDN in patients with secondary forms of hypertension and thus this should be thoroughly screened for prior to offering the procedure, especially in the setting of resistant hypertension
Optimisation of lifestyle and drug therapy is key to good hypertension management and should be undertaken prior to an invasive procedure such as RDN being offered
There are numerous pitfalls in the screening process for secondary hypertension which means that hypertension specialists should be involved in this component of the pathway
RDN can be offered by interventional radiologist, interventional cardiologists or angiologists who have had appropriate training
Clinical pathways for RDN must ultimately involve a multidisciplinary team overview with hypertension specialists, interventionists and imaging specialists combining efforts to ensure appropriate patient selection. This without question must involve patients in the decision-making process.
Introduction
Given the numerous, established methods to treat hypertension with lifestyle measures and the vast array of pharmacological options with numerous drug classes and multiple within-class drug alternatives, one might question the need for an additional strategy with invasive approaches to treat hypertension. Before considering what is on offer, it is important to establish that both lifestyle measures and drug therapy to treat hypertension are both exquisitely dependent on lifelong patient adherence to strategies to treat an asymptomatic condition – and frequently drug therapy can result in adverse effects, thus causing hypertension-related symptoms for the first time. It has already been clearly demonstrated that clinician expectations with respect to long term commitment by patients to lifestyle and drug therapy strategies are rarely met [Citation1]. Noting the global prevalence of hypertension with alarmingly poor rates of hypertension control in both men (18%) and women (23%), it behoves us to examine strategies not only to improve hypertension detection and drug treatment but also to countenance options such as renal denervation (RDN) which are independent from behavioural manipulation [Citation2].
Selecting patients for interventional procedures
Which patients?
In the aftermath of the Symplicity HTN-3 study, a new generation of randomised, controlled, clinical trials has demonstrated the safety and efficacy of RDN with radiofrequency energy [RF-RDN] (in drug naïve and medicated patients) and with ultrasound [US-RDN] (in drug naïve and resistant hypertensive patients), and emerging data support the efficacy of ethanol mediated RDN too [Citation3–8]. In all these trials, patients with established secondary forms of hypertension were excluded and, given that most recruitment came from hypertension centres of excellence, patients were also thoroughly worked up to ensure undiagnosed secondary hypertension was excluded.
Thus there is no data to date on use of RDN in patients with secondary hypertension and there is even a suggestion that patients with primary aldosteronism are unlikely to respond to renal denervation due to reduced muscle sympathetic nerve activity [Citation9,Citation10]. Patients with resistant hypertension appear an attractive population for whom RDN should be considered given they are highest risk of events and have most to gain [Citation11]. On the other hand, patients should not be required to have resistant hypertension to receive treatment with RDN (or other interventional approaches) especially if this offers the best opportunity to attain hypertension control.
Other than those with secondary hypertension, patients in whom RDN is (relatively) contraindicated include those with reduced renal function (eGFR < 40 ml/min) and those with renovascular disorder (either renal artery stenosis or fibromuscular dysplasia) and small renal arteries (diameter < 3 mm) and structural renal abnormalities. Furthermore RDN should be avoided in those patients with valvular heart disease and pregnant females. Presently it is thought that patient with isolated systolic hypertension are less likely to benefit from RDN and these patients have been excluded from the recent randomised clinical trials [Citation12].
Which procedure?
To date, US-RDN (Paradise™, Recor Medical, Palo Alto, California, USA) and RF-RDN (SPYRAL™, Medtronic, Minneapolis, Minnesota, United States) are advanced in both their clinical evaluation programmes and with commercialisation efforts. Some geographies already have reimbursement codes for the procedures (e.g. Germany; The Netherlands) and thus availability of RDN as a therapeutic option will be initially governed largely by location and in any particular site, interventionalists will have a choice of using one or both platforms. The advantages of having both are to provide a greater range of anatomical suitability given that US-RDN with the Paradise system presently requires renal artery dimensions of 4–8 mm diameter and >25 mm in length. Multielectrode RF-RDN with the SPYRAL catheter can access vessels 3–8 mm in diameter and can navigate complex anatomy.
Presently there is data to suggest equivalent efficacy (with no safety concerns) using these two catheters from a single small study and therefore choice of catheter will ultimately come down to operator preference given that costs are comparable and procedural duration is similar between the technologies [Citation13].
Work up of patients for intervention
Reversible secondary causes of hypertension should be established before offering a costly invasive procedure to treat hypertension as their presence will alter the management pathway. Patients with uncontrolled hypertension can be selected for interventional therapy following the pathway outlined in . A reasonable time frame for such a work-up would be 3–6 months given constraints in socialised medicine systems. The findings from the work up should be discussed in a multidisciplinary team meeting with appropriate specialists (interventionists and hypertension specialists) with documentation of the outcome and decision of whether or not to proceed to interventional therapy of hypertension.
Table 1. Work up pathway for selection of hypertensive patients for interventional therapy.
Identifying patients for device therapy of hypertension
A reasonable initial criterion for the practising clinician is to ask whether or not my patient can achieve hypertension control without the need for a high-cost invasive procedure. With increasing emphasis on the role of patient choice, an alternative view would be to offer RDN to those who express a preference for it as long as this provides the most pragmatic route to achieving hypertension control. Notably studies demonstrate willingness of patients to have device therapy of hypertension in preference to lifelong medication and this is unrelated to their BP levels or number of prescribed medications [Citation18,Citation19].
BP measurement
It is critical to ensure that the patient is truly hypertensive when measured according to appropriate standards both in the office and out of office (see ). This requires correct technique of measurement as well as trustworthy technology. Presently only oscillometric monitors are validated for home BP measurement although an emerging class of cuffless, wearable BP monitors will at some point in the foreseeable future also provide valuable out of office BP metrics [Citation21,Citation22].
Table 2. Thresholds for diagnosis & treatment of hypertension with out of office monitoring.
The safety and efficacy of RDN has not been demonstrated in individuals with masked hypertension (although it is likely to be effective) or white coat hypertension to date. Whilst office measurement of BP is a starting point in this process, ambulatory BP monitoring (ABPM) and standardised home BP monitoring are critical adjuncts to assist with understanding BP on a continuous basis.
Home BP monitoring
Patients should be educated how measure BP at home using validated, digital, oscillometric equipment with the appropriate cuff size and with the correct technique [Citation23]. This includes measurement in either arm (or the arm with higher BP if there is an interarm difference), whilst seated with their back supported, after resting for five minutes. They should not have consumed caffeine or smoked or have exercised within 30 min of measurement. Blood pressure should be measured at least twice, 1 min apart, in the mornings and evenings in order to evaluate BP levels, and/or capture treatment effects in advance of the next clinic visit. Home BP can be used to both diagnose and guide treatment of hypertension and provides superior prognostic information compared to office BP (see ) [Citation24].
ABPM
ABPM is proven to be cost effective and is recommended by international guidelines as a key component of the diagnostic pathway in patients who are being considered for medication to treat their hypertension [Citation23,Citation25,Citation26]. In multiple studies ABPM has been proven superior to office BP in predicting cardiovascular morbidity and mortality in both patients with hypertension and population cohorts [Citation27].
ABPM can confirm the diagnosis of white coat hypertension (prevalence up to 20% above the age of 70 years) as well as masked hypertension (prevalence 15%) [Citation28,Citation29]. In addition to provide diagnostic information on BP levels, ABPM is increasingly used to guide treatment decisions and monitor response to therapy though there is presently insufficient data for firm guidelines’ recommendation regarding optimal targets (see ) [Citation27]. There are significant limitations to ABPM use as outlined in along with proposed strategies to get around them.
Table 3. Pitfalls in the use of ABPM and options to solve them.
Lifestyle & medication optimisation
Importance of sodium
Primary hypertension and age-related increase in BP does not occur in populations consuming < 1 g of salt daily [Citation31]. Accordingly, high sodium intake is recognised to contribute to hypertension and increased cardiovascular morbidity and mortality [Citation32,Citation33]. Many clinicians overlook, and most patients are not always aware of, this fact and in general patients are unaware of the extent of their own salt intake. Globally average intake of sodium in adults is reckoned to be approximately 4 g per day (salt 10 g per day) [Citation34]. International and country-specific guidelines recommend a reduced salt intake of < 6 g daily in individuals with hypertension [Citation23,Citation26,Citation35–38]. This requires that patients be counselled regarding the sources of salt in daily life, both apparent (e.g. salt-shakers) and hidden (e.g. processed foods, condiments).
Reduction in salt intake is recommended to reduce BP levels and save lives in population cohorts: it is estimated that a modest 30% reduction in sodium intake, could save 40 million lives globally within 25 years [Citation39]. Furthermore, a recent metanalysis has demonstrated that interventions to reduce salt intake result in clinically meaningful blood pressure reduction (4 mmHg per 6 g salt) in the short term [Citation40].
Salt restriction is thus a reasonable approach to improving hypertension control in individuals prior to selecting them for renal denervation and is more likely to demonstrate larger effects on BP levels in salt sensitive individuals (older patients, black and Asian ethnicity) [Citation41]. Methods to evaluate salt intake and their drawbacks are outlined in .
Table 4. Pitfalls in the assessment of salt intake.
Optimising drug adherence
It would be reasonable to ensure that efforts to improve hypertension control through timely uptitration of medication although constraints upon primary and secondary healthcare provision in different geographies may mean that patients wait months for follow up appointments to have medication review. As such telemedicine programmes have been demonstrated to result in improved hypertension care but are not in widespread use [Citation45].
In the case of adherence to drug therapy of hypertension, there are two key obstacles to therapeutic success:
Covert non-adherence: the patient does not take antihypertensive medication either from the outset (initiation failure) or does not take them as prescribed with missed doses (implementation failure) but does not admit this to physician – this is exceedingly common and more of an issue with a greater burden of prescribed antihypertensive medication [Citation46].
Overt non-adherence: the patient complains of intolerance to antihypertensive medication and states this to physician – adverse effects can be explicable (e.g. cough with ACE-inhibitor, ankle swelling with calcium channel blocker) or unpredictable (feeling awful, fogging). This is less common but clinically very challenging as strategies to manage this are time consuming and costly although efficacy has been proven [Citation47].
Improving covert non-adherence in hypertensive patients is challenging and whilst therapeutic drug monitoring assists in the identification of this problem (See ), these are no data to support long term cost effectiveness or efficacy of adherence strategies although they should not be overlooked entirely [Citation46]. These include approaches such as pill reminders and e-health monitoring systems coupled with self-monitoring of BP. A highly useful strategy would be to simplify medication regimens where possible through use of once daily single pill combination (SPC) formulations which are known to both improve adherence and reduce cardiovascular outcomes in hypertensives compared to monotherapy regimens [Citation52–54]. Importantly the use of SPC for hypertension is now endorsed by major recent guidelines in the US and Europe although availability of two- and three-drug SPC varies widely across Europe and the UK [Citation23,Citation55].
Table 5. Pitfalls in Assessing & managing adherence.
Screening for secondary hypertension
This should be limited to those patients in whom a secondary cause of hypertension is more likely as it would not be cost effective to screen all comers, especially those with milder forms for hypertension. Clinical features that should prompt investigation for a secondary form of hypertension are outlined in and summarised in .
Figure 1. Summary of causes of secondary hypertension and key screening investigations. A:CR: albumin:creatinine ratio; Ca: calcium; CKD: chronic kidney disease; CTA: computed tomography angiogram; DUS: duplex ultrasound; eGFR: estimated glomerular filtration rate; FHx: family history; LDDS: low dose dexamethasone suppression test; OSA: obstructive sleep apnoea; pMets: plasma metanephrines; PTH: parathyroid hormone; TSH: thyroid stimulating hormone; TTE: transthoracic echocardiogram; US: ultrasound.
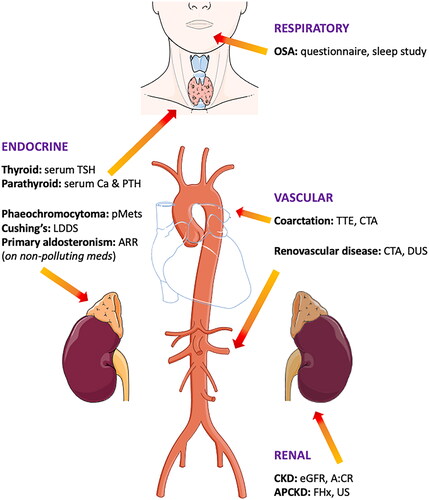
Table 6. Clinical features suggestive of secondary hypertension.
Renal parenchymal and renovascular disorder
Renal parenchymal disease is the most common cause of hypertension in children and adolescents and up to 10–15% of all adults [Citation38]. It is generally asymptomatic and best detected with biochemical assay (estimated glomerular filtration rate, urinary albumin:creatinine ratio), urine dipstick for blood/protein and renal tract ultrasound imaging.
Renovascular disorder due to either atherosclerotic renal artery disease or fibromuscular dysplasia is recognised as an important cause of secondary hypertension and investigation to rule this out is an important component in the work-up of resistant hypertension [Citation56].
Assessment of the renal arteries in the work up of renovascular hypertension has the added benefit on providing valuable information on anatomical suitability for the RDN procedure and presence/absence of accessory renal arteries. Imaging modalities include doppler ultrasound, Computed Tomographic and Magnetic Resonance angiography (CTA & MRA) and access to these is dependent on geography [Citation57]. There are caveats to assessment of renovascular disorder as outlined in .
Table 7. Pitfalls in the assessment of renovascular disorder.
Currently there is no evidence to suggest the efficacy of RDN or other interventional therapies in treated (i.e. post balloon angioplasty or renal artery stenting) renovascular disease and it is largely considered a contra-indication for RDN in the main artery.
Renal accessory arteries are common and stenoses of these vessels are largely non-functional though whether RDN is appropriate and effective in this setting is unclear as these patients were largely excluded from rennet clinical trials.
In contrast, presence of chronic kidney disease is not a contraindication to RDN or other interventional approaches until the patient reaches end-stage kidney disease and trials are ongoing to ascertain whether even in this group there is benefit from RDN for BP control) [Citation63].
Endocrine hypertension
Whilst some forms of adrenal hypertension (phaeochromocytoma, Cushing’s disease) are rarely encountered (), it is believed that primary aldosteronism (PA) accounts for 5–10% of all hypertension and is generally underdiagnosed [Citation67,Citation68]. Recent Endocrine Society guidelines to improve case detection and management of primary hyperaldosteronism have been published which standardise the approach to diagnosis [Citation69]. Complexities surrounding the diagnosis and management of hypertension of adrenal origin are outlined in .
Table 8. Endocrine causes of hypertension.
Table 9. Pitfalls in the assessment of hypertension of adrenal origin.
Whilst adequate reversal of underlying endocrine drivers to hypertension are largely not a barrier to interventional BP therapies, this is not the case with medically treated (i.e. not suitable for unilateral adrenalectomy) primary aldosteronism, which is still considered to be a contra-indication for RDN and other modalities in the absence of dedicated data.
Obstructive sleep apnoea (OSA)
OSA is a common cause of hypertension in young adults (18–35 years) in whom other secondary cause are excluded and is also seen commonly in adults with resistant hypertension [Citation73,Citation74]. It should be screened for with sleep surveys (Epworth Sleep Scale, Berlin Questionnaire or STOP-BANG questionnaire) with higher scores correlating with increasing severity of OSA [Citation75]. The gold standard investigation is overnight polysomnography (PSG) which requires a hospital stay. Alternatively home-based sleep tests are a reasonable alternative which have equivalent diagnostic sensitivity to PSG and are popular with patients [Citation76]. Pitfalls in screening for OSA are outlined in .
Table 10. Pitfalls in screening for OSA.
Notably, RDN has been shown to improve both office and ABP as well as sleep apnoea severity in a small randomised controlled study in 60 patients with resistant hypertension and moderate to severe OSA [Citation79]. At present there is insufficient data to recommend treatment with RDN in this setting and more research is needed and OSA should be diagnosed and treated conventionally with lifestyle measures and usual interventions (weight loss, continuous positive airways pressure, mandibular advancement devices etc). In the clinical trial setting, adequate treatment of OSA (with improvement in hyponoea/apnoea index) for at least 3 months has not been a contra-indication for inclusion and thus this is a reasonable approach in clinical practice.
Conclusion
It is important to exclude secondary forms of hypertension prior to selecting patients for device-based therapy as there is no evidence to support their use in this setting. The work up can be complicated, and it is best left to high volume specialised units to fully investigate adrenal hypertension and also to make management decisions regarding interventional therapy of renovascular hypertension. In addition, there is no guarantee that a patient entering a work up pathway for RDN will necessarily be suitable for the procedure either due to ambulatory or home BP levels or not meeting anatomical criteria. Indeed in the recent randomised controlled clinical trials of RDN, only 10–20% of enrolled patients met full inclusion criteria meaning that many patients who are keen to have RDN will ultimately not be suitable if centres offering the treatment adhere strictly to protocol for delivering the therapy [Citation3–6,Citation8].
Given that access to specialised care is frequently limited, hypertensive patients then run the risk of uncontrolled hypertension during work up for interventional therapy and this should be avoided where possible for 2 important reasons. Firstly, it has been demonstrated that delays in intensifying BP control when SBP levels are >150 mmHg leaves patients at substantially increased risk of CV events and death [Citation80]. This mandates clinicians to treat hypertension proactively and not delay initiation/uptitration of antihypertensive medications (i.e. avoid therapeutic inertia) during initial patient assessment. Secondly increasing evidence support the importance of durable BP control and that SBP time in target range is an independent predictor of major adverse cardiovascular events [Citation81]. This warrants close follow up of patients with uncontrolled hypertension which has major resource implications and may best be served with e-health monitoring programmes to ensure ongoing appropriate medicines management to maintain hypertension control.
Thus, patients who are being evaluated for a device-therapy programme should be informed that they may not be suitable for the procedure for anatomical or BP reasons or that a secondary form of hypertension may be discovered. Furthermore, they should be notified that there is a 30% chance of non-response to RDN. Finally, whilst patients are being worked up, clinicians must continue to try to improve BP control with the aforementioned approaches and not delay initiation or uptitration of antihypertensive medication and leave their patient at increased cardiovascular risk.
Disclosure statement
Melvin D LOBO: Personal consultancy with Ablative Solutions, Medtronic, Recor Medical and Aktiia. Grant funding from Medtronic & Recor Medical. Manish SAXENA: Dr Saxena reports personal consultancy with Recor Medical Inc., Novartis, Astra Zeneca, Alnylam, Vifor Pharma, C4 Research. He has received Institutional grant from Recor Medical Inc., Ablative Solutions Inc., Applied Therapeutics, MSD. Gurvinder RULL & Vikas KAPIL: None to report.
Additional information
Funding
References
- Pathak A, Poulter NR, Kavanagh M, et al. Improving the management of hypertension by tackling awareness, adherence, and clinical inertia: a symposium report. Am J Cardiovasc Drugs. 2022;22(3):1–11.
- Zhou B, Carrillo-Larco RM, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021; 398(10304): 957–980.
- Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476–2486.
- Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335–2345.
- Bohm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–1451.
- Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346–2355.
- Mahfoud F, Renkin J, Sievert H, et al. Alcohol-Mediated renal denervation using the peregrine system infusion catheter for treatment of hypertension. JACC Cardiovasc Interv. 2020;13(4):471–484.
- Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390(10108):2160–2170.
- Miyajima E, Yamada Y, Yoshida Y, et al. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension. 1991;17(6 Pt 2):1057–1062.
- Schmieder RE, Bosch A. Editorial comment: renal denervation. Hypertens Res. 2022;45(2):241–243.
- Wei FF, Zhang ZY, Huang QF, et al. Diagnosis and management of resistant hypertension: state of the art. Nat Rev Nephrol. 2018;14(7):428–441.
- Barbato E, Azizi M, Schmieder RE, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC council on hypertension and the european association of percutaneous cardiovascular interventions (EAPCI). Eur Heart J. 2023;44(15):1313–1330.
- Fengler K, Rommel KP, Lapusca R, et al. Renal denervation in isolated systolic hypertension using different catheter techniques and technologies. Hypertension. 2019;74(2):341–348.
- Elmer PJ, Obarzanek E ,Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomised trial. Ann Intern Med. 2006;144(7):485–495.
- Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(1):e004473.
- Arar MY, Hogg RJ ,Arant BS, Jr., Seikaly MG. Aetiology of sustained hypertension in children in the southwestern United States. Pediatr Nephrol. 1994;8(2):186–189.
- Chetan D, Mertens LL. Challenges in diagnosis and management of coarctation of the aorta. Curr Opin Cardiol. 2022;37(1):115–122.
- Schmieder RE, Hogerl K, Jung S, et al. Patient preference for therapies in hypertension: a cross-sectional survey of german patients. Clin Res Cardiol. 2019;108(12):1331–1342.
- Schmieder RE, Kandzari DE, Wang TD, et al. Differences in patient and physician perspectives on pharmaceutical therapy and renal denervation for the management of hypertension. J Hypertens. 2021;39(1):162–168.
- Mancia Chairperson G, Kreutz Co-Chair R, Brunstrom M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023. doi: 10.1097/HJH.0000000000003480.
- Mukkamala R, Stergiou GS, Avolio AP. Cuffless blood pressure measurement. Annu Rev Biomed Eng. 2022;24:203–230.
- Stergiou GS, Palatini P, Parati G, et al. 2021 European society of hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–1302.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248.
- George J, MacDonald T. Home blood pressure monitoring. Eur Cardiol. 2015;10(2):95–101.
- Mancia Chairperson G, Kreutz Co-Chair R, Brunström M, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the european society of hypertension endorsed by the european renal association (ERA) and the international society of hypertension (ISH). J Hypertens. 2023. doi: 10.1097/HJH.0000000000003480.
- Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357.
- Huang QF, Yang WY, Asayama K, et al. Ambulatory blood pressure monitoring to diagnose and manage hypertension. Hypertension. 2021;77(2):254–264.
- Conen D, Aeschbacher S, Thijs L, et al. Age-specific differences between conventional and ambulatory daytime blood pressure values. Hypertension. 2014;64(5):1073–1079.
- Hansen TW, Kikuya M, Thijs L, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25(8):1554–1564.
- Verdecchia P ,Angeli F ,Borgioni C ,Gattobigio R ,Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension. 2007;49(4):777–783.
- Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–1978.
- Mente A, O’Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371(7):601–611.
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–623.
- Sharman JE, Ordunez P, Brady T, et al. 2022 World hypertension league, resolve to save lives and international society of hypertension dietary sodium (salt) global call to action. J Hum Hypertens. 2023;37(2):155–159.
- Barroso WKS, Rodrigues CIS, Bortolotto LA, et al. Brazilian guidelines of hypertension - 2020. Arq Bras Cardiol. 2021;116(3):516–658.
- Joint Committee for Guideline R. Chinese guidelines for prevention and treatment of Hypertension-A report of the revision committee of chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. 2018;16(3):182–241.
- Umemura S, Arima H, Arima S, et al. The japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–1481.
- Williams B, Mancia G, Spiering W, et al. ESC/ESH guidelines for the management of arterial hypertension. European Heart Journal. 2018;313:603–698.
- Kontis V, Cobb LK, Mathers CD, et al. Three public health interventions could save 94 million lives in 25 years. Circulation. 2019;140(9):715–725.
- Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–634.
- Pilic L, Pedlar CR, Mavrommatis Y. Salt-sensitive hypertension: mechanisms and effects of dietary and other lifestyle factors. Nutr Rev. 2016;74(10):645–658.
- Rust P ,Ekmekcioglu C. Impact of salt intake on the pathogenesis and treatment of hypertension. Adv Exp Med Biol. 2017;956:61–84.
- Holbrook JT ,Patterson KY ,Bodner JE, et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40(4):786–793.
- Maughan RJ ,Leiper JB. Sodium intake and post-exercise rehydration in man. Eur J Appl Physiol Occup Physiol. 1995;71(4): 311–319.
- Omboni S, McManus RJ, Bosworth HB, et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension: an international expert position paper. Hypertension. 2020;76(5):1368–1383.
- Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124(7):1124–1140.
- Antoniou S, Saxena M, Hamedi N, et al. Management of hypertensive patients with multiple drug intolerances: a Single-Center experience of a novel treatment algorithm. J Clin Hypertens (Greenwich). 2016;18(2):129–138.
- Burnier M ,Egan BM. Adherence in hypertension. Circul Res. 2019;124(7):1124–1140.
- Tanna S ,Ogwu J ,Lawson G. Hyphenated mass spectrometry techniques for assessing medication adherence: advantages, challenges, clinical applications and future perspectives. Clin Chem Lab Med 2020;58(5):643–663.
- Azizi M ,Pereira H ,Hamdidouche I, et al. Adherence to antihypertensive treatment and the blood pressure lowering effects of renal Denervation in the Renal Denervation for Hypertension (DENERHTN) Trial. Circulation. 2016;134(12):847–857.
- Glynn LG ,Murphy AW ,Smith SM ,Schroeder K ,Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev 2010;3:CD005182.
- Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55(2):399–407.
- Verma AA, Khuu W, Tadrous M, et al. Fixed-dose combination antihypertensive medications, adherence, and clinical outcomes: a population-based retrospective cohort study. PLoS Med. 2018;15(6):e1002584.
- Webster R, Murphy A, Bygrave H, et al. Implementing fixed dose combination medications for the prevention and control of cardiovascular diseases. Glob Heart. 2020;15(1):57.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104.
- Herrmann SM, Textor SC. Renovascular hypertension. Endocrinol Metab Clin North Am. 2019;48(4):765–778.
- Baumgartner I, Lerman LO. Renovascular hypertension: screening and modern management. Eur Heart J. 2011;32(13):1590–1598.
- Textor SC. Pitfalls in imaging for renal artery stenosis. Ann Intern Med 2004;141(9):730–731.
- Gornik HL ,Persu A ,Adlam D, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med 2019;24(2):164–189.
- Fodor L ,Premuzic V ,Ivkovic V, et al. Arterial stiffness in atherosclerotic renovascular hypertension. J Hypertens. 2014;32(11):2238–2245; discussion 45.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomised trial. Ann Intern Med. 2009;150(12):840–888, W150-1.
- Investigators A ,Wheatley K, Ives N, et al. Revascularization versus medical therapy for renal-artery stenosis. New Engl J Med. 2009;361(20):1953–1962.
- Schmieder RE. Renal denervation in patients with chronic kidney disease: current evidence and future perspectives. Nephrol Dial Transplant. 2023;38(5):1089–1096.
- Zennaro MC, Boulkroun S ,Fernandes-Rosa FL. Pathogenesis and treatment of primary aldosteronism. Nat Rev Endocrinol 2020;16(10):578–589.
- Nishioka H ,Yamada S. Cushing’s disease. J Clin Med 2019;8(11):1951.
- Medical Masterclass C ,Firth J. Endocrinology: phaeochromocytoma. Clin Med. 2019;19(1):68–71.
- Kayser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and Meta-Regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826–2835.
- Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the working group on endocrine hypertension of the european society of hypertension. J Hypertens. 2020;38(10):1919–1928.
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2016;101(5):1889–1916.
- Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233–247.
- Schilbach K ,Junnila RK ,Bidlingmaier M. Aldosterone to renin ratio as screening tool in primary aldosteronism. Exp Clin Endocrinol Diabetes. 2019;127(2-03):84–92.
- Kebebew E. Adrenal incidentaloma. New Engl J Med. 2021;384(16):1542–1551.
- Jinchai J, Khamsai S, Chattakul P, et al. How common is obstructive sleep apnea in young hypertensive patients? Intern Emerg Med. 2020;15(6):1005–1010.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277.
- Brown J, Yazdi F, Jodari-Karimi M, et al. Obstructive sleep apnea and hypertension: updates to a critical relationship. Curr Hypertens Rep. 2022;24(6):173–184.
- Corral J, Sanchez-Quiroga MA, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196(9):1181–1190.
- Malhotra A ,White DP. Obstructive sleep apnoea. Lancet 2002;360(9328):237–245.
- Hla KM ,Young T ,Finn L, et al. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep 2008;31(6):795–800.
- Warchol-Celinska E, Prejbisz A, Kadziela J, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-Concept phase II trial. Hypertension. 2018;72(2):381–390.
- Xu W, Goldberg SI, Shubina M, et al. Optimal systolic blood pressure target, time to intensification, and time to follow-up in treatment of hypertension: population based retrospective cohort study. BMJ. 2015;350:h158.
- Fatani N, Dixon DL, Van Tassell BW, et al. Systolic blood pressure time in target range and cardiovascular outcomes in patients with hypertension. J Am Coll Cardiol. 2021;77(10):1290–1299.
