Abstract
Background: Hypertension can be classified into different phenotypes based on systolic and diastolic blood pressure (BP) that carry a different prognosis and may therefore be differently associated with sympathetic activity. We assessed the association between cardiac autonomic function determined from continuous finger BP recordings and hypertensive phenotypes. Methods: We included 10,221 individuals aged between 18-70 years from the multi-ethnic HELIUS study. Finger BP was recorded continuously for 3-5 minutes from which cross-correlation baroreflex sensitivity (xBRS) and heart rate variability (HRV) were determined. Hypertension was classified into isolated systolic (ISH; ≥140/<90), diastolic (IDH; <140/≥90) and combined systolic and diastolic hypertension (SDH; ≥140/≥90). Differences were assessed after stratification by age (younger: ≤40, older: >40 years) and sex, using regression with correction for relevant covariates. For xBRS, values were log-transformed. Results: In younger adults with ISH, xBRS was comparable to normotensive individuals in men (ratio 0.92; 95%CI 0.84-1.01) and women (1.00; 95%CI 0.84-1.20), while xBRS was significantly lower in IDH and SDH (ratios between 0.67 and 0.80). In older adults, all hypertensive phenotypes had significantly lower xBRS compared to normotensives. We found a similar pattern for HRV in men, while in women HRV did not differ between phenotypes. Conclusions: In younger men and women ISH is not associated with a shift towards increased sympathetic control, while IDH and SDH in younger and all hypertensive phenotypes in older participants were associated with increased sympathetic control. This suggests that alterations in autonomic regulation could be a contributing factor to known prognostic disparities between hypertensive phenotypes.
Plain Language Summary
Hypertension can be classified into different phenotypes based on systolic and diastolic blood pressure (BP) that carry a different prognosis. Impaired autonomic regulation is important in the pathogenesis of hypertension and independently associated with adverse cardiovascular outcomes.
We analyzed 3-5 minutes continuous non-invasive finger blood pressure recordings performed in over 10.000 individuals participating in the HELIUS cohort study. From these measurements, short term heart rate variability (HRV) and cross correlation baroreflex sensitivity (xBRS) were determined using an automatic algorithm.
In our analysis we observed pronounced differences in the relation between autonomic regulation and hypertensive phenotypes that depend on age and sex.
Younger men and women (age 18-40 years) with isolated systolic hypertension had similar values for xBRS and HRV compared to normotensives, while isolated diastolic hypertension was associated with a shift towards increased sympathetic control. In contrast to our findings in younger individuals, all hypertensive phenotypes were associated with increased sympathetic control in older participants (age 40-70 years).
This supports earlier studies showing prognostic differences and suggests that alterations in sympathovagal balance could be a contributing factor to the disparities between phenotypes.
Introduction
Hypertension can be classified into isolated systolic hypertension (ISH), isolated diastolic hypertension (IDH) and combined systolic and diastolic hypertension (SDH) depending on the increase in either systolic or diastolic blood pressure (BP) [Citation1, Citation2]. Current treatment decisions are mainly based on systolic BP, however previous studies have shown important prognostic age and sex dependent differences between hypertensive phenotypes [Citation3]. These disparities have been attributed to different pathophysiological mechanisms, including differences in central and peripheral blood pressure in young individuals with ISH that are thought to result from alterations in wave reflection [Citation4]. However, other studies have shown that ISH also occurs in younger adults with a more adverse cardiovascular risk profile, including increased arterial stiffness and obesity [Citation5, Citation6]. Given these disparities, there is still an ongoing debate whether ISH is benign in younger adults [Citation7]. In older adults, ISH mainly results from increased arterial stiffening; and an increased systolic and pulse pressure have been consistently associated with an increased risk for cardiovascular events [Citation8,Citation9]. For isolated diastolic hypertension, it is still unclear whether is its associated with an increased risk of cardiovascular disease and from which threshold treatment is indicated [Citation10].
The sympathetic nervous system is one of the key (patho)physiological mechanisms in BP regulation [Citation11, Citation12]. The sympathetic overdrive results in elevated blood pressure levels by decreasing parasympathetic control, leading to less cardiac variability and an increased peripheral vascular tone, heart rate and stroke volume [Citation13]. The balance between sympathetic and parasympathetic autonomic control can be non-invasively assessed using the cross-correlation baroreflex sensitivity (xBRS) and heart rate variability [Citation14, Citation15]. The xBRS quantifies changes in heart rate resulting from spontaneous changes in blood pressure; while heart rate variability measures variations in the duration of subsequent heart beats. Lower values of xBRS, SDNN and RMSDD are indicative of a shift towards increased sympathetic control, resulting from (a combination of) lower vagal activity or increased sympathetic activity. HRV has shown to be predictive of cardiovascular events and development of hypertension in the general population, while BRS has shown to be a predictor of mortality in patients with a history of myocardial infarction [Citation16–19]. As parameters of sympathetic and parasympathetic autonomic control, HRV and BRS may aid in understanding the discrepancies in prognosis between hypertensive phenotypes. At present little is known about the relation between measures of autonomic function and the different hypertensive phenotypes. We hypothesize that phenotypes with a more beneficial prognosis are associated with autonomic regulation comparable to normotensives, while phenotypes with a more adverse prognosis have decreased vagal control. We therefore assessed differences in xBRS and HRV in relation to different hypertensive phenotypes in participating in a multi-ethnic population-based cohort study and determined whether this depends on age and sex.
Methods
Study design and measurements
We used baseline data collected between 2011-2015 of the ongoing HEalthy LIfe in an Urban Setting (HELIUS) study, from which the design and aims have been described in detail elsewhere [Citation20]. Based on the municipality registers, people aged between 18-70 years were randomly invited, stratified by ethnicity. Only individuals from the major ethnic groups living in Amsterdam were invited (Dutch, Surinamese, Ghanaian, Turkish, or Moroccan origin). After a positive response, participants received a confirmation letter of the appointment for the physical examination, including a digital or paper version of the questionnaire (depending on the preference of the subject). In total, 22,165 participants completed the questionnaire, and visited the research location. Visits were conducted after an overnight fast and participants were asked to refrain from smoking prior to the visit. Participants were ask to bring all prescribed medication to the research site. For this analysis, we included all participants with available continuous finger BP blood measurements of sufficient quality, as described below. Office BP was determined using the average of two consecutive measurements with a validated semi-automatic oscillometric device (Microlife WatchBP Home; Microlife AG, Switzerland). An electrocardiogram (ECG) was performed using a MAC 1600 System (GE Healthcare). Diabetes was defined as either a fasting glucose ≥7 mmol/l or the use of glucose lowering medication. Following current guidelines, subjects were stratified based on office BP: normotensive (NT; <140/90 mmHg), isolated systolic hypertension (ISH; ≥140/<90 mmHg), isolated diastolic hypertension (IDH; <140/≥90 mmHg) and combined systolic and diastolic hypertension (SDH; ≥140/≥90 mmHg) [Citation1]. History of cardiovascular events was defined based on self-reported stroke, myocardial infarction, and coronary or peripheral revascularization. The use of blood pressure lowering medication, excluding beta-blockers, was determined based on ATC codes. Beta blockers were analyzed as a separate covariate due to their direct effect on heart rate.
Analysis of sympathovagal balance
In a subset of the participants, additional non-invasive continuous finger blood pressure measurements were performed using the Nexfin device (Edwards Lifesciences, Irvine, California) for 3-5 minutes after at least 10 minutes of rest. Beat to beat data of SBP and the inter-beat interval (IBI) were further analyzed using custom written software in Matlab (R2019a; the MathWorks, Inc), to determine xBRS, the standard deviation of normal-to-normal intervals (SDNN) and the square root of the mean squared successive differences between adjacent normal-to-normal intervals (RMSDD) as described earlier in detail [Citation21]. In brief, the beat to beat data was first filtered using a local moving median filter with a length of 9 beats. To exclude ectopic beats or measurements errors, beats with an IBI which exceed the local median around that beat with a bandwidth 25% of the mean IBI of the complete recording were excluded. Measurements were excluded from the analysis if 1) they were shorter than 180s, 2) had more than 20% excluded beats, 3) they lacked a stable segment of a least 30 beats without internal calibration or 4) if the participant did not have sinus rhythm on the ECG. Baroreflex sensitivity was determined as the change in heartrate (ms) per change in (mmHg) over 10 second segments of IBI and SBP intervals using the cross-correlation method [Citation22]. Short-term heart rate variability was determined from the filtered IBI following current standards [Citation15].
Statistical analysis
Office BP was used to categorize participants in the different hypertensive phenotypes. Given the earlier observed sex and age-related differences in prognosis of the different phenotypes, analysis were stratified by sex [Citation3,Citation23]. Age differences were analyzed continuously and dichotomously after stratification into younger (≤40 years) and older (>40 years) participants. Baseline characteristics were depicted for the different categories as percentages, mean and SD or median and IQR depending on the distribution of the variables. We first analyzed age differences between phenotypes using a linear regression model with sex-specific splines to model the relation between age and phenotype of which the order was chosen based on the Akaike information criterion. We included a correction term for BMI, the use of BP-lowering medication and beta-blockers. Estimates were derived from this model at the age of 30 and 60 years, with respect to the overall mean BMI (27.6 kg/m2). Next, differences in xBRS/HRV for each subgroup were assessed using an ANOVA test with post-hoc comparison with normotensive participants serving as reference. These models were performed with correction for age, ethnicity, BMI, BP-lowering drugs and beta-blockers. A sensitivity analysis was conducted in participants who did not use BP-lowering drugs or beta-blockers. Continuous variables were winsorized to the 1st and 99th percentile prior to the analysis, xBRS values were log transformed and results were presented as ratios. All statistical analyses were performed using R version 4.0.3 using the tableone version 0.13.0 and the regression modeling strategies version 0.13.0 packages. P-values of <0.05 were considered significant.
Results
There were 10,221 participants with available BP, antihypertensive medication, BMI, and ECG data in which xBRS/HRV could be determined (supplemental figure 1). An overview of the characteristics of the participants stratified by age and phenotype is given in . The distribution of hypertensive phenotypes and baseline characteristics was similar in the complete set (supplementary table 1). The prevalence of ISH was 9.9%; IDH 4.2% and SDH 11.8%. Overall, 14.8% of the participants used BP-lowering medication and 5.9% a beta-blocker. In participants aged below 40 years the number participants with hypertension was lower in all phenotypes, with a prevalence of ISH of 3.1%, IDH 2.8% and SDH 4.1%, in contrast to respectively 14.0%, 5.0% and 16.6% in older participants (≥40 years). In the younger subgroup, 1.8% of the participants used BP-lowering medication, and 0.6% of the participants used a beta-blocker. In the older subgroup medication use was higher with 22.7% using BP-lowering medication and 9.1% using a beta-blocker. BMI was higher in all hypertensive phenotypes compared to normotensives, but the differences were most pronounced in younger women (≤40 years) with a BMI of 32.7 kg/m2 (SD 7.2) in SDH compared to 25.8 kg/m2 (SD 5.2) in normotensives. Younger men with ISH only had a slightly elevated BMI 27.2 kg/m2 (SD 4.3), while it was 35.5 kg/m2 (SD 8.3) in women.
Table 1. Baseline characteristics, stratified by age and hypertensive phenotype. Data are presented as mean (SD), median [IQR] or n (%). BP: blood pressure; BMI: body mass index, CV: cardiovascular, SAS: South-Asian Surinamese, AS: African Surinamese. NT: normotensive, ISH: isolated systolic hypertension, IDH: isolated diastolic hypertension, SDH: systolic and diastolic hypertension. *Fasting glucose was missing in 45 participants (0.4%), in which case diabetes status was based on medication use.
Overall, geometric mean xBRS was 11.1 ms/mmHg (geometric SD 1.84), mean SDNN 51.8 ms (SD 24.4) and mean RMSDD 46.3 ms (SD 27.5). shows the continuous analysis of age versus xBRS/HRV stratified by phenotype, with correction for BMI, diabetes, beta-blockers and use of BP-lowering medication. At the age of 30 years, xBRS was similar for men in NT and ISH, with values of 16.6 ms/mmHg (95%CI 16.1-17.1) in NT, 15.5 ms/mmHg (95%CI 13.8-17.6) in ISH, while it was significantly lower in IDH: 12.7 ms/mmHg (95%CI 11.0-14.6) and SDH: 10.9 ms/mmHg (95%CI 9.7-12.3). In women, we observed a similar pattern with a xBRS of 18.1 ms/mmHg (95%CI 17.6-18.6), 18.3 ms/mmHg (95%CI 14.5-23.1), 12.9 (95%CI 10.9-15.3) ms/mmHg and 11.9 ms/mmHg (95%CI 9.9-14.3) in respectively NT, ISH, IDH, and SDH. Beyond the age of 50 years, values for xBRS became comparable in all hypertensive phenotypes ranging between 6.7-7.4 ms/mmHg and 6.9-7.7 ms/mmHg for 60 year old hypertensive men and women, as compared to 8.8 ms/mmHg (95%CI 8.5-9.1) and 8.5 ms/mmHg (95%CI 8.3-8.8) in normotensive men and women. We observed that SDNN and RMSDD were similar for ISH and NT in younger men, while above 50 years values became comparable to IDH and SDH. For women, the differences between phenotypes were less pronounced, with overlapping confidence intervals for most estimates.
Figure 1: Relation between age and xBRS, SDNN, RMSDD for different hypertensive phenotypes. Lines depicted estimates from linear regression model with correction for BMI (visualized for the population mean BMI of 27.6 kg/m2), diabetes, BP-lowering medication use and beta-blockers, shaded area indicate 95% confidence interval. xBRS: cross-correlation baroreflex sensitivity, SDNN: standard deviation of normal-to-normal intervals, RMSDD: the squared root of the mean squared successive difference between adjacent normal-to-normal intervals.
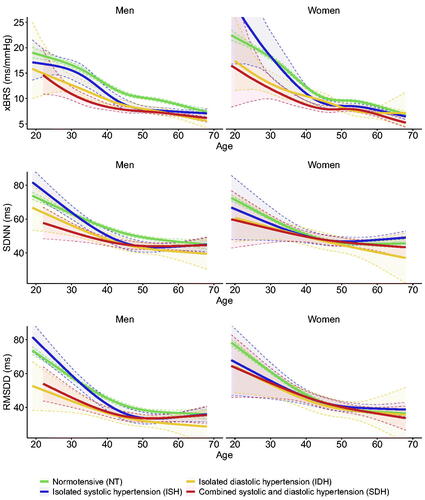
In the regression analysis, xBRS was significantly different between phenotypes in both younger and older participants of both sexes (p < 0.001 for all models, ). In younger men and women aged below 40 years with ISH, xBRS was comparable to same sex normotensives; with a ratio of 0.92 (95%CI 0.84-1.01) and 1.00 (95%CI 0.84-1.20) in men and women. Participants with IDH and SDH had significantly lower values for xBRS in both men and women, ranging between 0.67-0.80. In older men and women aged above 40 years, we observed a stepwise decrease in xBRS, with the lowest values for SDH of 0.74 (95%CI 0.70-0.77) and 0.75 (95%CI 0.71-0.79 in women). For SDNN and RMSDD, values were significantly different (p = 0.006 and p < 0.001) between phenotypes in both younger and older men, while in women we only observed significant differences for RMSDD in older women (p = 0.015). In the sensitivity analysis in participants without BP-lowering drugs or beta-blockers, changes in SDNN in RMSDD were larger in younger women. The other results were all comparable to the main analysis (supplementary table 2).
Table 2. Differences in xBRS and HRV between phenotypes as determined using linear regression models with correction for age, ethnicity, BMI, diabetes, BP-lowering drugs and beta-blockers. Coef. denotes coefficient from linear regression model, values for xBRS were log transformed prior to analyses and the coefficients depict ratio. xBRS: cross-correlation baroreflex sensitivity, SDNN standard deviation of normal-to-normal intervals, RMSDD: the squared root of the mean squared successive difference between adjacent normal-to-normal intervals. NT: normotensive, ISH: isolated systolic hypertension, IDH: isolated diastolic hypertension; SDH: systolic and diastolic hypertension, est: estimate, p-val: p-value.
Discussion
The main finding of our analysis is that xBRS and HRV are significantly different between hypertensive phenotypes. The continuous analysis of xBRS and HRV by age across different hypertensive phenotypes shows that the relation between hypertensive phenotype and autonomic function changes fundamentally between 40 to 50 years of age. This finding was confirmed by separate regression models, where ISH in individuals aged below 40 years had comparable values to normotensive men and women for both xBRS and HRV, suggesting that their increase in BP is not associated with alterations in autonomic regulation. In contrast, we observed significantly lower values for xBRS in individuals with IDH and SDH, while in individuals above 40 years of age, xBRS values were lower for all hypertensive phenotypes compared to normotensive participants. Earlier studies have shown that the hypertension and high normal blood pressure associated with increased sympathetic activity in younger and older adults using different techniques, [Citation12] including the observation of sympathetic activation in ISH and SDH in older adults [Citation24]. We conform these findings in older adults in a large cohort using autonomic analysis of xBRS and HRV, and extend these findings be showing differences in autonomic function in younger adults with ISH and IDH.
Our results suggest that measures of cardiac autonomic function, including xBRS and HRV, that are known to be associated with cardiovascular outcomes, may be useful in the evaluation of hypertension associated cardiovascular risk [Citation16–18]. When comparing xBRS and HRV, we noticed that differences in HRV between hypertensive phenotypes were less pronounced and had a larger intra-individual variation, especially in women. This may be explained by age-related changes in HRV as a result of sinus node degeneration, lowering its discriminating value [Citation25–27]. In contrast, xBRS is a more functional measure of baroreflex sensitivity. Although xBRS significantly decreases with age, our analysis still shows that differences between hypertensive phenotypes remained present making it a more widely applicable parameter of autonomic regulation [Citation24].
The risk associated with both ISH and IDH and the necessity of starting antihypertensive treatment is subject of debate.[Citation10,Citation28] Epidemiological studies have shown an increased risk in young individuals with ISH compared to normotensive adults, albeit not to the same extent as compared to individuals with SDH.[Citation3,Citation29] Our study shows that younger adults with ISH had similar xBRS and HRV levels compared to normotensive adults, suggesting that their increase in blood pressure is not caused by alterations in autonomic control. Additional factors, such as arterial stiffness and an impedance mismatch that results in heightened brachial BP readings while central BP remains normal, [Citation4,Citation6,Citation30] could present alternative pathophysiological mechanisms underlying ISH.
Regardless of age, SDH was consistently associated with a lower xBRS compared to individuals with ISH and IDH. A possible explanation could be a higher frequency of white coat effects that may lead to a less pronounced increase in sympathetic activity as compared to sustained hypertension.[Citation31,Citation32] However, the lower xBRS in individuals with SDH may also be related to the, on average, higher systolic BP values as compared to individuals with ISH in the present cohort. In line, this may also explain the higher xBRS in individuals with IDH compared to SDH, as both systolic and diastolic BP values were, on average, higher in those with SDH in the younger and older age categories. Still, both young and older individuals with IDH had significantly lower xBRS compared to normotensive individuals, suggesting an increased cardiovascular risk. This is in line with previous findings that individuals with IDH have an elevated risk to progress to sustained hypertension, [Citation33] and a study from the UK biobank showing that older (mean age 55 years) individuals with IDH have an increased cardiovascular risk [Citation34]. However, it contrasts findings by Yano et al in the Chicago Heart study, which showed a more beneficial prognosis of IDH in women aged below 50 compared to men, and a recent analysis of the MESA study showing that there was no consistent relation between IDH and adverse cardiovascular outcomes in older adults [Citation3]. In both instances results could have been confounded by the initiation of BP-lowering treatment during follow-up, while the MESA study included an older study population where lower diastolic BP values may be dampened by increased diastolic run-off as a result of arterial stiffness. This would also fit with our observation that younger individuals with IDH have lower xBRS compared to those with ISH, while in older individuals xBRS was decreased in both IDH and ISH to a similar extend.
A strength of this study is the use of a multi-ethnic population with a large sample size. Although due to the small subgroups after stratification by age, sex and phenotypes we were not able to analyses ethnic differences in xBRS and HRV between phenotypes, however our data support the applicability in different ethnicities. xBRS and HRV data were not available in all participants of the HELIUS cohort due to logistical reasons, we do not expect this to negatively influence the results of our study as the distribution of phenotypes was similar between the subset with available recordings and the complete cohort. Another strength of this study is the use of an automated xBRS-HRV analysis algorithm, reducing the inter-individual variability [Citation21]. For xBRS, we used the cross-correlation method to assess cardiac baroreflex based on spontaneous blood pressure variation. The design of our study did not allow to obtain other measurements of autonomic function such as measurement using pharmacological induced BP changes or measurement of muscle sympathetic nerve activity, as this is cumbersome to perform on a large scale [Citation35]. Although our method is not the gold standard for measurement of BRS, it has been validated against the gold standard using phenylephrine induced BP changes [Citation14]. Furthermore, a previous analysis of the finger BP data within our cohort has shown that recordings longer then 180s are sufficient for a reliable estimation of HRV and BRS at large scale using an automatic algorithm [Citation21]. Finally, while our results could be hampered by medication use, we adjusted separately for BP-lowering medication and beta blockers to reduce their potential confounding effect. In addition, the sensitivity analysis in participants who did not use beta-blockers nor other BP-lowering drugs yielded similar results.
In conclusion, in this study we observed that younger adults with ISH have similar HRV and xBRS values compared to normotensive individuals. However, in both younger and older participants IDH and SDH were associated with increased sympathetic control, with similar values to SDH in older adults. The observed differences in cardiac autonomic regulation are in line with earlier studies which showed a more beneficial prognosis of ISH in younger men and women. With increased age sympathetic activity increases, but we show that hypertension is associated with decreased xBRS for all phenotypes, supporting the role of sympathetic overdrive in the pathogenesis of hypertension. For future research purposes we suggest to examine the predictive value of combined assessment of HRV and xBRS in the progression of ISH and IDH towards SDH and the development of cardiovascular complications.
Supplemental Material
Download MS Word (92 KB)Disclosures
None of the authors have a conflict of interest to report.
Data availability
The HELIUS data are owned by the Amsterdam UMC, location AMC in Amsterdam, the Netherlands. Any researcher can request the data by submitting a proposal as outlined at http://www.heliusstudy.nl/.
Additional information
Funding
References
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018 Aug;39(33):1–9. doi: 10.1093/eurheartj/ehy339.
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American College of Cardiology/American Heart Association Task Force on Clinical pr. Hypertension. 2018 Jun 1;71(6):e13–115.
- Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, et al. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: The Chicago heart association detection project in industry study. J Am Coll Cardiol. 2015;65(4):327–35. doi: 10.1016/j.jacc.2014.10.060.
- O’Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth. Vasc Med. 2000;5(3):141–5. doi: 10.1177/1358836X0000500303.
- Grebla RC, Rodriguez CJ, Borrell LN, Pickering TG. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999–2004 US National Health And Nutrition Examination Survey. J Hypertens [Internet]. 2010;28(1). Available from: https://journals.lww.com/jhypertension/Fulltext/2010/01000/Prevalence_and_determinants_of_isolated_systolic.5.aspx
- Eeftinck Schattenkerk DW, van Gorp J, Vogt L, Peters RJG, van den Born BJH. Isolated systolic hypertension of the young and its association with central blood pressure in a large multi-ethnic population. The HELIUS study. Eur J Prev Cardiol. 2018;25(13):1351–9. doi: 10.1177/2047487318777430.
- Palatini P, Rosei EA, Avolio A, Bilo G, Casiglia E, Ghiadoni L, et al. Isolated systolic hypertension in the young: A position paper endorsed by the European Society of Hypertension. J Hypertens. 2018;36(6):1222–36. doi: 10.1097/HJH.0000000000001726.
- Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. Does the relation of blood pressure to coronary heart disease risk change with aging?: The Framingham Heart Study. Circulation. 2001;103(9):1245–9. doi: 10.1161/01.cir.103.9.1245.
- Pastor-Barriuso R, Banegas JR, Damin J, Appel LJ, Guallar E. Systolic Blood Pressure, Diastolic Blood Pressure, and Pulse Pressure: An Evaluation of Their Joint Effect on Mortality. Ann Intern Med [Internet]. 2003 Nov 4;139(9):731–9. Available from: doi: 10.7326/0003-4819-139-9-200311040-00007.
- Jacobsen AP, Al Rifai M, Arps K, Whelton SP, Budoff MJ, Nasir K, et al. A cohort study and meta-analysis of isolated diastolic hypertension: Searching for a threshold to guide treatment. Eur Heart J. 2021;42(21):2119–29. doi: 10.1093/eurheartj/ehab111.
- Dibona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61(3):556–60. doi: 10.1161/HYPERTENSIONAHA.111.00633.
- Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–14. doi: 10.1161/CIRCRESAHA.114.302524.
- Fisher JP, Paton JFR. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens [Internet]. 2012;26(8):463–75. Available from: doi: 10.1038/jhh.2011.66.
- Wesseling KH, Karemaker JM, Castiglioni P, Toader E, Cividjian A, Settels JJ, et al. Validity and variability of xBRS: Instantaneous cardiac baroreflex sensitivity. Physiol Rep. 2017;5(22):1–11.
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81.
- Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: Insights into pathogenesis of hypertension: The Framingham Heart Study. Hypertension. 1998;32(2):293–7. doi: 10.1161/01.hyp.32.2.293.
- Hillebrand S, Gast KB, De Mutsert R, Swenne CA, Jukema JW, Middeldorp S, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–9. doi: 10.1093/europace/eus341.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351(9101):478–84. doi: 10.1016/s0140-6736(97)11144-8.
- De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex Sensitivity Predicts Long-Term Cardiovascular Mortality After Myocardial Infarction Even in Patients With Preserved Left Ventricular Function. J Am Coll Cardiol. 2007;50(24):2285–90. doi: 10.1016/j.jacc.2007.08.043.
- Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJG, Zwinderman AH, et al. Cohort profile: The Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, the Netherlands. BMJ Open. 2017;7(12):1–11. doi: 10.1136/bmjopen-2017-017873.
- Collard D, Westerhof BE, Karemaker JM, Stok WJ, Postema PG, Krediet CTP, et al. Automated analysis of finger blood pressure recordings provides insight in determinants of baroreflex sensitivity and heart rate variability—the HELIUS study. Med Biol Eng Comput. 2023;61(5):1183–91. doi: 10.1007/s11517-023-02768-4.
- Westerhof BE, Gisolf J, Stok WJ, Wesseling A KH, Karemaker JM. Time-domain cross-correlation baroreflex sensitivity: Performance on the EUROBAVAR data set. J Hypertens. 2004;22(7):1371–80. doi: 10.1097/01.hjh.0000125439.28861.ed.
- Hozawa A, Ohkubo T, Nagai K, Kikuya M, Matsubara M, Tsuji I, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self-measurement of blood pressure at homel: The ohasama study. Arch Intern Med. 2000;160(21):3301–6. doi: 10.1001/archinte.160.21.3301.
- Grassi G, Seravalle G, Bertinieri G, Turri C, Dell’Oro R, Stella ML, et al. Sympathetic and reflex alterations in systo-diastolic and systolic hypertension of the elderly. J Hypertens. 2000;18(5):587–93. doi: 10.1097/00004872-200018050-00012.
- Almeida-Santos MA, Barreto-Filho JA, Oliveira JLM, Reis FP, da Cunha Oliveira CC, Sousa ACS. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr [Internet]. 2016;63:1–8. Available from: https://www.sciencedirect.com/science/article/pii/S0167494315300881 doi: 10.1016/j.archger.2015.11.011.
- Tegegne BS, Man T, van Roon AM, Snieder H, Riese H. Reference values of heart rate variability from 10-second resting electrocardiograms: the Lifelines Cohort Study. Eur J Prev Cardiol. 2020;27(19):2191–4. doi: 10.1177/2047487319872567.
- Tulppo MP, Mäkikallio TH, Seppänen T, Laukkanen RT, Huikuri H V. Vagal modulation of heart rate during exercise: Effects of age and physical fitness. Scand Cardiovasc Journal, Suppl. 1997;31(45):12.
- McEniery CM, Franklin SS, Wilkinson IB, Cockcroft JR. Isolated systolic hypertension in the young: a need for clarity. J Hypertens. 2013;31(9).
- Lee H, Yano Y, Cho SMJ, Park JH, Park S, Lloyd-Jones DM, et al. Cardiovascular Risk of Isolated Systolic or Diastolic Hypertension in Young Adults. Circulation. 2020;141(22):1778–86. doi: 10.1161/CIRCULATIONAHA.119.044838.
- McEniery CM, Yasmin, Wallace S, Maki-Petaja K, McDonnell B, Sharman JE, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46(1):221–6. doi: 10.1161/01.HYP.0000165310.84801.e0.
- Neumann SA, Jennings JR, Muldoon MF, Manuck SB. White-coat hypertension and autonomic nervous system dysregulation. Am J Hypertens. 2005;18(5):584–8. doi: 10.1016/j.amjhyper.2004.11.034.
- Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DASG. Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol [Internet]. 2002;40(1):126–32. Available from: doi: 10.1016/S0735-1097(02)01931-9.
- Coca A. Blood pressure and cardiovascular disease: Are diastolic values 80-89 mmHg enough to define hypertension? Eur Heart J. 2021;42(21):2130–2. doi: 10.1093/eurheartj/ehab109.
- McGrath BP, Kundu P, Daya N, Coresh J, Selvin E, McEvoy JW, et al. Isolated Diastolic Hypertension in the UK Biobank: Comparison of ACC/AHA and ESC/NICE Guideline Definitions. Hypertension. 2020;699–706. doi: 10.1161/HYPERTENSIONAHA.120.15286.
- Grassi G, Pisano A, Bolignano D, Seravalle G, D’Arrigo G, Quarti-Trevano F, et al. Sympathetic nerve traffic activation in essential hypertension and its correlates systematic reviews and meta-analyses. Hypertension. 2018;72(2):483–91. doi: 10.1161/HYPERTENSIONAHA.118.11038.
