Abstract
Objectives: Evidence suggests that renal function increasingly deteriorates in patients with apparently treatment-resistant hypertension (ATRH) in comparison with those who have non-resistant arterial hypertension (NAH). We aimed to assess the long-term decline in renal function between these patient groups and identify specific risk factors contributing to the progression of renal dysfunction. Methods: Data for 265 patients with ATRH and NAH in a hypertension excellence centre were retrospectively evaluated. Demographic characteristics, co-morbidities, laboratory findings, secondary causes of hypertension, medication and exposure to contrast agents were assessed. To address differences between groups, adjustment with linear mixed-effect models was used. Results: Data from the first 4 years of follow-up were evaluated. After adjustment for age and diabetes, which were identified as independent risk factors for renal dysfunction progression in the study cohort, the mean decrease in estimated glomerular filtration rate per year was steeper with ATRH than with NAH (−1.49 vs. −0.65 mL/min/1.73 m2 per year; difference in slope, 0.83 mL/min/1.73 m2 per year; 95% confidence interval [CI]: 0.25–1.41, p = 0.005). In subgroup analyses, without Holm–Bonferroni correction, the prescription of MRA indicated a faster decline in renal function in ATRH. Following correction, no specific therapeutic risk factor was associated with faster progression of renal dysfunction. Conclusions: Renal function declines twice as fast with ATRH compared with NAH, independently of age and diabetes. Larger studies are needed to reveal risk factors for renal dysfunction in patients with hypertension.
PLAIN LANGUAGE SUMMARY
High blood pressure (arterial hypertension) is a significant risk factor for kidney function decline. Resistant hypertension represents a subtype of hypertension that is difficult to treat and requires multiple antihypertensive agents to achieve effective blood pressure control. Recent research suggests that individuals with resistant hypertension are at greater risk of kidney dysfunction.
This study analyses data from adult patients with arterial hypertension and resistant hypertension followed-up for a mean duration of 6.4 years.
A faster decline in kidney function was observed in patients with resistant hypertension. This suggests that renal function in these patients should be closely monitored.
After statistical evaluation, no medication was found to be associated with an increased risk of kidney failure progression. However, two specific medications, spironolactone and eplerenone, raised suspicion and require further exploration in larger prospective studies.
Introduction
Arterial hypertension is the cause in ∼30% of chronic kidney disease (CKD) cases and, together with diabetes is responsible for most cases of chronic kidney failure worldwide [Citation1,Citation2]. Apparent treatment-resistant hypertension (ATRH) requires the use of ≥3 antihypertensive agents [Citation3]. Compared with non-resistant arterial hypertension (NAH), ATRH is associated with a greater risk of cardiovascular (CV) events and approximately 1.5 times higher risk of CKD [Citation4,Citation5].
ATRH is also associated with a faster decline in renal function in the earlier stages of CKD, and its prevalence increases with severity of CKD. Most studies have examined renal function decline based on renal end points, such as a defined decrease in estimated glomerular filtration rate (eGFR; e.g. 50%), increased creatinine, or the need to initiate renal replacement therapy (RRT) [Citation6]. Albuminuria also is a strong predictor of CKD progression and CV complications and is widely used in these studies [Citation7–9]. Precise quantification of the annual difference in the decline in the eGFR slope has occasionally been used in patients with hypertension [Citation10,Citation11]. And to our knowledge, data reflecting the overall annual decline in eGFR slope in patients with ATRH are still insufficient.
To fill this gap, we aimed to use the annual change in the eGFR slope as a precise long-term measure of kidney function in patients with ATRH compared with patients with NAH.
The secondary aims of this study were to assess other factors in both groups, including exposure to contrast imaging, renal denervation and antihypertensive, hypolipidemic, and antidiabetic treatments and identify those associated with a faster decline in renal function.
Materials and methods
Patient selection
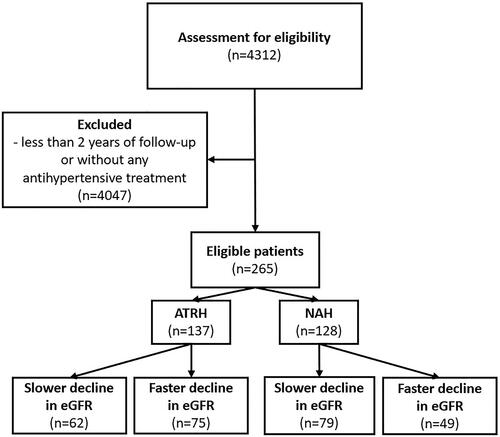
We retrospectively reviewed the medical records of 265 patients with arterial hypertension, ages 27–93 years, and all of European ancestry. Patients had been followed from May 2007 to December 2018 in an outpatient clinic of the Hypertension Excellence Centre of the University Hospital in Olomouc, Czech Republic. Patients with clinical follow-ups >2 years were enrolled. Every patient had signed informed consent at the examination and with regard to the anonymous processing of the data as a part of routine follow-up. This trial adheres to the principles outlined in the Declaration of Helsinki. The local Ethics Committee granted ethical approval for data evaluation. exempted the study from review, given that the nature of the data did not warrant it. Every patient had been treated with at least one antihypertensive drug. Patients were allocated to the ATRH or NAH group, with ATRH defined as uncontrolled hypertension despite the use of ≥3 antihypertensive agents (including a diuretic) at optimal dose () [Citation3].
Figure 1. Flow chart of the study. ATRH: apparently treatment-resistant hypertension; eGFR: estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration formula 2021); NAH: non-resistant arterial hypertension.
Direct and indirect methods for evaluating adherence were used during follow-up [Citation12–14]. Nonetheless, the treatment adherence may have changed considerably during the long-follow-up, and patients with partial adherence and secondary hypertension were also included in the study. For this reasons, we use the term ‘apparently treatment-resistant hypertension’ (ATRH) for the sake of precision.
Data collection
The frequency of the outpatient check-ups was not uniform, and every patient had an individual plan of examinations in the department based on their clinical status. All patients were evaluated for demographic characteristics (age, sex and duration of follow-up), common co-morbidities and biochemical findings (taken as the average of all check-ups during follow up). Impaired kidney function was attributed to hypertensive and diabetic kidney disease. All potential diseases that might contribute to progression of renal impairment were noted, if data were available. Creatinine was measured using Jaffe’s reaction until 25 October 2010, after which measurements were based on the more accurate enzymatic method. All eGFR values were calculated using the standardised Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation 2021 [Citation15].
A composite parameter, ‘manifest CV disease,’ was defined as the presence of at least one of the following: ischaemic heart disease, history of myocardial infarction or stroke and peripheral vascular disease.
At least once during follow-up, every patient had secondary causes of hypertension assessed (e.g. hormonal disturbances, renal artery stenosis and sleep apnoea), and if diagnosed, received appropriate treatment. If the treatment was invasive or mechanical related to secondary cause of hypertension (e.g. adrenalectomy for primary aldosteronism, continuous positive airway pressure for sleep apnoea or angioplasty of the renal artery) and the hypertension resolved, the patient was excluded from the study. If the hypertension improved but still required treatment with at least one antihypertensive agent, the patient was retained in the study group.
Office blood pressure and heart rate measurements were obtained by trained staff three times with the patients in the sitting position, after at least 5 min of quiet rest, and the mean of the second and third measurements was used as office blood pressure. Ambulatory blood pressure monitoring (ABPM) was performed according to current guidelines [Citation16].
Medication evaluated in the study included antihypertensive, antidiabetic and hypolipidemic agents. Because of minor changes during follow-up, the medication used for the longest period for each patient, was evaluated for this study.
Imaging studies and invasive procedures included computed tomography, magnetic resonance imaging, coronary angiography, right-sided catheterisation of the heart and renal denervation. Only data from the University Hospital of Olomouc were available, and imaging studies from other hospitals were not accessible. All deidentified data are available upon reasonable request.
Statistical analysis
Because of heterogeneity between the two patients populations, their clinical characteristics differed significantly. Logistic regression models were used to identify demographic and clinical individual risk factors for renal dysfunction. Linear mixed-effects models with random slope and intercept adjusted to calculated risk factors were used to control for the differences and assess progression of renal dysfunction. The least-squares method was used to determine the eGFR slopes over time.
To estimate individual risk factors for renal dysfunction, we categorised patients in each group based on the rate of renal function decline, with one subgroup having faster deterioration (defined as eGFR decrease > −1.0 mL/min/1.73 m2 per year) and one with slower deterioration (eGFR decrease < −1.0 mL/min/1.73 m2 per year). An eGFR decrease cut-off of 1 mL/min/1.73 m2 per year was chosen as the threshold value of approximate physiological decline in renal function in the overall healthy population, based on available population studies, which allowed for development of these subgroups [Citation17–19].
Results for categorical variables are described using absolute and relative frequencies, those for quantitative normally distributed variables are presented as mean ± standard deviation (SD), and results for quantitative non-normally distributed variables are presented as median with the interquartile range (25th–75th percentile). For comparing groups of patients, the Mann–Whitney U test was used for continuous variables and the Fisher’s exact test for categorical variables. Because of the large amount of data, Holm–Bonferroni correction was used to eliminate Type I error in multiple statistical tests.
All tests were two-tailed with a significance level of 0.05. The statistical analyses were performed using SPSS software version 28 (IBM, Armonk, NY).
Results
Patients were followed-up for a mean of 6.4 years (minimum 2 years, maximum 10.6 years). A total of 234 patients were followed-up >4 years, 18 patients <4 years and 13 patients <3 years. Laboratory data during the first 4 years of follow-up were evaluated.
Patients with ATRH (n = 137), when compared with patients classified as NAH (n = 128), had shorter intervals between check-ups (median 6.1 vs. 7.0 months), greater age (58 vs. 51 years), higher body mass index (BMI; 32.1 vs. 28.9 kg/m2), lower baseline eGFR (77 vs. 87 mL/min/1.73 m2), a higher rate of diabetes (40% vs. 9%), and a higher rate of manifest CV disease in the patient history (19.7% vs. 6.3%), a larger proportion of the cohort underwent ABPM, had higher mean office systolic blood pressure (SBP) and lower mean diastolic blood pressure (DBP) of ABMP during the daytime and the whole day throughout the follow-up. They also were, more likely to have undergone contrast imaging, and renal denervation (see , which depicts baseline clinical characteristics and renal function). No patient was on RRT.
Table 1. Baseline clinical characteristics, renal function and average blood pressure values throughout follow-up in patients in the ATRH and NAH groups.
The ATRH and NAH groups differed significantly in their use of most antihypertensive medications. The exceptions were angiotensin-converting enzyme inhibitors (ACEis), loop diuretics and central antihypertensives (see and Supplementary Table 1, Supplemental Digital Content, the medication of patients in ATRH and NAH groups).
Table 2. Medication of patients in the ATRH and NAH groups.
Compared with the NAH group, patients with ATRH had higher mean urea levels, uric acid, glycaemia, and glycated haemoglobin (HbA1c), and lower levels of albumin, LDL cholesterol, total cholesterol and magnesium (see Supplementary Table 2, Supplemental Digital Content, which shows biochemical measures in the ATRH and NAH groups). The incidence of secondary hypertension did not differ between groups. The most common diagnoses were primary aldosteronism (ATRH n = 24, NAH n = 12; adrenalectomy: ATRH n = 5, NAH n = 9), and renovascular hypertension (ATRH n = 6, NAH n = 4; percutaneous transluminal angioplasty: ATRH n = 5, NAH n = 4). Two patients with NAH underwent adrenalectomy for pheochromocytoma, and one patient with ATHR had hyperparathyroidism that required thyroidectomy. No patient had hypercortisolism, or newly diagnosed hypo or hyperthyroidism.
Renal function trajectory
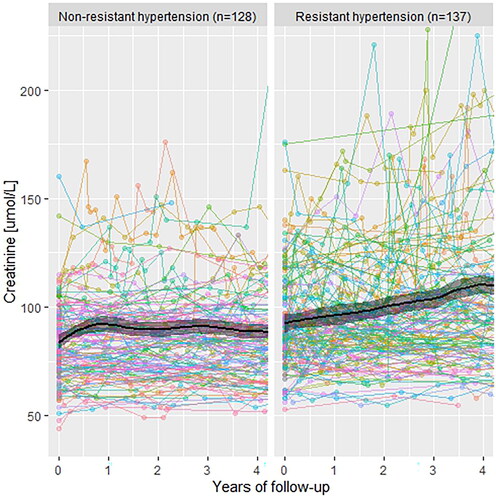
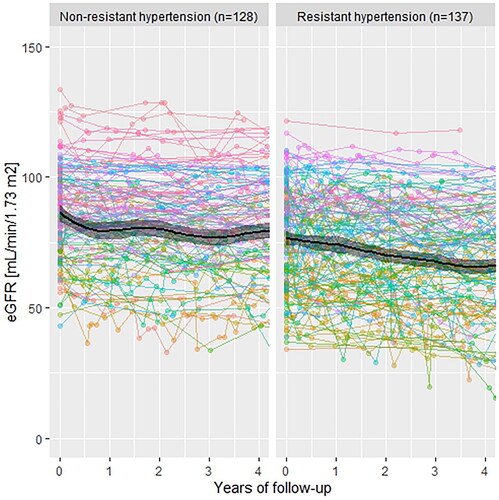
During 4 years of follow-up, patients with ATRH had a higher absolute mean annual increase in creatinine (+4.45 µmol/L per year) compared with patients in the NAH (+1.275 µmol/L per year). Similarly, the mean annual eGFR decline was faster in patients with ATRH (−2.725 mL/min/1.73 m2 per year compared with −1.85 mL/min/1.73 m2 per year; and ).
Figure 3. Development of estimated glomerular filtration rate (eGFR and CKD-EPI) during follow-up. CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration formula 2021.
From all between-group differences, logistic regression models established age and diabetes as statistically significant independent risk factors for renal deterioration. To mitigate these differences between groups, linear mixed-effect models were used to assess the trajectory of renal function.
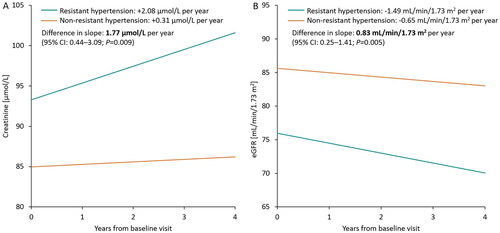
After adjustment for age and diabetes, the mean yearly creatinine increase was higher in the ATRH group compared with the NAH group (+2.08 µmol/L per year vs. +0.31 µmol/L per year). The between-group difference in slope was 1.77 µmol/L per year (95% confidence interval [CI]: 0.44–3.09, p = 0.009). The mean annual decrease in eGFR was steeper in the ATRH group (−1.49 mL/min/1.73 m2 per year vs. −0.65 mL/min/1.73 m2 per year with NAH), and the between-group difference in slope was 0.83 mL/min/1.73 m2 per year (95% CI: 0.25–1.41, p = 0.005), for a renal function decline that was ∼2.3 times steeper in the ATRH group (). The urine albumin: creatinine ratio (ACR) did not differ between the groups during follow-up.
Individual risk factors for renal dysfunction
In the ATRH group, according to the cut-off of −1 mL/min/1.73 m2 per year in development of renal function, 55% (n = 75) had faster renal function decline (> −1.0 mL/min/1.73 m2 per year), and 42% (n = 62) had slower renal function decline (< −1.0 mL/min/1.73 m2 per year). In the NAH group, 38% (n = 49) had faster decline, and 62% (n = 79) had slower decline.
All previously assessed parameters were compared between these groups. Following Holm − Bonferroni correction, none differed significantly (see Supplementary Tables 3 − 5, Supplemental Digital Content, which display baseline clinical characteristics, medication and biochemical findings in subgroups according to the progression of renal function). Without Holm − Bonferroni correction, only the use of MRA was significantly higher in patients with ATRH with faster decline renal function (p < 0.011).
Discussion
Using annual eGFR change as a measure, we found that patients with ATRH had a 2.3-fold faster decline in renal function than patients with NAH, even after adjustment for age and diabetes. Patients with ATRH had a significantly higher mean annual decrease in eGFR (−1.49 mL/min/1.73 m2 per year vs. −0.65 mL/min/1.73 m2 per year with NAH).
Various studies have shown associations between resistant arterial hypertension (RAH) and a higher incidence of CV and renal outcomes compared with non-resistant hypertension. The most frequently used parameter of kidney dysfunction is a composite renal endpoint that usually includes a combination of newly diagnosed CKD, specific decline in eGFR, end-stage kidney disease (ESRD), and/-or initiation of RRT or kidney transplantation. Annual eGFR slope development is frequently used in studies of sodium-glucose cotransporter 2 (SGLT2) inhibitors [Citation10,Citation20–28], but only rarely in studies of ATRH or the effects of antihypertensive, hypolipidemic or antidiabetic agents [Citation29–32].
Nevertheless, previous studies support that evaluating the annual eGFR slope trajectory offers additional information for identifying populations with faster progression of CKD. In their robust meta-analysis, Kovesdy et al. included 1,080,223 participants from 22 studies and evaluated two parameters, current eGFR and past annual eGFR slope decline, to predict progression to ESRD. Participants were categorised into subgroups according to baseline eGFR levels of 20, 30 or 40 mL/min/1.73 m2, and the results showed that both current values and past slope decline were independent predictors of 5-year risk of ESRD [Citation33].
In another meta-analysis covering 3,758,551 participants with eGFR ≥60 mL/min/1.73 m2 and 122,664 participants with eGFR <60 mL/min/1.73 m2, Grams et al. found that a slower eGFR annual decline by 0.75 mL/min per every 2 years was associated with a lower risk of ESRD in both groups [Citation34].
A few studies of hypertension and kidney disease have allowed for analysis of patient subgroups with ATRH in relation to CKD, such as the data for 3367 patients with hypertension and CKD from the Chronic Renal Insufficiency Cohort. Patients were followed-up for more than 6 years, and investigators found that the presence of ATRH (in 40.4%) predicted a higher mortality and adverse CV and renal events, defined as the need for RRT, or an estimated 50% decline in eGFR from baseline. This was independent of several parameters, such as age, sex, race, study centre, diabetes mellitus, smoking status, CV disease, BMI, haemoglobin, LDL, eGFR and 24-h proteinuria. ATRH prevalence was higher with more advanced CKD [Citation6,Citation35,Citation36]. This finding is in agreement with the result of Tanner et al. who reported that the prevalence of ATRH in a population of 10,700 patients with hypertension was higher in advanced stages of CKD [Citation37].
Several studies confirm the link between RAH and CKD [Citation5]. Muntner et al. evaluated subgroup of 1870 patients followed-up for an average 4.9 years from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Rates of coronary heart disease, stroke, heart failure, peripheral artery disease, all-cause mortality and progression to ESRD defined as kidney disease death, renal transplantation or long-term dialysis initiation, were significantly higher compared with results for patients without ATRH. Diuretic treatment was not required to establish a diagnosis of ATRH in that trial, in variance from our study [Citation38].
Viazzai et al. in their study of 29,923 patients with Type 2 diabetes and 4 years of follow-up, found that ATRH was associated with a significant annual eGFR slope decrease [Citation39]. Kaboré et al. found an association between faster eGFR slope decline and increased risk for ATRH. They evaluated data for 6848 patients over age 65 years followed-up for 4 years, of whom 3865 were treated for current hypertension. The mean eGFR slope decline was 1.5 ± 2.9 mL/min/1.73 m2 per year, and 27.7% of participants had a decline >3 mL/min/1.73 m2 per year, for an odds-ratio for development ATRH of 1.89 (1.09–3.29). A total of 10.1% had an annual decline >5 mL/min/1.73 m2 per year, for an odds-ratio for ATRH of 1.99 (1.19–3.35). Compared with our study, these authors used a similar evaluation of renal function trajectory with a similar length of follow-up, but focused on the incidence of newly diagnosed ATRH according to the rate of decline in renal function, whereas we evaluate the converse of this relationship [Citation11].
In this study, higher mean urea, uric acid, glycaemia and HbA1c levels in the ATRH group were associated with lower baseline eGFR, more frequent diuretic use and a higher incidence of diabetes. We infer that the lower LDL and total cholesterol values reflected aggressive hypolipidemic treatment due to more frequent manifest CV disease, as did the slightly lower albumin level in the ATRH group [Citation40,Citation41]. Lower serum magnesium levels in the ATRH group were probably caused by more frequent diuretic treatment.
In terms of medication, without applying Holm-Bonferroni correction, a significantly higher number of patients with a faster decline in renal function were prescribed MRA (spironolactone and eplerenon) in the ATRH group. However, subsequent application of the Holm–Bonferroni correction revealed no significant difference. It is important to note that our study involved a relatively small cohort of affected patients; therefore, results pertaining to impact of MRA on renal function in ATRH patients remain inconclusive. A retrospective trial by Desai et al. that included 80,598 patients with ATRH, assessed the impact on CV events, incidence of hyperkalemia, gynaecomastia, and deterioration of kidney function when comparing the use of MRA (N = 6626) as a fourth agent with beta-blockers (N = 73,972). In relation to kidney function, the mean follow-up duration in the MRA group (201.4 d) and the beta-blocker group (254.3 d) was shorter than in our trial. The study did not observe any difference in CV events, but a significant risk of kidney function deterioration was identified in patients treated with MRA (HR, 1.63 [95% CI, 1.34–1.99] [Citation42]. Larger trials are needed to determine the benefits or disadvantages of specific agents that might affect renal function in patients with ATRH.
Strengths of the study
The study focused on a real-life heterogeneous high-risk population with long-term close follow-up. Multiple risk factors associated with kidney disfunction were considered.
Limitations of the study
The most important limitation was the relatively low number of patients in the study, and no risk factor significantly influencing renal function was detected. Check-ups were not standardised, and every patient had an individual follow-up plan. The study was observational and retrospective; therefore, direct causality between risk factors cannot be inferred. Because of the heterogeneity of the study groups, linear mixed-effect models were used to assess renal function deterioration. Data regarding contrast examination were available only from the study hospital, potentially excluding contrast examinations performed elsewhere. Finally, the study did not involve novel classes of medications currently available, such as the latest generations of MRA (finerenone) [Citation43], and only 4 patients were taking SGLT2 inhibitors and angiotensin receptor/neprilysin inhibitor.
Conclusion and perspectives
Patients with ATRH have a 2.3-fold faster decline in renal function (annual eGFR slope) compared to patients with NAH, independently of age and the presence of diabetes. The findings emphasise that renal function in patients with ATRH must be closely monitored. Further evaluation in larger prospective studies of possible factors influencing renal function in patients with ATRH, particularly focusing on the use of MRA spironolactone and eplerenone, is necessary. The annual eGFR slope development is a practical tool for evaluating the risk of CKD progression and warrants implementation in more studies.
Supplemental Material
Download MS Word (413.3 KB)Acknowledgements
Zdeněk Ramík and Jan Václavík originated the study protocol. Zdeněk Ramík, Tomáš Kvapil, Libor Jelínek, Eva Kociánová, Monika Kamasová, Zdeněk Lys and Martin Drápela contributed to the design and data collection. Klára Benešová and Jiří Jarkovský analysed the data and contributed to the method section. Zdeněk Ramík and Jan Václavík wrote the manuscript with contributions from all authors.
The study results were unveiled in 2022 at the European Society of Cardiology Congress in Barcelona, Spain [Citation44], and XXXIX. Days of Young Internists in Martin, Slovakia, where it achieved the 1st place among all participants. In 2023, data were presented at the 21st European Congress of Internal Medicine, held jointly with the 12th International Congress of Internal Medicine in Athens, Greece [Citation45].
Disclosure statement
Z. Ramík, J. Václavík, T. Kvapil, L. Jelínek and K. Benešová, report grants from the Palacky University Olomouc, Faculty of Medicine under Grant [IGA_LF_2021_002] and [IGA_LF_2022_032]. E. Kociánová, M. Kamasová, Z. Lys, M. Drápela and J. Jarkovský, has nothing to disclose. The language editing fee was covered by grant [17/RVO-FNOs/2023] and [MH CZ – DRO (FNOs/2023)] from the Ostrava University, Faculty of Medicine. There are no relevant relationships with the industry and no competing interests to declare.
Additional information
Funding
References
- Levin A, Stevens PE, Bilous RW, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
- Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi:10.1053/j.ajkd.2014.01.416.
- Calhoun DA, Jones D, Textor S, et al. Resistant. Hypertension. 2008;51(6):1403–1419. doi:10.1161/HYPERTENSIONAHA.108.189141.
- Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34(16):1204–1214. doi:10.1093/eurheartj/ehs368.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642. doi:10.1161/CIRCULATIONAHA.111.068064.
- Thomas G, Xie D, Chen HY, et al. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the chronic renal insufficiency cohort study. Hypertension. 2016;67(2):387–396. doi:10.1161/HYPERTENSIONAHA.115.06487.
- Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. doi:10.1016/S2213-8587(18)30313-9.
- Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104. doi:10.1053/j.ajkd.2019.06.009.
- Lambers Heerspink HJ, Kröpelin TF, Hoekman J, et al. Drug-induced reduction in albuminuria Is associated with subsequent renoprotection. J Am Soc Nephrol. 2015;26(8):2055–2064. doi:10.1681/ASN.2014070688.
- Heerspink HJL, Jongs N, Chertow GM, et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):743–754. doi:10.1016/S2213-8587(21)00242-4.
- Kaboré J, Metzger M, Helmer C, et al. Kidney function decline and apparent treatment-resistant hypertension in the elderly. PLoS One. 2016;11(1):e0146056. doi:10.1371/journal.pone.0146056.
- Berra E, Azizi M, Capron A, et al. Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension. 2016;68:297–306. doi:10.1161/HYPERTENSIONAHA.116.07464.
- Jelínek L, Václavík J, Ramík Z, et al. Directly measured adherence to treatment in chronic heart failure: LEVEL-CHF registry. Am J Med Sci. 2021;361(4):491–498. doi:10.1016/j.amjms.2020.12.004.
- Fay KS, Cohen DL. Resistant hypertension in people with CKD: a review. Am J Kidney Dis. 2021;77(1):110–121. doi:10.1053/j.ajkd.2020.04.017.
- Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi:10.1056/NEJMoa2102953.
- Parati G, Stergiou GS, Asmar R, et al. European society of hypertension guidelines for blood pressure monitoring at home: a summary report of the second international consensus conference on home blood pressure monitoring. J Hypertens. 2008;26(8):1505–1526. doi:10.1097/HJH.0b013e328308da66.
- Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419–428, PMID: 19768194 PMCID: PMC2744545.
- Davies DF, Shock NW. Age changes IN glomerular filtration rate, effective renal plasma flow, AND tubular excretory capacity IN adult males. J Clin Invest. 1950;29(5):496–507. doi:10.1172/JCI102286.
- Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278–285. doi:10.1111/j.1532-5415.1985.tb07117.x.
- Mc Causland FR, Claggett BL, Vaduganathan M, et al. Dapagliflozin and kidney outcomes in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified analysis of the DELIVER randomized clinical trial. JAMA Cardiol. 2023;9(2):144–152. doi:10.1001/jamacardio.2023.4664.
- Sharma A, Ferreira JP, Zannad F, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from the EMPEROR-Preserved trial. Eur J Heart Fail. 2023;25(8):1337–1348. doi:10.1002/ejhf.2857.
- Cherney DZI, Cosentino F, Dagogo-Jack S, et al. Ertugliflozin and slope of chronic eGFR. Clin J Am Soc Nephrol. 2021;16(9):1345–1354. doi:10.2215/CJN.01130121.
- Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(5):1237–1250. doi:10.1111/dom.13648.
- Chertow GM, Vart P, Jongs N, et al. Effects of dapagliflozin in stage 4 chronic kidney disease. J Am Soc Nephrol. 2021;32(9):2352–2361. doi:10.1681/ASN.2021020167.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi:10.1056/NEJMoa2024816.
- Jardine MJ, Zhou Z, Mahaffey KW, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020;31(5):1128–1139. doi:10.1681/ASN.2019111168.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi:10.1056/NEJMoa1811744.
- Cao H, Rao X, Jia J, et al. Effects of sodium-glucose co-transporter-2 inhibitors on kidney, cardiovascular, and safety outcomes in patients with advanced chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2023;60(3):325–335. doi:10.1007/s00592-022-01989-7.
- Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-Like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145(8):575–585. doi:10.1161/CIRCULATIONAHA.121.055459.
- Holtkamp FA, De Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–287. doi:10.1038/ki.2011.79.
- Vaduganathan M, Ferreira JP, Rossignol P, et al. Effects of steroidal mineralocorticoid receptor antagonists on acute and chronic estimated glomerular filtration rate slopes in patients with chronic heart failure. Eur J Heart Fail. 2022;24(9):586–1590. doi:10.1002/ejhf.2635.
- Mc Causland FR, Lefkowitz MP, Claggett B, et al. Angiotensin–neprilysin inhibition and renal outcomes across the spectrum of ejection fraction in heart failure. Eur J Heart Fail. 2022;24(9):1591–1598. doi:10.1002/ejhf.2421.
- Kovesdy CP, Coresh J, Ballew SH, et al. Past decline versus current EGFR and subsequent ESRD risk. J Am Soc Nephrol. 2016;27(8):2447–2455. doi:10.1681/ASN.2015060687.
- Grams ME, Sang Y, Ballew SH, et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol. 2019;30(9):1746–1755. doi:10.1681/ASN.2019010008.
- Denker M, Boyle S, Anderson AH, et al. Chronic renal insufficiency cohort study (CRIC). Clin J Am Soc Nephrol. 2015;10(11):2073–2083. doi:10.2215/CJN.04260415.
- Lash JP, Go AS, Appel LJ, et al. Chronic renal insufficiency cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin JAm Soc Nephrol. 2009;4(8):1302–1311. Available from: https://journals.lww.com/cjasn/Fulltext/2009/08000/Chronic_Renal_Insufficiency_Cohort__CRIC__Study_.4.aspx doi:10.2215/CJN.00070109.
- Tanner RM, Calhoun DA, Bell EK, et al. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8(9):1583–1590. doi:10.2215/CJN.00550113.
- Muntner P, Davis BR, Cushman WC, et al. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-Stage renal disease. Hypertension. 2014;64(5):1012–1021. doi:10.1161/HYPERTENSIONAHA.114.03850.
- Viazzi F, Piscitelli P, Ceriello A, et al. Resistant hypertension, time‐updated blood pressure values and renal outcome in type 2 diabetes mellitus. J Am Heart Assoc. 2017;6(0 ):e006745. doi:10.1161/JAHA.117.006745.
- Pignatelli P, Farcomeni A, Menichelli D, et al. Serum albumin and risk of cardiovascular events in primary and secondary prevention: a systematic review of observational studies and Bayesian meta-regression analysis. Intern Emerg Med. 2020;15(1):135–143. doi:10.1007/s11739-019-02204-2.
- Ronit A, Kirkegaard-Klitbo DM, Dohlmann TL, et al. Plasma albumin and incident cardiovascular disease. Arterioscler Thromb Vasc Biol. 2020;40(2):473–482. doi:10.1161/ATVBAHA.119.313681.
- Desai R, Park H, Brown JD, et al. Comparative safety and effectiveness of aldosterone antagonists Versus beta-Blockers as fourth agents in patients With apparent resistant hypertension. Hypertension. 2022;79(10):2305–2315. doi:10.1161/HYPERTENSIONAHA.122.19280.
- Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi:10.1056/NEJMoa2025845.
- Ramik Z, Vaclavik J, Kocianova E, et al. Long-term decline of renal function in patients with resistant and non-resistant arterial hypertension. Eur Heart J. 2022;43(2):ehac544.2226. doi:10.1093/eurheartj/ehac544.2226.
- Ramik Z, Vaclavik J, Kvapil T, et al. Renal function IN comparison TO non-resistant arterial hypertension. J Hypertens. 2022;40(1):e287. doi:10.1097/01.hjh.0000838504.91361.bc.