Abstract
Introduction
Sex differences in blood pressure (BP), hypertension and hypertension mediated cardiovascular complications have become an increasingly important focus of attention. This narrative review gives an overview of current studies on this topic, with the aim to provide a deeper understanding of the sex-based disparities in hypertension with essential insights for refining prevention and management strategies for both men and women.
Methods and results
We searched Medline, Embase and the Cochrane libray on sex differences in BP-trajectories and hypertension prevalence. In the past decade various population-based studies have revealed substantial sex-disparities in BP-trajectories throughout life with women having a larger increase in hypertension prevalence after 30 years of age and a stronger association between BP and cardiovascular disease (CVD). In general, the effects of antihypertensive treatment appear to be consistent across sexes in different populations, although there remains uncertainty about differences in the efficacy of BP lowering drugs below 55 years of age.
Conclusion
The current uniform approach to the diagnosis and management of hypertension in both sexes neglects the distinctions in hypertension, while the differences underscore the need for sex-specific recommendations, particularly for younger individuals. A major limitation hampering insights into sex differences in BP-related outcomes is the lack of sex-stratified analyses or an adequate representation of women. Additional large-scale, longitudinal studies are imperative.
Introduction
Hypertension has emerged as the most significant risk factor for cardiovascular disease (CVD), with its incidence doubling over the past two decades [Citation1–3]. Sex differences in hypertension have become an increasingly important focus of attention, not only because of known disparities in prevalence, but also because of increased recognition of sex differences in blood pressure (BP) trajectories, associated CVD risk and treatment benefits. However, in clinical practice, the definition of hypertension in adults is independent of sex, with hypertension being defined as BP levels of ≥140/90 mmHg or ≥130/80 mmHg in both women and men, depending on the adherence to European or American guidelines [Citation4–7]. The objective of this review is to provide a narrative literature overview of sex differences in hypertension prevalence and BP trajectories, the risk of hypertension mediated organ damage, the association with CVD, and the efficacy of antihypertensive medication. Since we are aware of the fact that differences observed in our study may not only reflect biological factors (sex), as suggested by our choice of the term sex, but also sociocultural factors (gender), we chose to use the terms ‘women’ and ‘men’ over the use of the terms ‘male’ and ‘female’.
Methods
To examine the existing literature on sex disparities in hypertension prevalence and BP trajectories and their association with CVD and end-organ damage, we searched Medline, Embase, and the Cochrane Library for articles on sex differences in hypertension prevalence and BP trajectories in adults (age 18 years and above). Systematic reviews and large-scale, population-based studies with more than 1,000 participants were included. To offer a visual representation of sex differences in BP trajectories, we additionally included baseline data of the HEalthy Life in an Urban Setting (HELIUS) study, a multi-ethnic, population-based cohort study including more than 20,000 participants living in Amsterdam, The Netherlands [Citation8]. Written informed consent was obtained from all participants in the HELIUS study. The study received approval from the medical ethical review board of the Amsterdam UMC, location AMC and this study adhered to the principles outlined in the Declaration of Helsinki. We modelled the sex-specific association between BP levels and age using general additive models with cubic splines, after exclusion of participants using antihypertensive medication. The cubic splines were added since the relationship between BP levels and age is non-linear. Additionally, we created density plots of the distribution of BP levels across the population, stratified by sex. To evaluate sex-specific differences in the efficacy of antihypertensive medication, we searched for studies and meta-analyses that reported on sex-specific outcomes from randomised controlled trials. Sex differences in the underlying pathophysiology of hypertension and pregnancy-related hypertensive disorders fall outside the scope of this paper and have been recently reviewed elsewhere [Citation4,Citation9].
Sex differences in blood pressure trajectories
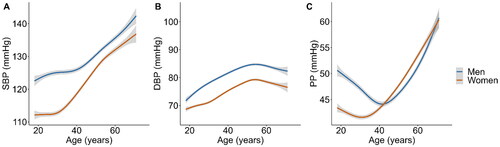
On average, systolic blood pressure (SBP) and diastolic blood pressure (DBP) are lower in women compared to men, but the differences in SBP and DBP levels changes across the life course and is marked by distinct phases [Citation10,Citation11]. The initial phase involves a rapid elevation in SBP from childhood to adolescence, aligning with growth in body weight and height. From early adulthood onwards, SBP levels gradually increase to slow down or even decline in the last decade of life [Citation12,Citation13]. In contrast, DBP levels peak in middle age, followed by a gradual decline, resulting in an elevation of pulse pressure after 50 years of age [Citation10,Citation11]. Although the direction of these patterns can be observed in both men and women, recent studies have demonstrated significant sex differences in BP trajectories throughout the life course [Citation13]. While no sex differences are apparent in children before puberty [Citation14], SBP increase is much steeper in men between 15 and 20 years, while from 20 years of age onwards SBP increases more rapidly in women [Citation13]. These sex differences in BP trajectories are also observed in data of the HELIUS study. The data show a larger increase in SBP in women from 30 years of age onwards to well beyond the 5th decade of life (), resulting in a decrease in the SBP gap between men and women. Studies show that identical cut-off values are a better predictor of the risk of future hypertension in young women compared to younger men (aged <45 years), which may be attributed to the generally lower BP values in women in this age-category [Citation15]. The larger incline in SBP over time also means that pulse pressure levels rise more steeply in women when compared to men, leading to a higher prevalence of isolated systolic hypertension at older age. This has also been demonstrated in the Framingham Heart Study, which included 4,993 participants with a mean follow-up time of 28 years [Citation16], resulting in an increased prevalence of isolated systolic hypertension in elderly women as compared to men [Citation17–19]. These sex differences in BP levels during the life course impact the observed differences in both the prevalence and phenotype of hypertension in men and women.
Figure 1. Sex-specific association between blood pressure levels and age modelled using general additive models with cubic splines for SBP (a), DBP (B) and PP (C) levels. Data were used of 18,747 participants in the HELIUS study (n = 7,951 men, n = 10,523 women). SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: pulse pressure.
Sex differences in hypertension prevalence, treatment and control
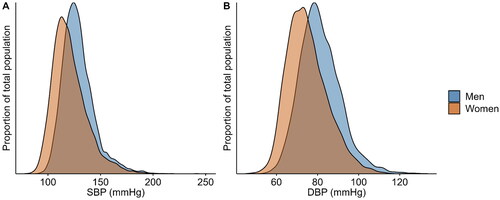
Although hypertension prevalence rates vary across studies, depending upon applied cut-off values and population characteristics, hypertension is, in general, less frequently observed in women compared to men. According to population-based data in the US, the prevalence is 51.7% in men and 42.8% in women aged ≥20 years using the stricter American guidelines [Citation20]. Studies adhering to European guidelines report age-standardized hypertension prevalence ranging from 26.4% − 43.3% in men and 17.5% − 30.6% in women living in Western Europe, and higher ranges of 42.6% − 55.9% in men and 34.0% − 46.9% in women living in Central and Eastern Europe, underscoring significant regional disparities in hypertension prevalence among men and women [Citation1]. Analysing data from the HELIUS study, we found an age-standardized prevalence of 36.8% in men and 31.4% in women, within the expected range for Western Europe and with a similar sex disparity to those observed in studies using data from the UK Biobank, the FINRISK and the CONSTANCES study [Citation21–23]. The lower hypertension prevalence in women can be attributed to their lower overall mean systolic and diastolic BP values [Citation1,Citation24,Citation25]. In contrast, the prevalence of white coat hypertension is higher in women compared to men [Citation26]. This is also demonstrated by the distribution of systolic and diastolic BP levels among men and women participating in the HELIUS study; the data show a leftward shift in the BP distribution curves in women (). With regard to hypertension control, studies generally report higher rates in women compared to men [Citation20,Citation24,Citation27], although this finding is not consistent in all studies [Citation28]. In the older population, hypertension control rates in men surpass those in women [Citation29–31]. The factors contributing to the lower rates of hypertension control among older women have not been completely clarified, but inadequate treatment may offer a partial explanation. Another potential contributor is the higher prevalence of depression in older women, which may result in lower adherence to antihypertensive medication [Citation32]. Besides, previous research has shown that older women are less likely to receive antihypertensive therapies than male patients for comparable BP values [Citation33]. Additionally, adverse effects from antihypertensive drugs are reported more often by women than by men in six out of ten groups of antihypertensive medication, which could impact adherence to antihypertensive treatment [Citation34]. The discrepancy in adverse effects can likely be attributed to variations in pharmacodynamics and pharmacokinetics due to differences in hormone levels and body compositions between the sexes, especially given that men and women typically receive the same dosage of antihypertensive medication [Citation35,Citation36].
Sex differences in hypertension-mediated organ damage
Hypertension leads to structural and functional changes in the microcirculation and increased stiffness of large arteries, resulting in a higher pulse wave velocity (PWV). Data from a population-based study involving 290 individuals indicate that women have an increased media/lumen ratio of small arteries compared to men after adjustment for confounders, indicating significant sex differences in microcirculation [Citation37]. Research on sex differences in PWV has shown no difference between sexes in normal PWV values after correcting for BP differences [Citation38]. This aligns with another study using cardiac MRI to determine PWV, which found no differences between men and women, except in individuals with BP levels exceeding 140/90 mmHg, where PWV was higher in women compared to men [Citation39]. Important examples of hypertension-mediated organ damage are the presence of left ventricular hypertrophy (LVH), white matter lesions (WML), and chronic kidney disease (CKD) [Citation4,Citation6]. Both population-based and randomised studies have shown that the prevalence of LVH is higher in women compared to men. In the LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) study for example, LVH was present in 80% of hypertensive women and 70% of hypertensive men [Citation40]. The Campania Salute Network study, including 12,329 participants with hypertension without CVD, further supports this observation, with a prevalence of LVH of 43.4% in women and 32.1% in men [Citation41], consistent with other studies [Citation42]. Additionally, distinct sex-specific ventricular remodelling patterns were noted, with women having more concentric remodelling and less eccentric hypertrophy compared to men [Citation43]. Not only is LVH more prevalent in women, but the development of cerebral WML, a marker of brain vasculature, is also more pronounced in women in population-based studies, affecting both deep and periventricular regions of the brain [Citation44,Citation45]. The existing literature on the interaction between sex, hypertension, and WML presents conflicting findings: some studies suggest a stronger association between hypertension prevalence and WML in women [Citation46], while others report the opposite [Citation47,Citation48]. Interestingly, studies on sex disparities in CKD prevalence show paradoxical results. While the proportion of women with CKD appears to be higher than the proportion of men in most populations [Citation49], men often have a steeper eGFR decline and a higher risk of end-stage kidney disease (ESKD) [Citation50–54]. This can be attributed to the possibility of CKD overdiagnosis in men with the use of commonly used eGFR equations and the higher life expectancy among women, which consequently elevates their likelihood of developing CKD, given that it predominantly affects the elderly population [Citation55]. Finally, studies examining the association between hypertension and the risk of CKD have demonstrated a more pronounced association in men as opposed to women [Citation56,Citation57]. In conclusion, sex differences across the different types of hypertension-mediated organ damage are not consistent and mechanisms underlying differences sometimes inconclusive, necessitating further research.
Association with cardiovascular diseases
Population-based cohort studies have consistently shown that men have a higher absolute risk of CVD compared to women [Citation58]. However, evidence on the BP associated relative risk of CVD and mortality is conflicting. A meta-analysis of population-based studies revealed a 15% increase in CVD risk per 10 mmHg rise in SBP for men and a 25% increase for women [Citation59], findings supported by other meta-analyses [Citation60,Citation61]. Additionally, women showed an increased risk of events starting at lower BP values compared to men, possibly explained by their lower mean BP values compared to men [Citation59]. Sex differences in the association between BP and events are also substantiated by a combined analysis for 4 population-based cohorts, encompassing 27,542 participants (54% women) without a history of CVD at baseline. This analysis found a steeper relationship between elevated BP levels and risk of CVD in women compared to men, a pattern consistent across various types of CVD, including myocardial infarction, heart failure, and stroke [Citation62]. These results align with findings from other studies, including the Tromsø Study and the UK Biobank study [Citation63,Citation64]. However, other studies failed to identify significant sex differences in the association between BP levels and CVD or observed disparities specific to the type of CVD [Citation65,Citation66]. Disparities in the association between baseline SBP levels and CVD between sexes have been analysed by examining the effect of a sex-specific 1 standard deviation (SD) difference in SBP, which could explain the disparities between different studies regarding the association between BP and CVD. This approach considers not only sex differences in mean SBP levels but also accounts for sex-based variations in the distribution of BP levels across the population. The absence of addressing sex disparities in both mean and standard deviation of baseline BP levels in other studies could potentially explain the differences observed between these studies and others, which underscores the necessity for further research using sex-specific SD differences in SBP when examining its association with CVD.
Effect of BP lowering medication on CVD outcomes
In current guidelines, there are no sex-specific recommendations for dosing or choice of medication, with the exception of the use of renin-angiotensin system (RAS)-inhibitors in younger women [Citation4,Citation6]. A recent meta-analysis on the efficacy of antihypertensive medication incorporating data of 51 randomised controlled trials involving 358,636 participants (42% women) indicates that the effects of antihypertensive medication on BP reduction and relative treatment effects for CVD are similar in both sexes and is consistent across different age categories and type of antihypertensive medications [Citation67]. The results of this meta-analysis were in line with other studies which found similar protection against major cardiovascular events in men and women [Citation68]. However, only a limited number of women aged <55 years was included in this meta-analysis, resulting in remaining uncertainty regarding sex differences in the effect of BP-lowering medication in younger populations.
Conclusion and discussion
In this narrative review, our objective was provide an overview of the existing literature concerning sex disparities in hypertension (). By its nature, this generated the potential to overlook certain aspects or perspectives that could be captured more effectively through a systematic or meta-analytical approach. However, by including various recent meta-analysis we feel that we have captured most of the recent literature on this subject, while generating additional room for reflection.
Figure 3. To model BP trajectories and calculate the prevalence of hypertension, we used data from participants of the HELIUS study (n = 7,951 men, n = 10,523 women).CVD: cardiovascular diseases; SBP: systolic blood pressure; BP: blood pressure.
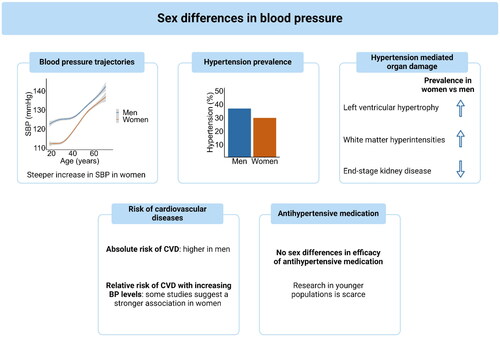
Various population-based studies have revealed substantial sex-disparities in BP trajectories throughout life with a larger increase in hypertension prevalence after 30 years of age and a different contribution to complications. The major limitation drawing conclusive insights into sex differences in BP-related outcomes is the lack of sex-stratified analysis or an adequate representation of women in studies especially in those <55 years of age. This emphasises the imperative need for adequately designed sex- and gender sensitive data collection and analyses in additional large-scale, longitudinal studies that have also included younger, pre-menopausal women. The current uniform approach taken in the identification and management of hypertension in adults might neglect inherent disparities in BP distribution and trajectories as well as hypertension mediated outcomes between men and women. For instance, while the higher absolute risk in men is already taken into account in various commonly used CVD risk charts and algorithms [Citation69], the higher hypertension associated CVD risk in women is not. Further, the fact that the most pronounced difference in BP trajectories is present in the young, implicates the need for sex-specific cut off values in guidelines, particularly for younger individuals.
Nevertheless, we show that further research is essential to gain a deeper understanding of these sex-based disparities in cardiovascular risk, which will be pivotal in refining prevention and management strategies for both men and women.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The HELIUS data are owned by the Amsterdam University Medical Centre, location AMC in Amsterdam, The Netherlands. Any researcher can request the data by submitting a proposal to the HELIUS Executive Board as outlined at http://www.heliusstudy.nl/en/researchers/collaboration. The HELIUS Executive Board will check proposals for compatibility with the general objectives, ethical approvals and informed consent forms of the HELIUS study. There are no other restrictions to obtaining the data and all data requests will be processed in the same manner.
Additional information
Funding
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):1–9. doi:10.1016/S0140-6736(21)01330-1.
- Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;388(10053):1659–1724. doi:10.1016/S0140-6736(15)00128-2.
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi:10.1016/S0140-6736(20)30752-2.
- Mancia Chairperson G, Kreutz Co-Chair R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023;41(12):1874–2071.
- National Institute for Health and Care Excellence: guidelines. Hypertension in adults: diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2022.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi:10.1161/HYP.0000000000000066.
- Meinert F, Thomopoulos C, Kreutz R. Sex and gender in hypertension guidelines. J Hum Hypertens. 2023;37(8):654–661. doi:10.1038/s41371-022-00793-8.
- Snijder MB, Galenkamp H, Prins M, et al. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open. 2017;7(12):e017873. doi:10.1136/bmjopen-2017-017873.
- Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37(5):1199–1208. doi:10.1161/01.hyp.37.5.1199.
- Wills AK, Lawlor DA, Matthews FE, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8(6):e1000440. doi:10.1371/journal.pmed.1000440.
- Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–315. doi:10.1161/01.cir.96.1.308.
- Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66(6):1108–1115. doi:10.1161/HYPERTENSIONAHA.115.05831.
- Ji H, Kim A, Ebinger JE, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5(3):19–26. doi:10.1001/jamacardio.2019.5306.
- Shen W, Zhang T, Li S, et al. Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: the bogalusa heart study. Hypertension. 2017;70(1):66–74. doi:10.1161/HYPERTENSIONAHA.117.09537.
- Vriend EMC, Bouwmeester TA, Franco OH, et al. Sex differences in blood pressure phenotypes over time - the HELIUS study. J Hypertens. 2024;42(6):977–983. doi:10.1097/HJH.0000000000003676.
- Cheng S, Xanthakis V, Sullivan LM, et al. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension. 2012;60(6):1393–1399. doi:10.1161/HYPERTENSIONAHA.112.201780.
- Martins D, Nelson K, Pan D, et al. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4(3):10–13. 20.
- Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. J Am Soc Hypertens. 2015;9(3):197–205. doi:10.1016/j.jash.2015.01.002.
- Berry KL, Cameron JD, Dart AM, et al. Large-artery stiffness contributes to the greater prevalence of systolic hypertension in elderly women. J Am Geriatr Soc. 2004;52(3):368–373.
- Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143(8):e254–e743. doi:10.1161/CIR.0000000000000950.
- Wan EYF, Fung WT, Schooling CM, et al. Blood pressure and risk of cardiovascular disease in UK biobank: a mendelian randomization study. Hypertension. 2021;77(2):367–375. doi:10.1161/HYPERTENSIONAHA.120.16138.
- Qiu C, Hu G, Kivipelto M, et al. Association of blood pressure and hypertension with the risk of Parkinson disease: the National FINRISK Study. Hypertension. 2011;57(6):1094–1100. doi:10.1161/HYPERTENSIONAHA.111.171249.
- Neufcourt L, Deguen S, Bayat S, et al. Gender differences in the association between socioeconomic status and hypertension in France: A cross-sectional analysis of the CONSTANCES cohort. PLoS One. 2020;15(4):e0231878. doi:10.1371/journal.pone.0231878.
- Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. 2017;390(10112):2549–2558.
- NCD Risk Factor Collaboration (NCD-RisC). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. 2019;394(10199):639–651. doi:10.1016/S0140-6736(19)31145-6.
- Franklin SS, Thijs L, Hansen TW, et al. White-coat hypertension: new insights from recent studies. Hypertension. 2013;62(6):982–987. doi:10.1161/HYPERTENSIONAHA.113.01275.
- Peters SAE, Muntner P, Woodward M. Sex Differences in the Prevalence of, and Trends in, Cardiovascular Risk Factors, Treatment, and Control in the United States, 2001 to 2016. Circulation. 2019;139(8):1025–1035. doi:10.1161/CIRCULATIONAHA.118.035550.
- Ong KL, Tso AW, Lam KS, et al. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51(4):1142–1148. doi:10.1161/HYPERTENSIONAHA.107.105205.
- Foti K, Wang D, Appel LJ, et al. Hypertension Awareness, Treatment, and Control in US Adults: trends in the Hypertension Control Cascade by Population Subgroup (National Health and Nutrition Examination Survey, 1999-2016). Am J Epidemiol. 2019;188(12):2165–2174. doi:10.1093/aje/kwz177.
- Osude N, Durazo-Arvizu R, Markossian T, et al. Age and sex disparities in hypertension control: the multi-ethnic study of atherosclerosis (MESA). Am J Prev Cardiol. 2021;8:100230.
- Daugherty SL, Masoudi FA, Ellis JL, et al. Age-dependent gender differences in hypertension management. J Hypertens. 2011;29(5):1005–1011. doi:10.1097/HJH.0b013e3283449512.
- Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psychiatry. 2008;65(2):172–178. doi:10.1001/archgenpsychiatry.2007.17.
- Bager JE, Manhem K, Andersson T, et al. Hypertension: sex-related differences in drug treatment, prevalence and blood pressure control in primary care. J Hum Hypertens. 2023;37(8):662–670. doi:10.1038/s41371-023-00801-5.
- Rydberg DM, Mejyr S, Loikas D, et al. Sex differences in spontaneous reports on adverse drug events for common antihypertensive drugs. Eur J Clin Pharmacol. 2018;74(9):1165–1173. doi:10.1007/s00228-018-2480-y.
- Rosano GMC, Lewis B, Agewall S, et al. Gender differences in the effect of cardiovascular drugs: a position document of the Working Group on Pharmacology and Drug Therapy of the ESC. Eur Heart J. 2015;36(40):2677–2680. doi:10.1093/eurheartj/ehv161.
- Tamargo J, Rosano G, Walther T, et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3(3):163–182. doi:10.1093/ehjcvp/pvw042.
- Bruno RM, Grassi G, Seravalle G, et al. Age- and sex-specific reference values for media/lumen ratio in small arteries and relationship with risk factors. Hypertension. 2018;71(6):1193–1200. doi:10.1161/HYPERTENSIONAHA.117.10634.
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values. Eur Heart J. 2010;31(19):2338–2350. doi:10.1093/eurheartj/ehq165.
- van Hout MJ, Dekkers IA, Westenberg JJ, et al. Normal and reference values for cardiovascular magnetic resonance-based pulse wave velocity in the middle-aged general population. J Cardiovasc Magn Reson. 2021;23(1):46. doi:10.1186/s12968-021-00739-y.
- Gerdts E, Okin PM, de Simone G, et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51(4):1109–1114. doi:10.1161/HYPERTENSIONAHA.107.107474.
- Gerdts E, Izzo R, Mancusi C, et al. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania Salute Network). Int J Cardiol. 2018;258:257–261. doi:10.1016/j.ijcard.2017.12.086.
- de Simone G, Devereux RB, Izzo R, et al. Lack of reduction of left ventricular mass in treated hypertension: the strong heart study. J Am Heart Assoc. 2013;2(3):e000144. doi:10.1161/JAHA.113.000144.
- Miller RJH, Mikami Y, Heydari B, et al. Sex-specific relationships between patterns of ventricular remodelling and clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21(9):983–990. doi:10.1093/ehjci/jeaa164.
- de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. doi:10.1136/jnnp.70.1.9.
- Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi:10.1161/01.str.27.8.1274.
- Bonberg N, Wulms N, Dehghan-Nayyeri M, et al. Sex-specific causes and consequences of white matter damage in a middle-aged cohort. Front Aging Neurosci. 2022;14:810296. doi:10.3389/fnagi.2022.810296.
- Alqarni A, Jiang J, Crawford JD, et al. Sex differences in risk factors for white matter hyperintensities in non-demented older individuals. Neurobiol Aging. 2021;98:197–204. doi:10.1016/j.neurobiolaging.2020.11.001.
- Sachdev PS, Parslow R, Wen W, et al. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30(6):946–956. doi:10.1016/j.neurobiolaging.2007.08.023.
- Murphy D, McCulloch CE, Lin F, et al. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med. 2016;165(7):473–481. doi:10.7326/M16-0273.
- Minutolo R, Gabbai FB, Chiodini P, et al. Sex differences in the progression of CKD among older patients: pooled analysis of 4 cohort studies. Am J Kidney Dis. 2020;75(1):30–38. doi:10.1053/j.ajkd.2019.05.019.
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi:10.1056/NEJMoa041031.
- Albertus P, Morgenstern H, Robinson B, et al. Risk of ESRD in the United States. Am J Kidney Dis. 2016;68(6):862–872. doi:10.1053/j.ajkd.2016.05.030.
- Ricardo AC, Yang W, Sha D, et al. Sex-related disparities in CKD progression. J Am Soc Nephrol. 2019;30(1):137–146. doi:10.1681/ASN.2018030296.
- Chesnaye NC, Dekker FW, Evans M, et al. Renal function decline in older men and women with advanced chronic kidney disease-results from the EQUAL study. Nephrol Dial Transplant. 2021;36(9):1656–1663. doi:10.1093/ndt/gfaa095.
- Carrero JJ, Hecking M, Chesnaye NC, et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–164. doi:10.1038/nrneph.2017.181.
- Vosters TG, Kingma FM, Stel VS, et al. Sex differences in CKD risk factors across ethnic groups. Nephrol Dial Transplant. 2024; doi:10.1093/ndt/gfae038.
- Wang D, Kou P-Q, Liao Y-Y, et al. Sex differences in impact of cumulative systolic blood pressure from childhood to adulthood on albuminuria in midlife: a 30-year prospective cohort study. BMC Public Health. 2023;23(1):666. doi:10.1186/s12889-023-15613-y.
- Boggia J, Thijs L, Hansen TW, et al. Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension. 2011;57(3):397–405. doi:10.1161/HYPERTENSIONAHA.110.156828.
- Wei YC, George NI, Chang CW, et al. Assessing sex differences in the risk of cardiovascular disease and mortality per increment in systolic blood pressure: a systematic review and meta-analysis of follow-up studies in the United States. PLoS One. 2017;12(1):e0170218. doi:10.1371/journal.pone.0170218.
- Roush GC, Fagard RH, Salles GF, et al. Prognostic impact of sex-ambulatory blood pressure interactions in 10 cohorts of 17 312 patients diagnosed with hypertension: systematic review and meta-analysis. J Hypertens. 2015;33(2):212–220. doi:10.1097/HJH.0000000000000435.
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi:10.1016/S0140-6736(04)17018-9.
- Ji H, Niiranen TJ, Rader F, et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation. 2021;143(7):761–763. doi:10.1161/CIRCULATIONAHA.120.049360.
- Albrektsen G, Heuch I, Løchen ML, et al. Risk of incident myocardial infarction by gender: interactions with serum lipids, blood pressure and smoking. The Tromsø Study 1979-2012. Atherosclerosis. 2017;261:52–59. doi:10.1016/j.atherosclerosis.2017.04.009.
- Remfry E, Ardissino M, McCracken C, et al. Sex-based differences in risk factors for incident myocardial infarction and stroke in the UK Biobank. Europ Heart J. Qual Care Clin Outcomes. 2023; doi:10.1093/ehjqcco/qcad029.
- Peters SA, Huxley RR, Woodward M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke. 2013;44(9):2394–2401. doi:10.1161/STROKEAHA.113.001624.
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi:10.1016/s0140-6736(02)11911-8.
- Bidel Z, Nazarzadeh M, Canoy D, et al. Sex-specific effects of blood pressure lowering pharmacotherapy for the prevention of cardiovascular disease: an individual participant-level data meta-analysis. Hypertension. 2023;80(11):2293–2302. doi:10.1161/HYPERTENSIONAHA.123.21496.
- Turnbull F, Woodward M, Neal B, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29(21):2669–2680. doi:10.1093/eurheartj/ehn427.
- Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi:10.1093/eurheartj/ehab484.