Abstract
Background
We conducted a comparative analysis of hypertension prevalence, progression, and treatment in two Finnish population-based cohorts comprising older adults born 20 years apart. The study covered data from pre- and post-HYVET Study eras and spanned the onset of the COVID-19 pandemic.
Methods
All 70-year-old home-dwelling citizens of Turku, in Southwest Finland, were invited to participate in the survey in 1990 (1920-born TUVA cohort) and in 2010 (1940-born UTUVA cohort) with a 25-year follow-up plan. The analyses included those with available data for systolic and diastolic blood pressure (BP), yielding 1015 TUVA and 888 UTUVA participants at baseline. Biomarkers associated with BP were analysed with t- and chi-square tests.
Results
At baseline, 83.4% of TUVA and 74.3% of UTUVA participants had uncontrolled BP, with respective antihypertensive medication usage at 36.0% and 55.9% (p < .001 for both between-cohort differences). Systolic BP exhibited an inverted U-shaped trajectory, with TUVA initially 7.8 mmHg higher at 155.4 mmHg than UTUVA (p < .001). However, by the ages 80–82, the difference in systolic BP trajectories between the cohorts was attenuated to 4.0 mmHg (p = .03). Diastolic BP differences were less clinically significant. UTUVA demonstrated higher use of all five conventional antihypertensive categories than TUVA (p ≤ .02 for all categories).
Conclusions
In the early years of older adulthood, the 1940-born cohort showed a positive trend in hypertension management, yet maintained a 74.3% baseline rate of uncontrolled BP. Furthermore, by the ages 81–82, the benefits observed over the 1920-born cohort had lessened, influenced by the COVID-19 pandemic or other lasting factors. Heightened efforts to improve hypertension treatment in older adults remain crucial in the post-HYVET era.
PLAIN LANGUAGE SUMMARY
We studied two generational cohorts of older adults from Finland, born 20 years apart, to examine changes in blood pressure readings over time, the prevalence of high blood pressure, and its treatment. Our investigation spanned periods both before and after the HYVET Study, a significant research effort demonstrating the benefits of treating hypertension in older adult patients, reducing the risk of stroke and other causes of mortality. Additionally, we considered the potential impact of the COVID-19 pandemic on blood pressure control.
We invited all 70-year-olds living at home in Turku, Southwest Finland, to participate in our survey in 1990 (the 1920-born cohort) and in 2010 (the 1940-born cohort), with plans to follow them for 25 years. We collected data on their blood pressure readings and the medications they were prescribed.
At the outset of our study, when participants were 70 years old, a higher proportion of individuals in the 1920-born cohort had uncontrolled high blood pressure compared to those in the 1940-born group. In addition, the participants born in 1940 showed increased usage and a wider selection of antihypertensive medications compared to the 1920-born cohort. Despite this, over 70% of the 70-year-olds even in the 1940-born cohort still had uncontrolled blood pressure. Furthermore, by the time these individuals reached their early 80s, the initial improvements in blood pressure control over the 1920-born cohort had somewhat diminished.
Our findings underscore the ongoing need for improvements in managing high blood pressure among older adults. This remains crucial as individuals age, emphasising the importance of continued research to develop better treatment approaches, even after landmark studies like HYVET.
Introduction
Hypertension is the leading cause of cardiovascular disease and premature death worldwide. It is a significant and often asymptomatic chronic disease that can escalate into a major risk factor for cardiac failure, stroke, chronic renal failure, and coronary artery disease if not treated effectively [Citation1]. The prevalence of hypertension increases with age and is particularly high among older adults [Citation2], reaching rates as high as 70% to 80% [Citation3]. As the older adult population continues to grow globally at an unprecedent rate, the high prevalence of hypertension is likely to persist or even increase [Citation4].
A fundamental aspect of preventing cardiovascular diseases has involved screening for high blood pressure (BP) and administering antihypertensive drugs. Until the past couple of decades, randomised controlled trials with older adults had either excluded individuals aged 80 and above or included too few participants in this age group to demonstrate a treatment benefit [Citation5], despite the heightened risk for complications or death from diseases related to hypertension in this vulnerable population. However, a significant shift occurred with the HYVET study. This groundbreaking research demonstrated that treating hypertension in older adult patients (with an average age of 83.6 years in the study) is clearly beneficial, decreasing the risk of death from stroke and death from any other cause. In the HYVET study, the overall mortality was 21% lower in patients treated with antihypertensive medications compared to the placebo group [Citation5].
Although the benefits of antihypertensive drug therapy are well-established, a significant percentage of older people with hypertension either go undiagnosed or receive inadequate treatment. A Dutch population-based study by Rossum et al. investigating older adults revealed that 54% had uncontrolled BP levels [Citation6]. The reasons for this are likely multifactorial, including inadequate clinical management, lack of follow-up, and patient non-compliance [Citation2]. Recently, these challenges have been compounded by the complexities introduced by COVID-19 and social distancing measures, further complicating BP management in older adults.
Research exploring the improvement, if any, in hypertension treatment for older adults before and after HYVET is limited. Particularly, studies examining these issues extending over the onset of the COVID-19 pandemic are practically non-existent. We aimed to fill these gaps in the literature by analysing information from two population-based older adult generational cohorts born 20 years apart, with data encompassing both pre- and post-HYVET eras and spanning the onset of the COVID-19 pandemic. We hypothesised that the 1940-born cohort could exhibit better-controlled BP readings compared to the 1920-born cohort. Despite this anticipated improvement, we believed the management could remain insufficiently effective, risking continued under-treatment of hypertension in the older adult population, particularly in the setting of possibly poorer availability of health services during the COVID-19 era.
Materials and methods
Participants
The study sample included individuals from two distinct birth cohorts: those born in 1920 and 1940. In 1990, the Turku Older Adults Study (TUVA) invited all 1920-born residents of Turku, in Southwest Finland, to participate in a prospective cohort study (n = 1503). After excluding refusals, institutionalised individuals, and those with incomplete questionnaires, 1032 (68.7%) participants aged 70–71 were included in the initial study conducted between 1991 and 1992. 20 years later, the second birth cohort, born in 1940, was invited to participate in the New Turku Older Adults Study (UTUVA), conducted in 2011 and 2012. A total of 1344 70-year-olds met similar exclusion criteria as in TUVA, resulting in 956 (71.1%) participants in the UTUVA study. Finally, we excluded those without data for both systolic and diastolic BP readings at baseline, resulting in 1015 TUVA participants and 888 UTUVA participants for the analyses of the present paper. Each follow-up subcohort was treated as a direct subset of the baseline study, allowing participants to miss individual follow-up assessments but return for further evaluation at later time points. Flowcharts depicting the inclusion-exclusion criteria for the TUVA and UTUVA cohorts in the present paper can be seen in the Supplemental material, Figures S1 and S2.
The TUVA cohort was followed for disease progression and mortality for 25 years, with follow-up examinations being conducted at 10, 15, 20, and 25 years after baseline. The follow-up examinations of the UTUVA cohort have been carried out at 5 and 10 years after baseline thus far.
Health interview and health examination
Both cohorts underwent a similar examination protocol, which included postal questionnaires, interviews, and clinical examinations and has been described in detail in prior publications [Citation7–9]. In the TUVA study, baseline health examinations were conducted at health centres by experienced general practitioners and research nurses. For all other time points in both the TUVA and UTUVA studies, examinations were carried out at the research centre, where BP readings were measured by research nurses, except at the UTUVA baseline and its 5-year follow-up, where BP measurements were taken by research physicians. As some survey time points of TUVA did not have consecutive BP measurements available, we opted to use only the first BP reading for systolic and diastolic BP at all time points for both TUVA and UTUVA. The first reading was taken as sitting BP at all time points in the TUVA study except the 15-year follow-up, where it was taken as supine BP, as well as in all time points in the UTUVA study. As a sensitivity analysis, we also conducted the same statistical analyses using the second, sitting BP reading, for all time points where the first reading was taken as supine BP. These decisions were made considering the mixed evidence from previous literature regarding whether supine or sitting BP measurements tend to be higher [Citation10,Citation11] and to ensure consistency across time points within each cohort. BP was measured using the auscultatory method with a mercury sphygmomanometer at baseline and the 10-year follow-up in the TUVA study. For all other time points in the TUVA and UTUVA studies, an oscillometric OMRON device (Omron Matsusaka Co., Kyoto, Japan) was used, except for the 10-year follow-up of UTUVA, where an oscillometric Apteq AE701f (Rossmax International LtD, Taipei, Taiwan) device was utilised.
Medications were documented through information obtained from the questionnaire, individual interviews, and deciphered from prescriptions of each participant. Subsequently, they were classified according to specific ATC codes (Anatomical Therapeutic Chemical classification system, 2010 revision). All diuretics were considered antihypertensive medications, excluding loop diuretics. Various infrequently used antihypertensive medications, including centrally and peripherally acting antiadrenergic agents, ganglion-blocking agents, agents acting on arteriolar smooth muscle, and aliskiren, were categorised as ‘other antihypertensive medication’.
BP variable definitions
Uncontrolled BP was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg readings. This definition aligns with the most commonly used criteria in prior studies and conforms to the current guidelines, which recommend a target BP of <140/90 mmHg [Citation12,Citation13]. Hypertension was defined as either having uncontrolled BP, self-reported hypertension, or confirmed use of antihypertensive medication without indications other than hypertension. Other indications comprised heart failure, coronary artery disease, previous acute myocardial infarction, arrhythmia (defined as atrial fibrillation in the UTUVA cohort), other cardiac diseases, and kidney disease.
Statistical analyses
Statistical analyses were performed using R, version 4.3.0 (R Core Team, Vienna, Austria). Differences in BP within each cohort were statistically compared using the paired Student’s t-test. To examine differences in BP between cohorts, an unpaired t-test was employed. The chi-square test was used to compare categorical variables.
Ethics
The study protocol of the TUVA study was approved by the City of Turku Ethics Committee on Health Care on 19 December 1990 (Record No. 2/90). Subsequent approval dates (and record numbers), signed by the Ethical Committee of the Hospital District of Southwest Finland, were: 10-year follow-up on 28 February 2001 (1-2001), 15-year follow-up on 23 March 2005 (94/2005), 20-year follow-up on 17 March 2009 (19/2009), and 25-year follow-up on 1 October 2014 (ETMK: 150/1802/2014).
Similar approvals (and record numbers) for the UTUVA study, signed by the Ethical Committee of the Hospital District of Southwest Finland, were: baseline study on 16 February 2010 (ETMK: 2/180/2010), 5-year follow-up on 17 March 2015 (ETMK: 39/1801/2015), and 10-year follow-up on 17 December 2019 (ETMK: 117/1801/2019).
All participants signed informed consent according to the Declaration of Helsinki.
Results
The characteristics of both study cohorts at baseline are listed in . In both the TUVA (64.4% females) and UTUVA (59.6% females) cohorts, there were more female participants than male participants. The mean age of participants at baseline in the TUVA cohort was 70.9, only slightly lower than the mean age of 71.4 in the UTUVA cohort. The progression of systolic and diastolic BP in each cohort is shown in and , respectively.
Figure 1. The progression of systolic blood pressure in the 1920-born TUVA and 1940-born UTUVA cohorts. The numbers within the plot area represent study time point years.
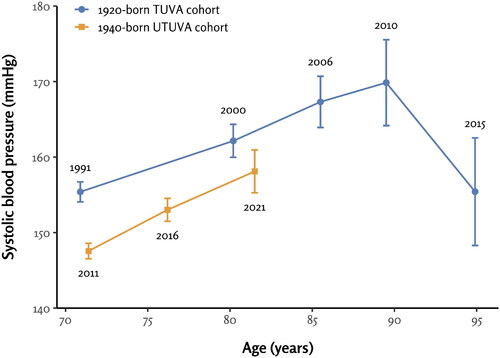
Figure 2. The progression of diastolic blood pressure in the 1920-born TUVA and 1940-born UTUVA cohorts. The numbers within the plot area represent study time point years.
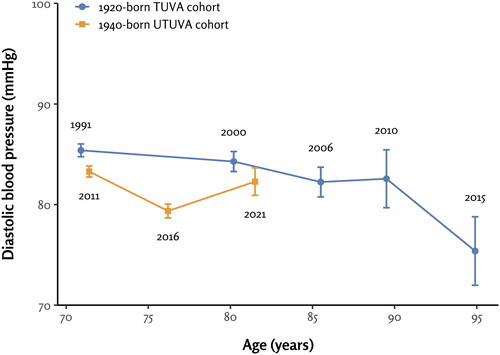
Table 1. Baseline characteristics of the study cohorts.
Comparison of BP values measured at different time points within the 1920-born TUVA cohort
In the TUVA cohort, systolic BP readings measured at the 10-year, 15-year, and 20-year follow-ups were higher than the baseline BP (p ≤ .003 for all). Systolic BPs measured at baseline and the 25-year follow-up did not differ from each other significantly (p = 1.00) ().
Table 2. Differences in mean systolic blood pressure (SBP) within and between the generational cohorts.
Correspondingly, diastolic BP readings measured at the 10-year (p = 0.01), 15-year (p < .001), 20-year (p = .04), and 25-year (p < .001) follow-ups were lower than the baseline diastolic BP ().
Table 3. Differences in mean diastolic blood pressure (DBP) within and between the generational cohorts.
Comparison of BP values measured at different time points within the 1940-born UTUVA cohort
In the UTUVA cohort, systolic BP readings measured at the 5-year and 10-year follow-ups were significantly higher than the baseline systolic BP (p < .001 for both comparisons) ().
Correspondingly, diastolic BP measured at the 5-year follow-up was significantly lower than the baseline diastolic BP (p < .001). The diastolic BPs measured at baseline and the 10-year follow-up did not differ significantly from each other (p = .14) ().
Comparison of BP values between the 1920-born TUVA and 1940-born UTUVA cohorts
The mean systolic BP was 7.8 mmHg higher among the TUVA participants than UTUVA participants at the age of approximately 71 years at baseline (p < .001). By the ages 80–82, the difference in systolic BP trajectories between the cohorts was attenuated to 4.0 mmHg, which remained statistically significant (p = .03) ().
Correspondingly, the mean diastolic BP was 2.1 mmHg higher at baseline (p < .001) and 2.0 mmHg higher at the 10-year follow-up (p = .02) among the TUVA participants compared to the UTUVA participants ().
Comparison of prevalence of hypertension between the 1920-born TUVA and 1940-born UTUVA cohorts
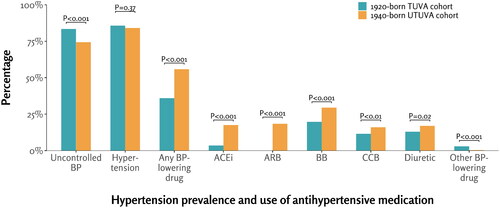
At baseline, 83.4% of participants in TUVA and 74.3% in UTUVA had uncontrolled BP (p < .001). 36.0% of participants in TUVA and 55.9% in UTUVA used antihypertensive medication (p < .001). The prevalence of hypertension was 85.7% in TUVA and 84.1% in UTUVA (p = .37) ().
Figure 3. Prevalence of hypertension and breakdown of antihypertensive medication use in the 1920-born TUVA and 1940-born UTUVA cohorts at the mean age of 71 years. BP: blood pressure; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BB: beta-blocker; CCB: calcium channel blocker. Other BP-lowering drugs are specified in methods section. Uncontrolled BP was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg. Hypertension was defined as either having uncontrolled BP, self-reported hypertension, or confirmed use of antihypertensive medication without indications other than hypertension.
Comparison of antihypertensive medication use between the 1920-born TUVA and 1940-born UTUVA cohorts
The use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers, and diuretics was more frequent in the UTUVA cohort than in the TUVA cohort (p ≤ .02 for all categories). The TUVA cohort showed higher usage of rarely used antihypertensive medications (specified in Methods section) compared to UTUVA (p < .001) ().
Sensitivity analyses
To investigate potential survivor bias, we examined the BP values specifically among individuals who reached the final time-point of the TUVA study at 95 years of age. Nevertheless, this subgroup displayed BP trajectories consistent with the overall TUVA cohort (Supplemental material, Figures S3 and S4).
We also conducted all statistical analyses using the second, sitting BP reading, for the 15-year follow-up of TUVA and all UTUVA time points. The mean sitting systolic BPs in UTUVA and its 5- and 10-year follow-ups were 145.3 ± 15.9 mmHg, 145.8 ± 20.3 mmHg, and 160.5 ± 22.2 mmHg, respectively. In sitting systolic BP comparisons, only the 10-year follow-up differed from the baseline within the UTUVA cohort (p < .001), but it did not differ from the respective 10-year follow-up of TUVA (difference in sitting systolic BP between the cohorts: 1.6 mmHg, p = .56). Within the TUVA cohort, the sitting diastolic BPs measured at the 15-year follow-up (mean: 85.9 ± 13.8 mmHg) were slightly higher than in the main analyses of this article, and they did not differ significantly from the baseline sitting diastolic BP readings (p = .95). Otherwise, these sensitivity analyses did not reveal clinically meaningful differences compared to the main analyses presented in this paper (data not shown).
Discussion
The objective of this population-based longitudinal study was to compare the prevalence, progression, and treatment of hypertension in two Finnish generational cohorts, each comprising older adult participants born 20 years apart. The study covered data from both pre- and post-HYVET eras and spanned the onset of the COVID-19 pandemic. In 2011, the analysis revealed that 71-year-olds were more inclined to use antihypertensives, and their BP was better controlled compared to a similar cohort born two decades earlier, whose BP readings were measured in 1991. This finding suggests a positive trend in hypertension management among the more recent cohort during their early older adult years. However, as the cohorts advanced in age to 80–82 years, this advantage appeared to lessen in the later-born cohort.
Prevalence and progression of hypertension
The definition of hypertension has evolved over the years. In the seventies, it was not uncommon to define hypertension solely based on diastolic BP, with a threshold set, for instance, at 100 mmHg [Citation14]. In later years, the definition broadened to include both systolic BP and diastolic BP. However, as late as in the nineties, there was no generally accepted definition of hypertension in older adults. The definition could vary significantly in older adult population studies, ranging from such readings as >160/95 mmHg to even >195/100 mmHg [Citation15]. Finally, in the 2000s, age-adjusted BP targets were discarded, dismissing the unverified yet persistent clinical belief that it is acceptable for systolic BP to be ‘100 + your age’ [Citation16].
We observed a high prevalence of hypertension at baseline among 71-year-olds, with 85.7% of the 1920-born TUVA participants and 84.1% of the 1940-born UTUVA participants exhibiting hypertension. These findings, while substantial, align with other studies reporting similarly high hypertension rates in their respective populations [Citation2,Citation4,Citation17]. The baseline prevalence of hypertension did not differ significantly between the cohorts, despite a notably lower (7.8 mmHg difference) baseline systolic BP in UTUVA compared to TUVA. This discrepancy is likely attributed to the more prevalent use of antihypertensive agents in the UTUVA cohort at baseline.
We observed a consistent increase in systolic BP at the 10-, 15-, and 20-year follow-ups within the TUVA cohort, reaching a peak at the age of 90 years. Subsequently, however, the systolic BP declined to a nadir at the age of 95 years, resulting in an inverted U-shaped trajectory. A similar hypertension disease progression was reported in a Polish population-based study, where systolic BP values increased until the age range of 80-84, after which they exhibited a decline [Citation3]. The reasons for this decrease in systolic BP in the oldest old could be multifactorial. First, we addressed potential survivor bias by plotting the BP values of the sub-population that reached the final time-point of the TUVA study at 95 years of age. However, this group exhibited a trajectory of systolic BP values similar to that of the overall study population. Second, physiological factors contributing to the observed decline of systolic BP include changes in arterial stiffness, vascular compliance, and the autonomic nervous system in older adults [Citation6,Citation17–22]. Frail individuals may also experience reduced cardiac output, resulting in lower BP values [Citation23]. Additionally, the oldest old often experience multiple comorbidities, which might result in polypharmacy and more pronounced effects of antihypertensive medication [Citation24]. Third, we cannot exclude the statistical play of chance either, given that this remaining subset of 95-year-old participants was substantially smaller (n = 55) than the original study population (n = 1015).
The 1940-born UTUVA participants had a significantly lower baseline mean systolic BP (147.6 mmHg) than the 1920-born TUVA cohort (155.4 mmHg). However, we observed that this initial difference somewhat attenuated at the 10-year follow-up when the participants were approximately 80-82 years old. The 10-year follow-up of UTUVA was conducted in 2021, a year significantly affected by the COVID-19 pandemic, which was notably reflected in the attendance rate. While 438 participants signed the informed consent at that UTUVA follow-up, only 249 ultimately attended the examination. The rise in systolic BP observed at the UTUVA 10-year follow-up might be attributed to the impact of the COVID-19 pandemic in two different ways. First, the decreased attendance rate influenced by COVID-19 could have caused non-response bias. However, it remains unclear why the BP of those attending the examination would have been higher than that of those staying home. Second, enforced house confinement, limited mobility, and reduced access to routine medical appointments during this period have likely hindered the older adult participant’s ability to effectively manage their medication while coping with isolation at home. This hypothesis is further supported by a study by Brindel et al. which asserted that regular contact with a general practitioner and routine screenings are crucial factors for both hypertension awareness and treatment [Citation2]. Possible discontinuation of treatment at older ages could be another explanatory factor, perhaps driven by various reasons such as side effects, comorbidities, or preferences. It is also worth considering factors related to prescribers. Even after the HYVET study, altering prescribing patterns past the age of 80 could be unexpectedly challenging. Moreover, changes in these habits are not actively promoted by pharmaceutical companies either, given that patents for common antihypertensives have expired long ago. Finally, we cannot exclude the possibility that the effectiveness of BP-lowering-medication might diminish around the age of 80 years. The forthcoming follow-ups of the UTUVA cohort will hopefully illuminate the enigma of the rise in systolic BP observed around this age, despite the availability of modern antihypertensive arsenal.
In terms of diastolic BP, the study revealed relatively stable values over the age range of 70-90 years. The 1940-born UTUVA participants exhibited slightly lower diastolic BP readings at baseline and the 10-year follow-up compared to the 1920-born TUVA cohort, with a difference of approximately 2 mmHg. Subsequently, a decline in diastolic BP was observed in the TUVA cohort for which further BP data were available. The most prominent decline occurred between the ages of 90 and 95 years, reaching its nadir at 95 years, a pattern similar to that observed in systolic BP. Consistent with these findings, other studies [Citation3,Citation6] have also observed a similar decline in diastolic BP among older participants. Previous research suggests that this phenomenon can be attributed to the stiffening of large arteries and the progression of atherosclerosis within the older population [Citation6,Citation25].
Profile of antihypertensive medications in each study cohort
In this study, we presented both the overall percentage of BP-lowering medication use and the breakdown of different antihypertensive agents at the baseline of the studied cohorts. In the 1920-born TUVA cohort, only 36% of participants were using antihypertensive medication, while in the 1940-born UTUVA cohort, the figure had risen to 56%. A study by Rossum et al. reported a slightly higher percentage of subjects using antihypertensive medication, at 66% [Citation6]; nevertheless, all these percentages remain relatively low and fall short of the desired levels. Despite the positive trend in antihypertensive medication use, 74% of the UTUVA participants still had uncontrolled BP at baseline, defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg. Several other studies have reported similar results indicating considerable amount of ineffective treatment [Citation1,Citation2,Citation4,Citation6]. This pattern of ineffective treatment is particularly concerning, as it is associated with an increased risk of cardiovascular diseases and mortality among the older population [Citation5,Citation26].
The 1940-born UTUVA cohort exhibited significantly higher medication use than the TUVA cohort across all five major categories of antihypertensive agents: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, diuretics, and beta blockers. At the baseline of the 1920-born TUVA cohort in 1991, beta blockers were the most prescribed medication, followed by diuretics and calcium channel blockers. A small percentage of participants used angiotensin-converting enzyme inhibitors, and none could use angiotensin receptor blockers, which became available only 4 years later with the approval of losartan by the U.S. Food and Drug Administration (FDA) in 1995 [Citation27]. Similarly, in the UTUVA cohort, beta blockers were prescribed most frequently, with angiotensin receptor blockers already constituting the second most used medication. Since then, the use of beta blockers as the initial medication to combat hypertension has been questioned due to the lack of efficacy in preventing deaths, strokes, and heart attacks compared to other classes of medicines [Citation28].
Limitations
First, the single measurement of BP coupled with the potential presence of white-coat hypertension in some participants could have led to an overestimation of hypertension in our study. In addition, varying BP measurement methods employed at different time points may present challenges in making direct comparisons. However, this variability is often unavoidable in longitudinal studies spanning several decades. Second, the UTUVA cohort’s 10-year follow-up in 2021, marked by the impact of the COVID-19 pandemic, saw a notable decline in attendance. Only 249 participants attended the physical examination at that time point. Third, there is a possibility that individuals who did not participate in the study had different BP levels compared to the participants, potentially introducing selection bias into the formation of the study cohorts. Fourth, our findings are based on two birth cohorts from a specific geographical area in Finland, possibly limiting the generalisability of the results. However, while acknowledging potential differences in genetic and socioeconomic factors, we believe that our results provide valuable insights into the management of hypertension in older adults that can be applicable across diverse populations.
Conclusion
In conclusion, our study highlights significant undertreatment or inadequate management of hypertension in the older population. In 2011, 71-year-olds demonstrated improved antihypertensive use and better-controlled BP compared to a cohort born two decades earlier, suggesting an initial positive trend. However, by ages 81–82, this advantage lessened in the later-born cohort. The underlying causes, influenced by the COVID-19 pandemic or indicative of a lasting trend, require further investigation. The findings of our study emphasise the urgent need for heightened efforts to improve hypertension treatment among older adults.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The analysis codes for this study have been deposited in the Zenodo repository with the following DOI: 10.5281/zenodo.11495302.
Additional information
Funding
References
- Perez-Fernandez R, Mariño AF, Cadarso-Suarez C, et al. Prevalence, awareness, treatment and control of hypertension in Galicia (Spain) and association with related diseases. J Hum Hypertens. 2007;21(5):366–373. doi: 10.1038/sj.jhh.1002158.
- Brindel P, Hanon O, Dartigues JF, et al. Prevalence, awareness, treatment, and control of hypertension in the elderly: the Three City study. J Hypertens. 2006;24(1):51–58. doi: 10.1097/01.hjh.0000198028.84353.86.
- Zdrojewski T, Wizner B, Więcek A, et al. Prevalence, awareness, and control of hypertension in elderly and very elderly in Poland: results of a cross-sectional representative survey. J Hypertens. 2016;34(3):532–538; discussion 538. doi: 10.1097/HJH.0000000000000823.
- Muli S, Meisinger C, Heier M, et al. Prevalence, awareness, treatment, and control of hypertension in older people: results from the population-based KORA-age 1 study. BMC Public Health. 2020;20(1):1049. doi: 10.1186/s12889-020-09165-8.
- Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369.
- van Rossum CT, van de Mheen H, Witteman JC, et al. Prevalence, treatment, and control of hypertension by sociodemographic factors among the Dutch elderly. Hypertension. 2000;35(3):814–821. doi: 10.1161/01.hyp.35.3.814.
- Upmeier E, Lavonius S, Lehtonen A, et al. Serum lipids and their association with mortality in the elderly: a prospective cohort study. Aging Clin Exp Res. 2009;21(6):424–430. doi: 10.1007/BF03327441.
- Upmeier E, Vire J, Korhonen MJ, et al. Cardiovascular risk profile and use of statins at the age of 70 years: a comparison of two Finnish birth cohorts born 20 years apart. Age Ageing. 2016;45(1):84–90. doi: 10.1093/ageing/afv187.
- Upmeier E, Korhonen MJ, Rikala M, et al. Older statin initiators in Finland - cardiovascular risk profiles and persistence of use. Cardiovasc Drugs Ther. 2014;28(3):263–272. doi: 10.1007/s10557-014-6517-x.
- Privšek E, Hellgren M, Råstam L, et al. Epidemiological and clinical implications of blood pressure measured in seated versus supine position. Medicine (Baltimore). 2018;97(31):e11603. doi: 10.1097/MD.0000000000011603.
- Lu LC, Wei TM, Li S, et al. Differences in blood pressure readings between supine and sitting positions in hypertensive patients. Acta Cardiol. 2008;63(6):707–711.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339.
- Sakhuja S, Colvin CL, Akinyelure OP, et al. Reasons for uncontrolled blood pressure among US adults: data from the US National Health and Nutrition Examination Survey. Hypertens Dallas Tex 1979. 2021;78(5):1567–1576.
- Sourander LB, Ruikka I, Kasanen A. A health survey on the aged with a 5-year follow-up. Acta Sociomed Scand Suppl. 1970;3:1–41.
- Österlind PO. Medical and social conditions in the elderly: gender and age differences - the Umeå longitudinal study [PhD dissertation]. Umeå: University of Umeå; 1993. Accessed December 17, 2023. Available from: https://www.diva-portal.org/smash/get/diva2:808716/FULLTEXT01.pdf.
- Izzo JL, Levy D, Black HR. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35(5):1021–1024. doi: 10.1161/01.hyp.35.5.1021.
- Oliveira IM, Duarte YADO, Zanetta DMT. Prevalence of systemic arterial hypertension diagnosed, undiagnosed, and uncontrolled in elderly population: SABE study. J Aging Res. 2019;2019:3671869–3671811. doi: 10.1155/2019/3671869.
- Cohn JN, Finkelstein SM. Abnormalities of vascular compliance in hypertension, aging and heart failure. J Hypertens Suppl Off J Int Soc Hypertens. 1992; 10(6):S61–S64.
- Lim MA, Townsend RR. Arterial compliance in the elderly: its effect on blood pressure measurement and cardiovascular outcomes. Clin Geriatr Med. 2009;25(2):191–205. doi: 10.1016/j.cger.2009.01.001.
- Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10 Suppl 1(s1):S127–S136. doi: 10.1111/j.1447-0594.2010.00592.x.
- Marigold JRG, Arias M, Vassallo M, et al. Autonomic dysfunction in older people. Rev Clin Gerontol. 2011;21(1):28–44. doi: 10.1017/S0959259810000286.
- Bulpitt CJ, Cameron JD, Rajkumar C, et al. The effect of age on vascular compliance in man: which are the appropriate measures? J Hum Hypertens. 1999;13(11):753–758. doi: 10.1038/sj.jhh.1000879.
- Dai X, Hummel SL, Salazar JB, et al. Cardiovascular physiology in the older adults. J Geriatr Cardiol. 2015;12(3):196–201. doi: 10.11909/j.issn.1671-5411.2015.03.015.
- Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: a comprehensive review. J Clin Hypertens. 2016;18(1):10–18. doi: 10.1111/jch.12624.
- Witteman JC, Grobbee DE, Valkenburg HA, et al. J-shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet Lond Engl. 1994;343(8896):504–507. doi: 10.1016/S0140-6736(94)91459-1.
- Bulpitt CJ, Beckett NS, Peters R, et al. Blood pressure control in the Hypertension in the Very Elderly Trial (HYVET). J Hum Hypertens. 2012;26(3):157–163. doi: 10.1038/jhh.2011.10.
- Ripley E, Hirsch A. Fifteen years of losartan: what have we learned about losartan that can benefit chronic kidney disease patients? Int J Nephrol Renov Dis. 2010;3:93–98.
- Wiysonge CS, Bradley HA, Volmink J, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1(1):CD002003. doi: 10.1002/14651858.CD002003.pub5.
