Abstract
Objective Real-life management of patients with hypertension and chronic kidney disease (CKD) among European Society of Hypertension Excellence Centres (ESH-ECs) is unclear : we aimed to investigate it. Methods A survey was conducted in 2023. The questionnaire contained 64 questions asking ESH-ECs representatives to estimate how patients with CKD are managed. Results Overall, 88 ESH-ECS representatives from 27 countries participated. According to the responders, renin-angiotensin system (RAS) blockers, calcium-channel blockers and thiazides were often added when these medications were lacking in CKD patients, but physicians were more prone to initiate RAS blockers (90% [interquartile range: 70–95%]) than MRA (20% [10–30%]), SGLT2i (30% [20–50%]) or (GLP1-RA (10% [5–15%]). Despite treatment optimisation, 30% of responders indicated that hypertension remained uncontrolled (30% (15–40%) vs 18% [10%–25%]) in CKD and CKD patients, respectively). Hyperkalemia was the most frequent barrier to initiate RAS blockers, and dosage reduction was considered in 45% of responders when kalaemia was 5.5–5.9 mmol/L. Conclusions RAS blockers are initiated in most ESH-ECS in CKD patients, but MRA and SGLT2i initiations are less frequent. Hyperkalemia was the main barrier for initiation or adequate dosing of RAS blockade, and RAS blockers’ dosage reduction was the usual management.
PLAIN LANGUAGE SUMMARY
What is the context? Hypertension is a strong independent risk factor for development of chronic kidney disease (CKD) and progression of CKD to ESKD. Improved adherence to the guidelines in the treatment of CKD is believed to provide further reduction of cardiorenal events. European Society of Hypertension Excellence Centres (ESH-ECs) have been developed in Europe to provide excellency regarding management of patients with hypertension and implement guidelines. Numerous deficits regarding general practitioner CKD screening, use of nephroprotective drugs and referral to nephrologists prior to referral to ESH-ECs have been reported. In contrast, real-life management of these patients among ESH-ECs is unknown. Before implementation of strategies to improve guideline adherence in Europe, we aimed to investigate how patients with CKD are managed among the ESH-ECs.
What is the study about? In this study, a survey was conducted in 2023 by the ESH to assess management of CKD patients referred to ESH-ECs. The questionnaire contained 64 questions asking ESH-ECs representatives to estimate how patients with CKD are managed among their centres.
What are the results? RAAS blockers are initiated in 90% of ESH-ECs in CKD patients, but the initiation of MRA and SGLT2i is less frequently done. Hyperkalemia is the main barrier for initiation or adequate dosing of RAAS blockade, and its most reported management was RAAS blockers dosage reduction. These findings will be crucial to implement strategies in order to improve management of patients with CKD and guideline adherence among ESH-ECs.
Introduction
Hypertension is a strong independent risk factor for development of chronic kidney disease (CKD) and progression of CKD to end-stage kidney disease (ESKD) [Citation1]. According to guidelines, the diagnosis of CKD in hypertensive patients is based on evaluation of kidney function (estimated glomerular filtration rate (eGFR)) and the use of the urinary albumin/creatinine ratio (UACR) [Citation1,Citation2]. These clinical practice guidelines have been widely disseminated for many years and recently re-emphasised [Citation1,Citation3,Citation4]. We recently conducted a survey among European Society of Hypertension excellence centres (ESH-ECs) among 27 countries. This survey indicated numerous deficits regarding CKD screening, use of nephroprotective drugs and it appeared that referral to nephrologists from general practitioners before referral to ESH-ECs was infrequent. However, results varied widely across countries. These deficits were mostly related to low rates of UACR screening and low use of renin-angiotensin system blockers (RASb), glucose co-transporter 2 inhibitors (SGLT2i) and mineralocorticoid receptor antagonists (MRA) [Citation5]. However, how these CKD patients are managed in ESH-ECS has not been reported so far and remains unknown. Wide dissemination of current guidelines is expected in ESH-ECs but whether these guidelines are implemented is also unknown. Physicians in these ESH-ECs are recognised experts in hypertension exploration and management. However, real-life management of patients with hypertension and CKD in these ESH-ECs is unclear. Several issues in patients with hypertension and CKD, including use of antihypertensive medications (especially RASb) when they are lacking, initiation and barriers to use medications such as RASb, MRA, SGLT2i and Glucagon-like peptide-1 receptor agonists (GLP1-RA) [Citation3], the prevalence of uncontrolled hypertension [Citation6], and management of non-severe hyperkalaemia [Citation7]. Before implementation of strategies to improve guideline adherence in Europe, it is crucial to investigate how patients with CKD and hypertension are managed among the ESH-ECs.
In the present study, we assessed how CKD patients are managed among the ESH-ECs and whether ESH-ECs management of these patients markedly differed across centres.
Methods
Design of the survey and participants
A survey was conducted in 2023 among the ESH-ECS network. Briefly, the questionnaire was drafted by the chair (JMH) and vice-chair (LV) of the Hypertension-Kidney Working Group (HT-Kidney-WG) in February 2023, thereafter validated by 3 other members of the ESH (AP, RK, PS), made accessible online and sent by emails between March and June 2023 to all members of the ESH-ECS network. Data-management and analyses were conducted between July and September 2023.
Contents of the survey
The content of the questionnaire has been published (Halimi et al. J Hypertens). Briefly, the questionnaire included ESH-ECS and patient characteristics, including renal diagnosis, use of RASb, SGLT2i and MRA in CKD patients prior to ESH-ECS referral and the specific management of CKD patients in the ESH-ECS (Supplemental Table 1).
Primary CKD was defined as CKD due to primary renal diseases such as glomerulonephritis, polycystic kidney disease, lupus erythematous (i.e. lupus nephritis) not related to cardiovascular and metabolic disorders. Secondary CKD was defined as CKD associated with hypertension, vascular disease or diabetes mellitus.
Variations among 27 countries from Europe and in the Middle East regarding management of CKD in the ESH-ECS
In this analysis, we assessed whether and to what extent to which RASb, SGLT2i, or MRA were added in CKD patients when these drugs were lacking, and whether a significant variability in management of CKD patients was present in ESH-ECS in Europe.
Statistical analyses
Descriptive data are presented as median (IQR, Interquartile Range) for quantitative variables and counts and percentages for categorical variables. Comparisons of parameters among centres were performed using Wilcoxon test, chi2 test or Fisher’s exact test as appropriate. Statistical analysis was performed using SAS (SAS 7.1 SAS Institute Inc., SAS Campus Drive, Cary, NC, USA).
Results
Survey responders among ESH-ECS across European and Middle East countries
Overall, 88 responses were provided from 27 countries (24 from Europe and 3 from the Middle East) (Supplemental Table 2). The survey was fully completed in 66/88 of cases (75.0%). Most of the patients seen in ESH-ECS belonging to the <50 (median: 25% [IQR: 20–30%]) and 50–69-year (40% [30–50%]) age groups. Type 2 diabetes mellitus was present in 33% (25–50%) of cases. Known cardiovascular disease was present in 25% (15–35%) (heart failure: 20% (10–30%)) of patients. Secondary kidney diseases (30% [20–45%]) were more frequent than primary kidney diseases (10% [5–15%])
Management of CKD patients in ESH-ECS
Overall, according to the responses to this questionnaire, RASb, calcium-channel blockers (CCB) and thiazides were often added when these medications were missing (), and this was especially true for CKD patients (). Physicians in these ESH-ECs were more prone to initiate ACEI or ARB (90% [70–95%]) than MRA (20% [10–30%]), SGLT2i (30% [20–50%]) or (GLP1-RA (10% [5–15%]) when these medications were missing ().
Table 1. Initial treatments among all patients and patients with CKD in ESH-ECS.
Table 2. Treatment of CKD patients and proportion of patient with uncontrolled hypertension.
After optimisation of treatments, 30% of responders indicated that uncontrolled hypertension was still present in 30% (15–40%) patients with CKD (vs 18% [10%–25%] in all patients) ().
Interestingly, there was no significant differences in the management of CKD patients among ESH-ECS responders ( and Citation2).
Barriers to RASb optimisation in CKD patients
Physicians were asked to classify potential barriers to RASb use from 1 (the most important or most frequent one) to 4 (the least frequent or least important one) among cough, hyperkalaemia, acute kidney injury (AKI) and low eGFR (usually < 30 ml/min/1.73m2). Hyperkalemia (32.3%) was usually considered the most frequent one, followed by cough (25%), AKI (15.4%) and low eGFR (12.3%) (, ).
Figure 1. Barriers to RAS blockers’ optimisation according to responders (%) (among hyperkalemia, cough, AKI and low GFR).
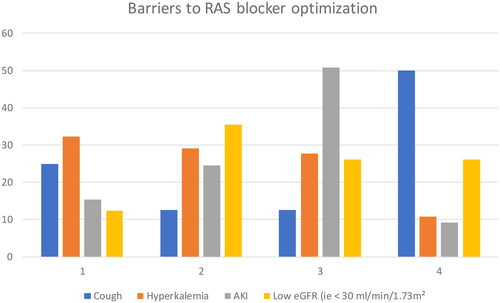
Table 3. barriers to RAS blockers in CKD patients.
Management of non-severe hyperkalaemia
Among patients treated with RASb, 45% of responders indicated that dosage reduction would be considered when kalaemia ranged between 5.5 to 5.9 mmol/L; 30% indicated that they would rather consider the addition of potassium binders, and 10% would not modify treatments. Of note, there were no significant differences among ESH-ECs responders ().
Table 4. Management of non-severe hyperkalemia.
Potential differences between ESH-EC with versus centres without a nephrologist
We did not find any significant difference regarding any of the studied parameters (initiation of medications: SGLT2i (p = .4176), MRA (p = .5491), GLP1-RA (p = .1152); barriers to the use of RAS blockers: cough (p = .1251), eGFR deemed too low (p = .8651), acute kidney injury (p = .5830), hyperkalaemia (p = .0649); management of mild to moderate hyperkalaemia: lowering dose of RASb (p = .6497), use of lowering potassium drugs (p = .4081), no change in the dose of RASb (p = .1881); uncontrolled hypertension among CKD patients (p = .7933)).
Discussion
The results of the present study indicate that ACEI/ARB, MRA and SGLT2i were initiated upon referral to ESH-ECS in 90%, 20% and 30%, respectively, according to the responders of this survey. Despite optimisation of treatments, uncontrolled hypertension was still present in 30% in CKD patients compared to 18% in other hypertensive patients without CKD. Hyperkalemia was the main identified barrier to RAAS blockade. For half of responders, management of moderate hyperkalaemia was reduction of RAAS dosage rather than addition of potassium binders or no treatment modification. No significant difference was noted regarding management of CKD patients among the ESH-ECS.
The results of the present study indicate that the initiation of ACEI/ARB was performed in 90% of patients with CKD, according to responders of this survey. The initiation of RAAS blockers by 90% of responders is justified by the results of several clinical trials among diabetic and nondiabetic patients [Citation8–10] and recent European guidelines [Citation1]. RAAS blockers have been shown to reduce proteinuria, the rate of eGFR decline, and the risk of renal failure, especially in protein uric patients [Citation1]. These results are not surprising and correspond to a standard of care for most patients with CKD. It is reassuring that guidelines regarding initiation of ACEI/ARB are respected.
In marked contrast, the initiation of MRA was performed in only 30% of patients with CKD with eGFR > 30 ml/min. According to these recent guidelines, the use of MRA was advocated in patients with CKD, when blood pressure (BP) was uncontrolled despite the use of 3 anti-hypertensive medications (ACEI or ARB, CCB and diuretics) at least when eGFR was > 30 ml/min/1.73m2 [Citation1]. Only 20% of responders indicated that they consider MRA use in patients with CKD. It is presently unclear whether MRA would be a steroidal MRA such as spironolactone or one such as the non-steroidal MRA finerenone. A recent meta-analysis of randomised clinical trials showed that spironolactone was superior in lowering BP than other antihypertensive medications, although the BP difference was modest and very few studies were available for analysis [Citation11]. Finerenone reduces BP and has significant beneficial effect vs placebo on the risk of end-stage kidney disease and heart failure among patients with CKD and type 2 diabetes mellitus [Citation12–15]. One of the main barriers for the use of MRA is the risk or at least the fear of hyperkalaemia. Real-world data show that hyperkalaemia is associated with increased mortality [Citation16]. In our study, the fear of hyperkalaemia was also the main barrier for RAAS blockers. Mild to moderate hyperkalaemia (<6.0 mmol/L) can be managed using potassium binders, lowering RAAS blockers dosage, adding thiazide or loop diuretics in patients with uncontrolled hypertension, or one can opt to monitor closely potassium levels. In our study, lowering RAAS blockers dosage was more frequently chosen than the other options [Citation17,Citation18]. In the literature, it was reported that the most frequent reason for dose reduction or discontinuation of RAAS blockers in CKD patients was hyperkalaemia; however, it was also observed that dose reduction or discontinuation of RAAS blockers could result in increased cardiovascular morbidity [Citation19–23]. Our findings that the initiation of MRA was infrequently performed in CKD patients and the discontinuation or dose reduction of RAAS blockers was usually considered, even among ESH-ECs, are in marked contrast with a recent consensus statement [Citation24] and the European guidelines [Citation1]. In the ESH guidelines, it was stated that « a potassium binder can be used to maintain normal or near normal serum potassium levels (<5.5 mmol/L) in order to allow optimal treatment with a RAS-blocker or a MRA to continue » [Citation1]. A potential reason could be the existence of a certain time lag before new recommendations are well applied in routine clinical practice.
Despite optimisation of treatments, uncontrolled hypertension was still present in 30% in CKD patients. In our study, uncontrolled hypertension was confirmed by ambulatory or home BP measurements. Similar numbers were observed in other studies [Citation6,Citation25]. The consequences have been widely identified, including heart failure, progression of renal disease, hypertensive encephalopathy and malignant hypertension [Citation26–28]. Moreover and in accordance with the literature, uncontrolled hypertension was more frequent in CKD patients than in patients without CKD in the present study [Citation6,Citation29].
Surprisingly, only 30% of the responders indicated that they add SGLT2i in patients with CKD. In marked contrast, recent guidelines recommend adding SGLT2i in diabetic and non-diabetic patients with CKD [Citation1,Citation30–33]. SGLT2i demonstrated significant beneficial effects on cardiovascular morbidity and mortality in addition to large reductions in kidney major events [Citation34–36]. These guidelines are recent but have been largely disseminated [Citation37,Citation38]. Barriers to the prescription of SGLT2i have not been assessed in the present study. However, the low use of SGLT2i is certainly influenced by restrictive health care and reimbursement policies in some countries, that may result in low prescription rates. It is probably true in other countries: it was noted that ‘there remain serious challenges to implementation, particularly in the United States where inequities in insurance coverage and high costs limit their use, particularly in vulnerable populations, ultimately widening health care disparities’ [Citation39]. In addition, the low reported rate of single RAAS blockade use might have influenced SGLT2i prescription by the ESH-ECS physicians, because RAAS blockade is considered to represent the first line of cardiorenal therapy. It is also important to consider that SGLT2i as well as GLP1-RA are relatively new treatments for nephrologists and hypertension specialists. Further studies to examine the trends in prescription rates in the future are warranted for the aforementioned agents.
Overall, no significant differences were noted in the management of CKD patients among the ESH-ECS but it does mean that difference do not exist, as the number of centres per country was small and heterogenous. In contrast, using the same survey, we observed a high degree of heterogeneity regarding screening and management of hypertensive patients with CKD in the countries of Europe and the Middle East before being referred to the ESH-ECs in our previous study (Halimi JM, in press, J Hypertension). Based on the present results and previous one in ESH-ECs (Halimi JM, in press, J Hypertension), a clearer view of the unmet needs regarding management of hypertensive patients before referral to ESH-ECS and within the ESH-ECS (): these unmet needs are clearly different: at the GP level, the 3 main unmet needs are: yearly screening (UACR and serum creatinine), increased use or maintenance of ACEI/ARB and introduction of SGLT2i, and reintroduction of these medications if they have been stopped after an acute event in patients with CKD; at the ESH-ECS levels, implementation of BP goals according to current guidelines, addition of SGLT2i and MRA according to local regulations and maintain ACEI/ARB and MRA in patients with mild to moderate hyperkalaemia ().
Table 5. Major unmet needs at the GP and ESH-ECS levels to improve management of patients with CKD.
The strength of this survey derives from the large number of ESH-ECS and countries involved in this survey. This is the first survey investigating the management of CKD patients referred to Hypertension Excellence centres in Europe.
This study has limitations. Many Excellent centres across Europe responded to this survey, and actually many more than most surveys in Excellence centres [Citation40,Citation41]; however, not all of them responded, and the reasons of non-response are unclear. Within the limits of this survey, it was not possible to obtain real data from patients followed in the same centres. The diagnosis of comorbidities was not rigorously assessed in this survey: it is only assumed that these conditions are defined using the best available guidelines as usually 80% of the responders in such surveys have a professional experience of 10 years or more. Participants willingly participated and therefore a selection bias is possible. It also explains the various number of centres within and across countries. In the present survey, the BP measurement methods and the proportion of ESH-ECs using ambulatory BP monitoring and home blood pressure were not explored.
In conclusion, the results of our survey indicate that RAAS blockers are initiated in 90% of centres in CKD patients but the initiation of other nephroprotective agents such as MRA and SGLT2i is less frequently done, according to responders. Despite optimisation of treatments, uncontrolled hypertension was still present in 30% in CKD patients. The most frequent barrier to RAAS blockade was hyperkalaemia, and for half of responders, management of moderate hyperkalaemia was RAAS blockers dosage reduction rather than addition of potassium binders or no treatment modification, in marked contrast to current guidelines. No significant difference was noted regarding management of CKD patients among the ESH-ECs.
Author contributions
Prof Jean-Michel Halimi had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Prof Reinhold Kreutz, Prof Jean-Michel Halimi, Prof Liffert Vogt, Alexandre Persu. Acquisition, analysis, or interpretation of data: Prof Reinhold Kreutz, Prof Jean-Michel Halimi, Prof Liffert Vogt, Prof Pantelis Sarafidis, Alexandre Persu.
Drafting of the manuscript: Prof Jean-Michel Halimi. Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Prof Jean-Michel Halimi.
Disclosure statement
The authors report no conflicts of interest relating directly to the contents of this article.
Jean-Michel Halimi reported fees for advisory boards or lectures from Alexion, Astra Zeneca, Bayer, Boehringer Ingelheim France, Servier, Vifor Fresenius Pharma.
Daniel Gordin reported lecture or advisory board honoraria from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, GE Healthcare, Fresenius, Novo Nordisk. He was supported by Liv och Hälsa Society, Medical Society of Finland (Finska Läkaresällskapet), Sigrid Juselius Foundation, Helsinki University Hospital, University of Helsinki, Wilhelm and Else Stockmann Foundation, Minerva Foundation Institute for Medical Research Clinician Scientist, and Academy of Finland.
Markus van der Giet reported lecture fee from Bayer, Astra Zeneca, Medtronic, Omron, Vifor, Berlin-Chemie, Novartis, Apontis, Streamed-Up, Akademie der Hochdruckliga; Advisory boards for Bayer, GSK, Apontis.
George Stergiou reported lecture and consulting fees from Astra Zeneca, Menarini, Sanofi-Aventis, Servier, Viatris.
Ilkka Kantola received lecture fees from Bayer and Boehringer-Ingelheim
Marit Solbu have received lecture honoraria from AstraZeneca and Boehringer-Ingelheim.
Additional information
Funding
References
- Mancia G, Kreutz R, Brunstrom M, et al. 2023 ESH guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874–2071.
- Kidney Disease: Improving Global Outcomes Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87.
- Gourdy P, Darmon P, Dievart F, et al. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc Diabetol. 2023;22(1):79. doi: 10.1186/s12933-023-01798-4.
- Kidney Disease: Improving Global Outcomes CKDWG. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4S):S117–S314.
- Halimi J-M, Sarafidis P, Azizi M, et al. Screening and management of hypertensive patients with chronic kidney disease referred to hypertension excellence centres among 27 countries. A pilot survey based on questionnaire. J Hypertens. 2024. doi: 10.1097/HJH.0000000000003756.
- Rossignol P, Massy ZA, Azizi M, et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet. 2015;386(10003):1588–1598. doi: 10.1016/S0140-6736(15)00418-3.
- Wagner S, Metzger M, Flamant M, et al. Association of plasma potassium with mortality and end-stage kidney disease in patients with chronic kidney disease under nephrologist care - the NephroTest study. BMC Nephrol. 2017;18(1):295. doi: 10.1186/s12882-017-0710-7.
- Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163(13):1555–1565. doi: 10.1001/archinte.163.13.1555.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16(10):3027–3037. doi: 10.1681/ASN.2004110919.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet. 1997;349(9069):1857–1863.
- Chen C, Zhu XY, Li D, et al. Clinical efficacy and safety of spironolactone in patients with resistant hypertension: a systematic review and meta-analysis. Medicine. 2020;99(34):e21694. doi: 10.1097/MD.0000000000021694.
- Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845.
- Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–2263. doi: 10.1056/NEJMoa2110956.
- Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–484. doi: 10.1093/eurheartj/ehab777.
- Sawami K, Tanaka A, Node K. Recent understandings about hypertension management in type 2 diabetes: what are the roles of SGLT2 inhibitor, GLP-1 receptor agonist, and finerenone? Hypertens Res. 2023;46(8):1892–1899. doi: 10.1038/s41440-023-01324-9.
- Sarafidis PA, Blacklock R, Wood E, et al. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin J Am Soc Nephrol. 2012;7(8):1234–1241. doi: 10.2215/CJN.01150112.
- Morales J, Palmer BF. Non-steroidal mineralocorticoid antagonists and hyperkalemia monitoring in chronic kidney disease patients associated with type II diabetes: a narrative review. Postgrad Med. 2024;136(2):111–119. doi: 10.1080/00325481.2024.2316572.
- Morales E, Cravedi P, Manrique J. Management of chronic hyperkalemia in patients with chronic kidney disease: an old problem with news options. Front Med. 2021;8:653634. doi: 10.3389/fmed.2021.653634.
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–1162. doi: 10.1001/archinternmed.2009.132.
- Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–S20.
- Yildirim T, Arici M, Piskinpasa S, et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3-5 in clinical practice: a safety concern? Ren Fail. 2012;34(9):1095–1099. doi: 10.3109/0886022X.2012.717478.
- Fu EL, Evans M, Clase CM, et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol. 2021;32(2):424–435. doi: 10.1681/ASN.2020050682.
- Walther CP, Winkelmayer WC, Richardson PA, et al. Renin-angiotensin system blocker discontinuation and adverse outcomes in chronic kidney disease. Nephrol Dial Transplant. 2021;36(10):1893–1899. doi: 10.1093/ndt/gfaa300.
- Hannedouche T, Rossignol P, Darmon P, et al. Early diagnosis of chronic kidney disease in patients with diabetes in France: multidisciplinary expert opinion, prevention value and practical recommendations. Postgrad Med. 2023;135(7):633–645. doi: 10.1080/00325481.2023.2256208.
- An J, Kurella Tamura M, Odden MC, et al. Prevalence of apparent treatment-resistant hypertension in chronic kidney disease in two large US health care systems. Clin J Am Soc Nephrol. 2022;17(10):1457–1466. doi: 10.2215/CJN.04110422.
- Halimi J-M, de Fréminville J-B, Gatault P, et al. Characteristics and prognosis of patients with hypertensive encephalopathy: a french nationwide cohort study. Hypertension. 2023;80(8):1716–1727. doi: 10.1161/HYPERTENSIONAHA.123.21226.
- Halimi J-M, de Fréminville J-B, Gatault P, et al. Long-term impact of cardiorenal syndromes on major outcomes based on their chronology: a comprehensive French nationwide cohort study. Nephrol Dial Transplant. 2022;37(12):2386–2397. doi: 10.1093/ndt/gfac153.
- Boulestreau R, Lorthioir A, Persu A, et al. Revisiting malignant hypertension: rationale and design of the ‘HAMA cohort’, on behalf of the ESH working group ‘hypertension and the kidney’. J Hypertens. 2023;41(3):453–458. doi: 10.1097/HJH.0000000000003357.
- Sarafidis PA, Georgianos PI, Zebekakis PE. Comparative epidemiology of resistant hypertension in chronic kidney disease and the general hypertensive population. Semin Nephrol. 2014;34(5):483–491. doi: 10.1016/j.semnephrol.2014.08.001.
- Halimi JM. [SGLT2 inhibitors: a new era for our patients]. Nephrol Ther. 2021;17(3):143–148. doi: 10.1016/j.nephro.2020.12.006.
- Sarafidis P, Papadopoulos CE, Kamperidis V, et al. Cardiovascular protection with sodium-glucose cotransporter-2 inhibitors and mineralocorticoid receptor antagonists in chronic kidney disease: a milestone achieved. Hypertension. 2021;77(5):1442–1455. doi: 10.1161/HYPERTENSIONAHA.121.17005.
- Sarafidis P, Ortiz A, Ferro CJ, et al. Sodium–glucose co-transporter-2 inhibitors for patients with diabetic and nondiabetic chronic kidney disease: a new era has already begun. J Hypertens. 2021;39(6):1090–1097. doi: 10.1097/HJH.0000000000002776.
- Sarafidis P, Schmieder R, Burnier M, et al. A European Renal Association (ERA) synopsis for nephrology practice of the 2023 European Society of Hypertension (ESH) guidelines for the management of arterial hypertension. Nephrol Dial Transplant. 2024;39(6):929–943.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720.
- Neal B, Perkovic V, Mahaffey KW, et al. Optimizing the analysis strategy for the CANVAS program: A prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017;19(7):926–935. doi: 10.1111/dom.12924.
- Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233.
- Rossing P, Caramori ML, Chan JCN, et al. Executive summary of the KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102(5):990–999. doi: 10.1016/j.kint.2022.06.013.
- Navaneethan SD, Zoungas S, Caramori ML, et al. Diabetes management in chronic kidney disease: synopsis of the KDIGO 2022 clinical practice guideline update. Ann Intern Med. 2023;176(3):381–387. doi: 10.7326/M22-2904.
- Mottl AK, Nicholas SB. KDOQI commentary on the KDIGO 2022 update to the clinical practice guideline for diabetes management in CKD. Am J Kidney Dis. 2024;83(3):277–287. doi: 10.1053/j.ajkd.2023.09.003.
- Weber T, Amar J, de Backer T, et al. Covid-19 associated reduction in hypertension-related diagnostic and therapeutic procedures in Excellence Centers of the European Society of Hypertension. Blood Press. 2022;31(1):71–79. doi: 10.1080/08037051.2022.2060182.
- Burnier M, Prejbisz A, Weber T, et al. Hypertension healthcare professional beliefs and behaviour regarding patient medication adherence: a survey conducted among European Society of Hypertension Centres of Excellence. Blood Press. 2021;30(5):282–290. doi: 10.1080/08037051.2021.1963209.
