Abstract
Aim
To explore the association between serum folate concentration and the prevalence of elderly diastolic hypertension. This study aims to identify potential relationships that could inform further research into the mechanisms underlying hypertension management.
Methods
Data from six NHANES cycles (2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018) were analysed for individuals aged over 60. Weighted logistic regression estimated odds ratios (ORs) and 95% confidence intervals (CIs). Subgroup and restricted cubic spline (RCS) regression explored the serum folate concentration and elderly diastolic hypertension relationship.
Results
This study included 9,419 participants (4,734 females and 4,685 males) with a mean age of 70.0 ± 7.0 years. Among them, 360 were diagnosed with diastolic hypertension. In the fully adjusted model, there was a negative correlation between serum folate concentration and the prevalence of diastolic hypertension (OR 0.65; 95% CI: 0.52–0.82). When serum folate concentration levels were divided into quartiles (in μg/dL), the ORs for diastolic hypertension corresponding to Q2 (1.29–1.98), Q3 (1.99–3.08), and Q4 (3.09–5.56) levels compared to Q1 (0.18–1.28) were 1.41 (95% CI: 0.60–3.33), 0.48(95% CI: 0.20–1.16), and 0.35 (95% CI: 0.16–0.74), respectively, with a P for trend <.05. Restricted cubic spline plots showed a negative correlation between serum folate concentration and the prevalence of diastolic hypertension (non-linearity: p = .495). Subgroup analysis indicated that the negative correlation between serum folate concentration and the prevalence of diastolic hypertension was more significant in female participants (interaction p = .009).
Conclusion
Higher serum folate concentration is associated with a lower prevalence of diastolic hypertension in the elderly.
PLAIN LANGUAGE SUMMARY
What is the context?
Diastolic hypertension, characterised by high blood pressure during the relaxation phase of the heartbeat.
It significantly elevates the risk of cardiovascular diseases such as heart attacks and strokes.
This study examines how serum folate levels relate to diastolic hypertension in the elderly, aiming to uncover correlations that inform future management strategies.
What is new?
This study investigated the relationship between serum folate concentration and the prevalence of diastolic hypertension in individuals aged over 60.
Analysing data from multiple cycles of the National Health and Nutrition Examination Survey (NHANES), researchers found a noteworthy correlation between higher serum folate levels and a lower prevalence of diastolic hypertension.
This association remained significant even after adjusting for various factors such as age, sex, and other health variables.
What is the impact?
The findings underscore the potential significance of folate intake in lowering the prevalence of diastolic hypertension among the elderly.
It suggests avenues for further research into nutritional interventions targeting hypertension in this vulnerable population, potentially leading to more effective preventive measures and improved health outcomes.
Introduction
Hypertension is a common chronic disease characterised by continuously elevated blood pressure levels, which significantly affects the cardiovascular system and increases the risk of serious complications such as stroke, heart disease, and kidney disease [Citation1–3]. According to the World Health Organisation (WHO), over 1 billion people globally suffer from hypertension, and this number continues to rise, posing a significant challenge in the field of global public health [Citation4, Citation5]. Particularly among individuals aged 60 and above, the prevalence of hypertension is higher, possibly due to age-related physiological changes such as decreased vascular elasticity and arterial wall stiffening, making the elderly more susceptible to hypertension [Citation6–8]. In addition to vascular physiological changes, the complexity of hypertension in the elderly is also compounded by multiple chronic diseases and medication therapies. Elderly individuals often suffer from various diseases such as diabetes, hyperlipidaemia, and obesity, which interact with hypertension, increasing the risk of cardiovascular events. Moreover, elderly individuals may require simultaneous administration of multiple medications, which may interact with each other, affecting the efficacy of blood pressure control [Citation9, Citation10].
Folate is an important component of the B vitamins and has a positive impact on cardiovascular health [Citation11–13]. Folate is involved in biological metabolic processes such as methylation reactions and DNA synthesis, playing a crucial role in maintaining the normal function of endothelial cells and the normal structure of blood vessel walls [Citation14,Citation15]. Clinical studies have shown that inadequate folate intake is associated with the occurrence and development of hypertension, while increasing folate intake helps reduce the risk of hypertension [Citation16–18]. However, the current research results on the relationship between serum folate levels and hypertension are inconsistent [Citation19–21], especially lacking sufficient evidence in the elderly population.
The rationale for investigating the relationship between serum folate concentration and hypertension in the elderly stems from the need to identify modifiable risk factors and potential therapeutic targets in this population for hypertension management. This study utilises data from the NHANES to examine the relationship between serum folate concentration and hypertension in the elderly, thereby adding evidence to the role of folate in cardiovascular health and hypertension management. While systolic hypertension is recognised as a primary risk factor for cardiovascular diseases in this demographic, the specific impacts of diastolic hypertension are less understood. Diastolic hypertension, although less prevalent, is still clinically relevant due to its potential association with adverse cardiovascular outcomes [Citation22,Citation23]. This study explores this relationship to better understand diastolic hypertension’s role, without implying causation. Understanding how folate relates to blood pressure regulation in the elderly may offer insights that could inform future hypothesis-driven research aimed at developing targeted interventions for hypertension.
Materials and methods
Data source
NHANES is a nationally representative cross-sectional program conducted by the National Centre for Health Statistics (NCHS) of the United States, held every 2 years, aimed at studying the risk factors and prevalence of common diseases in the U.S. population. The detailed dataset of NHANES is collected by trained personnel and includes face-to-face interviews, biochemical tests, and physical examinations. NHANES employs a complex, stratified, multi-stage sampling strategy to select participants. All individuals participating in the NHANES survey are eligible for two interviews. The first interview is conducted in their homes, and the second is a series of health examinations conducted at the Mobile Examination Centre (MEC). Written informed consent is obtained from all participants upon inclusion in the survey. More detailed information on NHANES survey design, codebooks, and methodology can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm, accessed on March 28, 2024).
Study population
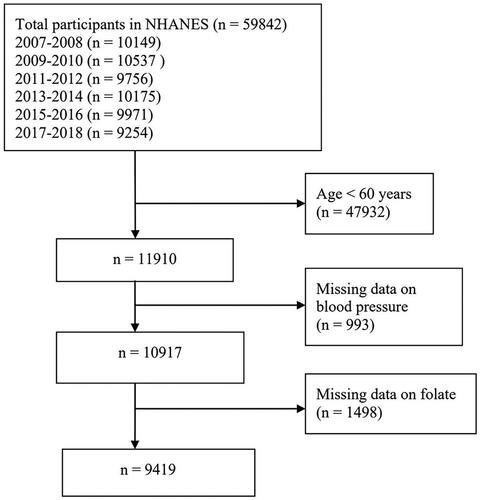
This study selected research data from six cycles of the NHANES database (2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018). The exclusion criteria were as follows: (1) age <60 years, (2) missing blood pressure data, (3) missing folate data. Finally, 9,419 subjects were analysed. illustrates the detailed flowchart of inclusion/exclusion criteria for the study population.
Measurement of serum folate
In NHANES, five forms of folate, including 5-methyltetrahydrofolate, folate, tetrahydrofolate, 5-formyltetrahydrofolate, and 5,10-methylenetetrahydrofolate, were measured using liquid chromatography–tandem mass spectrometry (LC–MS/MS). The assay involved the combination of serum specimens with ammonium formate buffer and internal standard mixture. Sample extraction and cleanup were performed using automated 96-probe solid-phase extraction (SPE) with 96-well phenyl SPE plates, with each 96-well plate requiring approximately 1 h. The separation of folate forms occurred within 6 min under isocratic flow conditions. Quantification was based on interpolation of five-point aqueous calibration curves using peak area ratios. Serum total folate was calculated by summing the concentrations of the aforementioned five folate forms. 5-methyltetrahydrofolate is the predominant biologically active form of serum total folate. Therefore, serum total folate and 5-methyltetrahydrofolate levels were included in the analysis [Citation24,Citation25]. Detailed descriptions of specimen collection and processing can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Definition of hypertension
At the Mobile Examination Centre, trained medical personnel conducted systolic and diastolic blood pressure (BP) measurements using a standard protocol. Three consecutive blood pressure measurements were taken by auscultation (after the participant had rested for 5 min while sitting), using a mercury sphygmomanometer and an appropriately sized cuff (based on upper arm circumference measurement). A fourth measurement was taken if the blood pressure measurement was interrupted, and all available blood pressure measurements were used to calculate the average systolic blood pressure (SBP) and diastolic blood pressure (DBP) for each participant. In this study, diastolic hypertension was defined as an average DBP ≥90 mmHg based on previous guidelines and literature [Citation26–28].
Covariates
Age, sex, BMI (Body Mass Index), education, marital status, race, poverty income ratio (pir), alcohol use, smoking, diabetes, physical activity, triglyceride, cholesterol, creatinine, and uric acid are considered as relevant covariates. Age and BMI were treated as continuous variables. Education was categorised into three groups: (1) Less than high school; (2) High school or equivalent; (3) College or above. Marital status was classified into six categories: (1) Married; (2) Widowed; (3) Divorced; (4) Separated; (5) Never married; (6) Living with partner. Race was divided into five categories: (1) Mexican American; (2) Other Hispanic; (3) Non-Hispanic White; (4) Non-Hispanic Black; (5) Other Race. Poverty Income Ratio (PIR) was classified into three categories: (1) <1.30; (2) 1.30–3.49; (3) ≥3.50. According to previous literature, alcohol use was classified into four categories: (1) Never drinking: no history of alcohol consumption or former drinkers; (2) Current heavy drinking (≥3 drinks/day for women, ≥4 drinks/day for men); (3) Current moderate drinking (≥2 drinks/day for women, ≥3 drinks/day for men); (4) Current light drinking: does not meet the above criteria [Citation29]. Smoking was classified into three categories: (1) Never: smoked less than 100 cigarettes in life; (2) Former: smoked more than 100 cigarettes in life and do not smoke at all now; (3) Current: smoked more than 100 cigarettes in life and smoke some days or every day [Citation30]. Physical activity was divided into two categories: According to the United States Physical Activity Guidelines, adults should engage in at least 150–300 min of moderate-intensity aerobic activity per week, 75–150 min of vigorous-intensity aerobic activity per week, or an equivalent combination of moderate and vigorous-intensity aerobic activity [Citation31]. In this study, if the intensity and duration of exercise meet the above requirements, it is considered as recommended activity. Otherwise, it is defined as insufficient activity.
Statistical analyses
According to the NHANES analysis tutorial, the complex survey design was taken into consideration, and Full Sample 2 Year MEC Exam Weight was applied in the analysis. Categorical variables were expressed as percentages (%) and compared using chi-square tests. Continuous data with normal distribution were presented as mean (±standard deviation (SD)) and compared using independent sample t-tests. For non-normally distributed data, the median (first quartile (P25) and third quartile (P75)) was used, and analysis was conducted using Wilcoxon rank-sum tests. Folate concentration was converted from ng/mL to µg/dL in the analysis. Folate concentrations were categorised into quartiles, from the lowest (first quartile, Q1) to the highest (fourth quartile, Q4), with Q1 as the reference group. Logistic regression analysis was used to evaluate the relationship between serum folate concentration as continuous and categorical variables and diastolic hypertension. Model 1 was the crude model without any variable adjustments. Model 2 was adjusted based on age, sex, BMI, education, triglyceride, cholesterol, creatinine, and uric acid. Model 3 was further adjusted for age, sex, BMI, education, triglyceride, cholesterol, creatinine, uric acid, marital status, race, PIR, alcohol use, smoking, diabetes, and physical activity. The potential nonlinear relationship between serum folate concentration and diastolic hypertension was explored using restricted cubic spline (RCS) analysis. In the RCS model, all the aforementioned confounders were adjusted. Additionally, subgroup analyses were conducted based on sex, education, marital status, race, PIR, alcohol use, smoking, diabetes, and physical activity. Finally, the likelihood ratio test was utilised to examine interactions among these subgroups. Statistical analyses were performed using R 4.2.2 and Free Statistics Software version 1.7. Descriptive statistics were conducted for all participants. A p-value <.05 in two-sided tests indicated statistical significance.
Results
Participants and demographic characteristics
A total of 9,419 eligible participants were included in our study, representing approximately 300.70 million of the total U.S. population, with 360 participants (3.8%) categorised as having diastolic hypertension due to diastolic blood pressure ≥90 mmHg. The median serum folate concentration was 1.98 µg/dL (Q1 = 1.28, Q3 = 3.08). Detailed baseline characteristics were presented in . The mean age of the participants was 70.0 ± 7.0 years, with 4,685 individuals (49.7%) being male, and the majority self-reported their race as Non-Hispanic White (4,616 individuals, 49.0%). At baseline, participants with higher folate levels tended to be older, female, college or above educated, widowed, Non-Hispanic White, with PIR ≥3.50, never drinkers, non-smokers, insufficient activity, with lower diastolic blood pressure, BMI, and cholesterol, and without diastolic hypertension and diabetes.
Table 1. Baseline characteristics of the study participants.
Association between serum folate concentration and diastolic hypertension
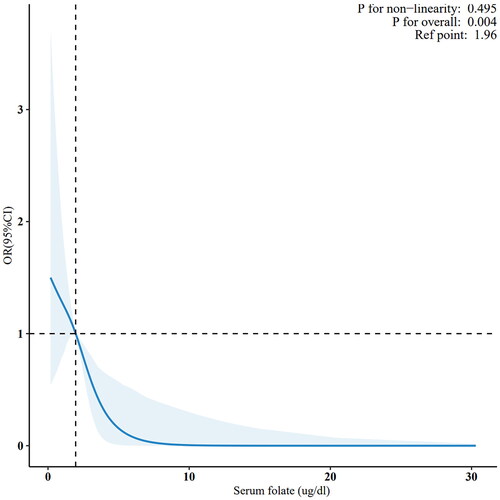
presents the results of multivariable logistic regression analysis examining the association between serum folate concentration and the prevalence of diastolic hypertension. When serum folate concentration was analysed as a continuous variable, a significant negative correlation was observed between serum folate levels and the prevalence of diastolic hypertension in the unadjusted Model 1 (OR: 0.63, 95% CI: 0.51–0.76; p < .001); this association remained significant after adjustments in Models 2 and 3. With increasing serum folate levels, the prevalence of diastolic hypertension decreased, with the fourth quartile having lower odds compared to the first quartile (OR: 0.35, 95% CI: 0.16–0.74) in Model 3 adjusted for age, sex, BMI, education, triglyceride, cholesterol, creatinine, uric acid, marital status, race, PIR, alcohol use, smoking, diabetes, and physical activity. Furthermore, all models exhibited a significant linear trend (trend p < .05). When considering all potential confounders, RCS plots showed a negative correlation between serum folate levels and the prevalence of diastolic hypertension (non-linearity: p = .495).
Table 2. The associations between serum folate and diastolic hypertension.
Subgroup analyses
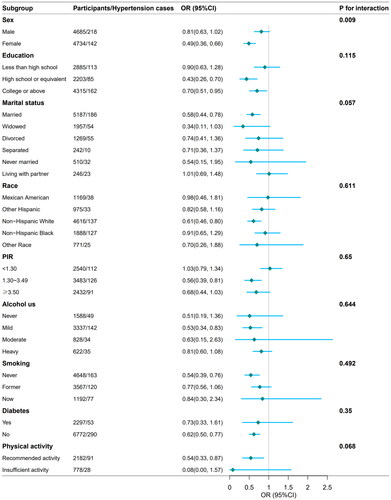
Stratification and interaction analyses were conducted to determine whether the association between serum concentration and the prevalence of diastolic hypertension remained consistent across several subgroups (see ). Consistent results were observed when stratifying by sex, education, marital status, race, PIR, alcohol use, smoking, diabetes, and physical activity. Compared to males, the negative association between serum folate concentration and elderly diastolic hypertension was more significant in the female population (OR 0.49; 95% CI: 0.36–0.66), with a p-value for interaction <.05 (see ).
Discussion
This study utilised data from the NHANES database spanning from 2007 to 2018, aiming to explore the relationship between serum folate concentration and the prevalence of elderly diastolic hypertension. A total of 9,419 participants aged 60 and above were included in the analysis. After adjusting for various potential confounding factors, logistic regression analysis revealed a significant negative correlation between serum folate concentration and the prevalence of diastolic hypertension. Specifically, higher serum folate concentration was associated with lower prevalence of diastolic hypertension, and this association remained independent of other factors such as age and sex. Stratified analysis further investigated this association and found it particularly significant among female participants, highlighting the potential role of serum folate in mitigating the prevalence of diastolic hypertension in the elderly population. These findings highlight the association between higher folate levels and lower prevalence of diastolic hypertension in the elderly, suggesting that folate may play a role in hypertension management strategies. Further research is needed to determine if increasing folate intake could contribute to the prevention of hypertension.
Figure 3. Subgroup analysis of the association between serum folate concentration and the prevalence of elderly diastolic hypertension. Age, sex, BMI, education, triglyceride, cholesterol, creatinine, uric acid, marital status, race, PIR, alcohol use, smoking, diabetes, and physical activity were adjusted in subgroup analysis. OR: odds ratio; 95% CI: 95% confidence interval.
Lee’s study investigated the relationship between serum folate concentration and hypertension in Korean adults. The study utilised data from the Korea National Health and Nutrition Examination Survey from 2016 to 2018, including 6,343 Korean adults. The study subjects were divided into five groups based on serum folate concentration, and multivariable logistic and linear regression models were used to analyse the relationship between serum folate concentration, blood pressure, and hypertension. The results showed that there was no statistically significant linear relationship between serum folate concentration and blood pressure, and after adjusting for all potential confounding factors, there was no significant difference in the prevalence of hypertension among populations with different serum folate concentration. Therefore, the conclusion drawn was that the association between high serum folate concentration and reduced hypertension in Korean adults is not significant [Citation19]. Two additional cross-sectional studies from China were conducted. In Shen’s study, 1,532 healthy non-pregnant individuals with normal blood pressure were included. Serum folate concentration was measured using immunoassay, and other biochemical indicators were measured using standardised clinical procedures. The study results showed a negative correlation between serum folate concentration and both systolic and diastolic blood pressure, which persisted even after adjusting for demographic, anthropometric, and biochemical factors [Citation20]. In Du’s study, 3,464 non-pregnant women of reproductive age were included. The results indicated a significant association between serum folate deficiency and the prevalence of prehypertension and hypertension among these non-pregnant Chinese women [Citation21]. All of these studies were conducted in Asian countries, which may limit their external validity. Our study focuses on the elderly population in the United States and utilises estimates from a nationally representative sample. The results indicate a negative correlation between serum folate concentration and the prevalence of elderly diastolic hypertension, which contradicts the findings of Lee’s study but aligns with those of Shen and Du, despite Shen and Du’s study populations not being exclusively elderly. The differences from Lee’s study results may be due to differences in baseline characteristics of the study populations, hence limited sample size being sufficient to yield different outcomes. In many countries, including the United States, mandatory folic acid fortification of grain products has been implemented to reduce the incidence of neural tube defects. This policy has led to higher baseline levels of serum folate in the population [Citation32,Citation33]. In contrast, countries without mandatory fortification may have lower average serum folate levels, which could influence the generalisability of our findings. The differences in folate status due to fortification policies need to be considered when comparing our results to those from other countries.
Folate, a member of the B-vitamin family, plays a crucial role in amino acid metabolism [Citation34,Citation35]. Adequate folate intake is essential for metabolism, cellular homeostasis, and DNA synthesis [Citation14,Citation36,Citation37] (as shown in ). Since its discovery in the 1940s, folate deficiency has been linked to many disease states, with epidemiological studies finding a negative correlation between blood folate concentration and cardiovascular health [Citation11–13]. This study indicates a negative correlation between serum folate concentration and the prevalence of diastolic hypertension. Potential mechanisms that may explain the observed association between higher folate levels and lower prevalence of diastolic hypertension include [Citation1]: Vasodilatory effects: Folate may impact vasodilation through various pathways, including promoting the synthesis and release of nitric oxide (NO). NO is a crucial vasodilator that promotes vasodilation, thereby lowering blood pressure levels. Ageing is associated with reduced endothelial function, manifested by diminished NO-dependent vasodilatory capacity in the skin vasculature. Folate and its metabolite 5-methyltetrahydrofolate (5-MTHF) have been reported to improve vascular function. Stanhewicz’s study demonstrated that folate increased skin vascular conductance and NO-dependent vasodilatory capacity in elderly but not young participants. Local injection of 5-MTHF and folate supplementation both increased vascular dilation in the elderly through NO-dependent mechanisms [Citation2,Citation38,Citation39]. Reduction of vascular inflammation and oxidative stress: Folate possesses antioxidative and anti-inflammatory properties, which can diminish endothelial inflammation and oxidative stress reactions, thereby improving the elasticity and stability of the vascular wall and lowering blood pressure levels. Cagnacci et al. found that oral administration of 15 mg/d of 5-methyltetrahydrofolate (5-MTHF) for 3 weeks reduced oxidative stress levels in postmenopausal women, and this reduction was closely associated with nocturnal blood pressure reduction [Citation3,Citation40,Citation41]. Lowering levels of homocysteine (Hcy) in the blood: Elevated levels of Hcy are closely associated with endothelial dysfunction and vascular wall damage, which may lead to increased blood pressure. Endothelial dysfunction is one of the early events in vascular diseases, and the accumulation of Hcy may exacerbate this dysfunction. Folate, as a key cofactor in homocysteine metabolism, promotes the conversion of Hcy to methionine, thereby aiding in reducing the accumulation of Hcy in the blood. Therefore, maintaining a higher serum folate concentration may help to preserve normal endothelial function, thus mitigating the adverse effects of elevated Hcy levels on vascular health and contributing to the control of blood pressure elevation [Citation4,Citation42,Citation43]. Regulation of angiotensin levels: Angiotensin II is a potent vasoactive substance that causes vasoconstriction and elevation of blood pressure. Folate may regulate blood pressure by affecting the angiotensin system. Early studies have indicated that folate can influence the activity of angiotensin-converting enzyme (ACE), thereby reducing the levels of angiotensin II. By modulating this biological process, folate may contribute to maintaining vascular relaxation and normal blood pressure levels. Although research in this area is still in its early stages, it provides important clues for understanding the potential mechanisms of folate in blood pressure regulation [Citation44,Citation45].
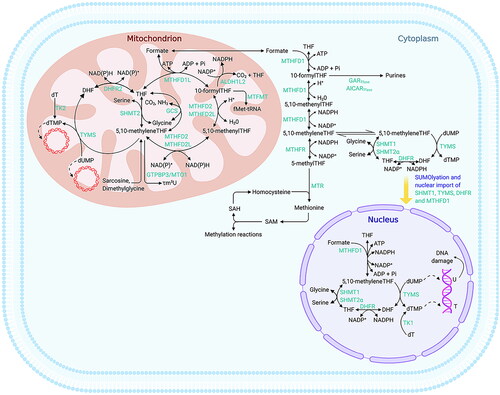
Figure 4. Folate-mediated one-carbon metabolism. (adapted from Xiu Y, Field MS. The roles of mitochondrial folate metabolism in supporting mitochondrial DNA synthesis, oxidative phosphorylation, and cellular function. Curr Dev Nutr. 2020;4(10):nzaa153). The compartmentalisation of folate-mediated one-carbon metabolism (FOCM) within cells involves distinct regions for various metabolic processes. Thymidylate (dTMP) synthesis occurs in the cytosol, nucleus, and mitochondria, whereas the synthesis of purines and methionine takes place exclusively in the cytosol. Mitochondrial FOCM generates formate, which is utilised by both cytosolic and nuclear FOCM, and provides biosynthetic precursors for mitochondrial DNA (mtDNA) synthesis and protein translation. Key enzymes and components involved in these processes include aminoimidazolecarboxamide ribonucleotide transformylase (AICARTfase), aldehyde dehydrogenase 1 family member L2 (ALDH1L2), dihydrofolate (DHF), dihydrofolate reductase (DHFR), deoxythymidine (dT), deoxythymidine monophosphate (dTMP), deoxyuridine monophosphate (dUMP), N-formylmethionine (fMet), glycinamide ribonucleotide transformylase (GARTfase), glycine cleavage system (GCS), mitochondrial GTP-binding protein 3 (GTPBP3), mitochondrial methionyl-tRNA formyltransferase (MTFMT), methylenetetrahydrofolate dehydrogenase (MTHFD), methylenetetrahydrofolate dehydrogenase 1-like (MTHFD1L), methylene-tetrahydrofolate reductase (MTHFR), mitochondrial tRNA translation optimisation 1 (MTO1), methionine synthase (MTR), phosphate (Pi), S-adenosylhomocysteine (SAH), S-adenosylmethionine (SAM), serine hydroxymethyltransferase (SHMT), small ubiquitin-like modifier (SUMO), thymidine (T), tetrahydrofolate (THF), thymidine kinase (TK), thymidylate synthase (TYMS), uracil (U), and modified nucleoside 5-taurinomethyluridine (τm5U).
To the best of our knowledge, this study represents the largest-scale investigation to date on the association between serum folate concentration and the prevalence of diastolic hypertension in the elderly population of the United States. It encompasses male and female participants from diverse racial backgrounds. In comparison to prior research, our study places greater emphasis on representing participants that are nationally representative of the US population, utilising 2 years of MEC weighting. Considering the complex multistage probability sampling method employed by NHANES, our conclusions are rendered more reliable. Our investigation is focused on diastolic hypertension in the elderly population, a subject that has not been extensively explored in previous studies. Additionally, we conducted subgroup analyses to enhance the robustness of our findings. Nonetheless, given the limited individual sample size in our survey, caution should be exercised in interpreting these findings, and further well-designed prospective studies are warranted to validate them.
Our study also has certain limitations [Citation1]: although we used a nationally representative database for our research, these results may not be directly generalisable to the general population worldwide. Therefore, further research is needed to validate our findings [Citation2]. Unknown or unmeasurable factors may introduce confounding, which cannot be completely ruled out [Citation3]. Due to the limitations of the cross-sectional design, we cannot establish a causal relationship between serum folate concentration and elderly diastolic hypertension. Therefore, future multicentre prospective studies are needed to confirm the potential causal relationship [Citation4]. Serum folate was measured only once, which may not fully reflect the long-term status.
Conclusion
In summary, utilising large-scale nationally representative cohort data from the United States, we found that higher levels of serum folate concentration are negatively associated with the prevalence of elderly diastolic hypertension. Specifically, higher levels of serum folate concentration are correlated with lower prevalence of elderly diastolic hypertension. Subgroup analysis further demonstrates a more significant negative association between serum folate concentration and the prevalence of diastolic hypertension among female participants. These findings highlight the potential role of serum folate concentration in association with diastolic hypertension in the elderly and suggest directions for future research to explore how folate may influence hypertension management strategies.
Ethics approval and consent to participate
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
This study utilised publicly available datasets, which are accessible at the following location: (https://wwwn.cdc.gov/nchs/nhanes/ accessed on March 28, 2024).
Additional information
Funding
References
- Wajngarten M, Silva GS. Hypertension and stroke: update on treatment. Eur Cardiol. 2019;14(2):111–115. doi: 10.15420/ecr.2019.11.1.
- Slivnick J, Lampert BC. Hypertension and heart failure. Heart Fail Clin. 2019;15(4):531–541. doi: 10.1016/j.hfc.2019.06.007.
- Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79(4):365–379. doi: 10.1007/s40265-019-1064-1.
- Dzau VJ, Balatbat CA. Future of hypertension: the need for transformation. Hypertension. 2019;74(3):450–457. doi: 10.1161/HYPERTENSIONAHA.119.13437.
- Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2.
- Laurent S, Boutouyrie P. Arterial stiffness and hypertension in the elderly. Front Cardiovasc Med. 2020;7:544302. doi: 10.3389/fcvm.2020.544302.
- Lee JH, Kim KI, Cho MC. Current status and therapeutic considerations of hypertension in the elderly. Korean J Intern Med. 2019;34(4):687–695. doi: 10.3904/kjim.2019.196.
- Oliveros E, Patel H, Kyung S, et al. Hypertension in older adults: assessment, management, and challenges. Clin Cardiol. 2020;43(2):99–107. doi: 10.1002/clc.23303.
- Rizzuto D, Melis RJF, Angleman S, et al. Effect of chronic diseases and multimorbidity on survival and functioning in elderly adults. J Am Geriatr Soc. 2017;65(5):1056–1060. doi: 10.1111/jgs.14868.
- Tinetti ME, Han L, McAvay GJ, et al. Anti-hypertensive medications and cardiovascular events in older adults with multiple chronic conditions. PLoS One. 2014;9(3):e90733. doi: 10.1371/journal.pone.0090733.
- Shabbir H, Shabbir I, Aslam M, et al. Fundamental aspects of vitamin B complex in human nourishment and fitness. Am J Food Sci Health. 2020;6(4):109–118.
- Sarwar MF, Sarwar MH, Sarwar M. Deficiency of vitamin B-complex and its relation with body disorders. In B-complex vitamins-sources, intakes and novel applications. 2021. p. 79–100.
- Nkemjika S, Ifebi E, Cowan LT, et al. Association between serum folate and cardiovascular deaths among adults with hypertension. Eur J Clin Nutr. 2020;74(6):970–978. doi: 10.1038/s41430-019-0533-7.
- Xiu Y, Field MS. The roles of mitochondrial folate metabolism in supporting mitochondrial DNA synthesis, oxidative phosphorylation, and cellular function. Curr Dev Nutr. 2020;4(10):nzaa153. doi: 10.1093/cdn/nzaa153.
- Froese DS, Fowler B, Baumgartner MR. Vitamin B12, folate, and the methionine remethylation cycle—biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019;42(4):673–685. doi: 10.1002/jimd.12009.
- Forman JP, Rimm EB, Stampfer MJ, et al. Folate intake and the risk of incident hypertension among US women. JAMA. 2005;293(3):320–329. doi: 10.1001/jama.293.3.320.
- Xun P, Liu K, Loria CM, et al. Folate intake and incidence of hypertension among American young adults: a 20-y follow-up study. Am J Clin Nutr. 2012;95(5):1023–1030. doi: 10.3945/ajcn.111.027250.
- Xiong Y, Huang J, Amoah AN, et al. Folate, vitamin B6, and vitamin B12 intakes are negatively associated with the prevalence of hypertension: a national population-based study. Nutr Res. 2023;112:46–54. doi: 10.1016/j.nutres.2023.02.006.
- Lee Y, Park S. Serum folate levels and hypertension. Sci Rep. 2022;12(1):10071. doi: 10.1038/s41598-022-13978-5.
- Shen M, Tan H, Zhou S, et al. Serum folate shows an inverse association with blood pressure in a cohort of Chinese women of childbearing age: a cross-sectional study. PLoS One. 2016;11(5):e0155801. doi: 10.1371/journal.pone.0155801.
- Du Y, Xia S, Zhang J, et al. Plasma folate deficiency increases the risk for abnormal blood pressure in Chinese women of childbearing age. Nutr Res. 2022;98:9–17. doi: 10.1016/j.nutres.2021.12.003.
- Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381(3):243–251. doi: 10.1056/NEJMoa1803180.
- Chrysant SG. The clinical significance of isolated diastolic hypertension. Postgrad Med. 2020;132(7):624–628. doi: 10.1080/00325481.2020.1788294.
- Wen J, Wang C, Giri M, et al. Association between serum folate levels and blood eosinophil counts in American adults with asthma: results from NHANES 2011–2018. Front Immunol. 2023;14:1134621. doi: 10.3389/fimmu.2023.1134621.
- Yao B, Lu X, Xu L, et al. Association of serum folate with prevalence of non-alcoholic fatty liver disease among adults (NHANES 2011–2018). Front Nutr. 2023;10:1141156. doi: 10.3389/fnut.2023.1141156.
- Akpa OM, Okekunle AP, Asowata JO, et al. Passive smoking exposure and the risk of hypertension among non-smoking adults: the 2015–2016 NHANES data. Clin Hypertens. 2021;27(1):1–12. doi: 10.1186/s40885-020-00159-7.
- Unger T, Borghi C, Charchar F, et al. International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
- Chen Y, Li Y, Liu M, et al. Association between systemic immunity-inflammation index and hypertension in US adults from NHANES 1999–2018. Sci Rep. 2024;14(1):5677. doi: 10.1038/s41598-024-56387-6.
- Li Y, Xiong B, Zhu M, et al. Associations of starchy and non-starchy vegetables with risk of metabolic syndrome: evidence from the NHANES 1999–2018. Nutr Metab (Lond). 2023;20(1):36. doi: 10.1186/s12986-023-00760-1.
- Liu B, Liu J, Pan J, et al. The association of diabetes status and bone mineral density among US adults: evidence from NHANES 2005–2018. BMC Endocr Disord. 2023;23(1):27. doi: 10.1186/s12902-023-01266-w.
- Zhang W, Xu R, Cai Z, et al. Association between physical activity and resistant hypertension in treated hypertension patients: analysis of the national health and nutrition examination survey. BMC Cardiovasc Disord. 2023;23(1):289. doi: 10.1186/s12872-023-03303-x.
- Williams J, Mai CT, Mulinare J, Centers for Disease Control and Prevention., et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification – United States, 1995-2011. MMWR Morb Mortal Wkly Rep. 2015;64(1):1–5.
- Mitchell LE. Folic acid for the prevention of neural tube defects: the US Preventive Services Task Force Statement on folic acid supplementation in the era of mandatory folic acid fortification. JAMA Pediatr. 2017;171(3):217–218. doi: 10.1001/jamapediatrics.2016.4983.
- Naderi N, House JD. Recent developments in folate nutrition. Adv Food Nutr Res. 2018;83:195–213. doi: 10.1016/bs.afnr.2017.12.006.
- Mahmood L. The metabolic processes of folic acid and Vitamin B12 deficiency. J Health Res Rev. 2014;1(1):5–9. doi: 10.4103/2394-2010.143318.
- Duthie SJ, Narayanan S, Brand GM, et al. Impact of folate deficiency on DNA stability. J Nutr. 2002;132(8 Suppl):2444S–2449S. doi: 10.1093/jn/132.8.2444S.
- Wagner C. Biochemical role of folate in cellular metabolism. Clin Res Regul Aff. 2001;18(3):161–180. doi: 10.1081/CRP-100108171.
- Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci (Lond). 2015;129(2):159–167. doi: 10.1042/CS20140821.
- Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev. 2017;75(1):61–70. doi: 10.1093/nutrit/nuw053.
- Cagnacci A, Cannoletta M, Xholli A, et al. Folate administration decreases oxidative status and blood pressure in postmenopausal women. Eur J Nutr. 2015;54(3):429–435. doi: 10.1007/s00394-014-0726-8.
- Jones P, Lucock M, Scarlett CJ, et al. Folate and inflammation–links between folate and features of inflammatory conditions. J Nutr Intermed Metab. 2019;18:100104. doi: 10.1016/j.jnim.2019.100104.
- Kaye AD, Jeha GM, Pham AD, et al. Folic acid supplementation in patients with elevated homocysteine levels. Adv Ther. 2020;37(10):4149–4164. doi: 10.1007/s12325-020-01474-z.
- Zhao W, Gao F, Lv L, et al. The interaction of hypertension and homocysteine increases the risk of mortality among middle-aged and older population in the United States. J Hypertens. 2022;40(2):254–263. doi: 10.1097/HJH.0000000000003002.
- Pushpakumar SB, Kundu S, Metreveli N, et al. Folic acid mitigates angiotensin-II-induced blood pressure and renal remodeling. PLoS One. 2013;8(12):e83813. doi: 10.1371/journal.pone.0083813.
- Masi S, Uliana M, Virdis A. Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. Vascul Pharmacol. 2019;115:13–17. doi: 10.1016/j.vph.2019.01.004.