Abstract
Background
In the in-clinic blood pressure (BP) recording setting, a sizable number of individuals with normal BP and approximately 30% of patients with chronic renal disease (CKD) exhibit elevated outpatient BP records. These individuals are known as masked hypertension (MHTN), and when they are on antihypertensive medications, but their BP is not controlled, they are called masked uncontrolled hypertension (MUHTN). The masked phenomenon (MP) (MHTN and MUHTN) increases susceptibility to end-organ damage (a two-fold greater risk for cardiovascular events and kidney dysfunction). The potential extension of the observed benefits of MP therapy, including a reduction in end-organ damage, remains questionable.
Aim and methods
This review aims to study the diagnostic methodology, epidemiology, pathophysiology, and significance of MP management in end-organs, especially the kidneys, cardiovascular system, and outcomes. To achieve the purposes of this non-systematic comprehensive review, PubMed, Google, and Google Scholar were searched using keywords, texts, and phrases such as masked phenomenon, CKD and HTN, HTN types, HTN definition, CKD progression, masked HTN, MHTN, masked uncontrolled HTN, CKD onset, and cardiovascular system and MHTN. We restricted the search process to the last ten years to search for the latest updates.
Conclusion
MHTN is a variant of HTN that can be missed if medical professionals are unaware of it. Early detection by ambulatory or home BP recording in susceptible individuals reduces end-organ damage and progresses to sustained HTN. Adherence to the available recommendations when dealing with masked phenomena is justifiable; however, further studies and recommendation updates are required.
PLAIN LANGUAGE SUMMARY
Blood pressure tells us how much force the heart exerts on the blood vessels as it pumps blood. Normal blood pressure should be 120/80 mmHg, which generally decreases when a person is sleeping or sitting. High blood pressure or hypertension occurs when the blood pressure is too high. Hidden or masked hypertension (MH) is a type of high blood pressure. Masked hypertension was described as having high blood pressure readings even though the doctor’s office or in-clinic showed normal blood pressure readings.
This review aimed to teach people about various kinds of high blood pressure, focusing on hidden (masked) hypertension and how to recognise it, as well as its consequences, treatment, and new information.
Introduction
Most observational research and intervention studies have defined hypertension (HTN) based on in-clinic blood pressure (BP) recordings. Home BP (HBP) or ambulatory BP monitoring (ABPM) recording offers a more precise assessment of the potential cardiovascular (CV) disease risk and overall mortality than BP records obtained in in-clinic settings [Citation1]. HTN is a prominent contributor to morbidity and mortality worldwide. Approximately one-third of individuals with HTN have normal BP records during clinical visits but experience elevated outside-clinic BP records [Citation2]. In addition to the evident correlation between normal BP and HTN-induced organ damage, masked HTN (MHTN) is associated with sustained HTN (SHTN). Evidence suggests that individuals with MHTN phenotypes are susceptible to HTN-mediated organ damage [Citation3]. BP phenotypes, namely sustained normotension (NT), white-coat coat HTN (WCHTN), sustained HTN (SHTN), and masked HTN (MHTN), have been characterised after the introduction of ABPM recording. In 2002, Pickering first used the term MHTN, characterised as having both NT in-clinic BP and high out-clinic BP or masked uncontrolled HTN (MUHTN) [Citation4]. The known phenotypes of BP are shown in .
Figure 1. Blood pressure phenotypes. BP: Blood pressure; MHTN: masked hypertension; MUHT: masked uncontrolled hypertension; HBP: home blood pressure; ABPM: ambulatory blood pressure monitoring.
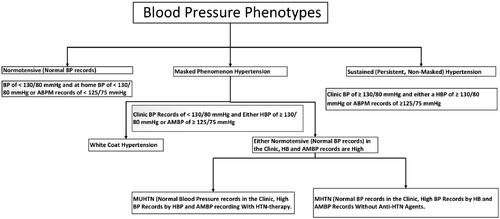
The masked phenomenon (MP), which includes MHTN and MUHTN, has been recognised as a clinical entity for more than two decades by Pickering [Citation5]. However, several features of MHTN/MUHTN remain unclear. It showed that > 33% of individuals diagnosed with chronic kidney disease (CKD) and exhibiting normal BP records during clinical assessments had increased BP levels during ambulatory blood pressure monitoring (ABPM) recording. Research findings indicate a significant association between a low estimated glomerular filtration rate (eGFR) and high proteinuria among people from the Chronic Renal Insufficiency Cohort (CRIC) study and an increased likelihood of MHTN. Furthermore, patients with MHTN were more susceptible to end-organ damage and dysfunction, such as elevated left ventricular mass and pulse wave velocity. The findings in individuals with CKD are consistent with previous research on individuals with normal kidney function. Studies have shown that individuals with MHTN have a two-fold higher likelihood of experiencing CV events than those with normal BP records in both clinical and ambulatory settings [Citation6,Citation7].
Although the focus on out-of-office BP recording in recent years has made the masked phenomenon more well-acknowledged, some practitioners are still unaware of it. Despite their increased susceptibility to adverse outcomes, patients with masked HTN have historically been excluded from HTN studies owing to their normal BP records during in-clinic BP recording. Hence, the extent to which HTN therapy extends its benefits in terms of reducing end-organ damage and unfavourable cardiovascular outcomes remains uncertain for people with MHTN. Furthermore, there are numerous questions about MHTN.
Different randomised controlled trials and review articles have been conducted to describe and assess antihypertensive medication’s potential in modifying BP patterns among individuals with MHTN and the effect of MP control on outcomes. This non-systematic comprehensive review aims to discuss the latest updates on MP pathogenesis, treatments, and their effects on CV and CKD onset and progression. PubMed, Google, Scopus, EMBASE, and Google Scholar were searched using keywords, texts, and phrases such as CKD and HTN, MP, HTN types, HTN definition, cardiovascular events and MHTN, CKD progression, masked HTN, MUHTN, and CKD onset. To make the search more practical and concentrated, we restricted the search to published articles published in the last ten years.
Masked Phenomenon epidemiology
MHTN is typically seen in approximately 8–20% of the general population [Citation2, Citation8], and a meta-analysis of 36 studies estimated an MHTN prevalence of approximately 19% among adults [Citation9]. In the Chronic Kidney Disease Japan Cohort (CKD-JAC) report, the incidence of MHTN was 31% [Citation10]. It was observed that MHTN prevalence was higher in CKD patients. In a study of 1492-CKD participants in the Chronic Renal Insufficiency Cohort (CRIC) study, 28% had MHTN [Citation11]. Among 617 participants from the African American Study of Kidney Disease and Hypertension (AASK) cohort, 25% had MHTN when defined by daytime ABPM recording, and approximately 43% had MHTN when both day and night-time ABPM records were considered [Citation12]. It is worth noting that the results are not entirely consistent; an earlier meta-analysis by Bangash and Agarwal found that only 8% of patients with CKD had MHTN [Citation13]. In the International Database of Ambulatory BP in Renal Patients (I-DARE) study, compared with CKD-JAC participants and AASK participants, those from AASK were likely to have MHTN. By contrast, the CRIC study found that Italian and Spanish participants were unlikely to have MHTN [Citation14]. The prevalence of MHTN is significantly higher in CKD; however, prevalence estimates can vary and may differ based on patient characteristics, race, and ethnicity. MHTN prevalence does not appear to exhibit significant variation when the diagnosis is made using self-measured BP or ABPM recording [Citation15]. Based on the guidelines provided by the European Society of Hypertension and the European Society of Cardiology, it has been observed that the MHTN prevalence typically ranges around 13% in research conducted on general population-based samples [Citation16].
Studies have revealed that the MHTN prevalence has been estimated at 10% [Citation17,Citation18], and in another study, out of 2915 participants, 21.9% had MHTN [Citation19]. Studies have reported that the prevalence of MP, as determined by ABPM and HBP recordings, was 11% [Citation20,Citation21] and 13% [Citation19,Citation20], respectively, without significant statistical differences [Citation5]. An analysis of data collected from a sample size of 9,316 individuals participating in the National Health and Nutrition Examination Survey conducted between 2005 and 2010 revealed that the estimated prevalence of MHTN among US adults was approximately 12.3% [Citation21]. The prevalence of MHTN was elevated among older, male, those diagnosed with diabetes, and those with evidence of pre-HTN features [Citation21]. The overall weighted mean prevalence of MP is 11% [Citation22]. MHTN incidence exhibited significant variations, as demonstrated by cross-sectional investigations, with rates ranging from 8% to 49% [Citation15,Citation16, Citation23,Citation24].
The observed heterogeneity in MHTN under investigation has been ascribed to various patient features, populations under study, and utilisation of diverse criteria for MHTN diagnosis [Citation15]. There is evidence that black individuals have a higher rate of MHTN [Citation14]. Several studies have been undertaken on various populations, including individuals with normotension who were recommended for the study, patients receiving treatment for HTN, and elderly individuals, notably men [Citation15].
The prevalence of MUHTN is 13% [Citation19,Citation20], and when expressed as a proportion of patients with normal in-clinic BP, the weighted mean prevalence is 32%. Others have reported that the prevalence of MUHTN was 43% [Citation2, Citation25]. When representing a fraction of patients with normal in-clinic BP records, it was noted to be 32% for normotensive (NT) [Citation26], 28% for MHTN [Citation27], and 43% for uncontrolled HTN participants [Citation25]. It was shown that ABPM had a higher sensitivity level than HBP recording, indicating that a significant percentage of patients had MHTN/MUHTN when ABPM recording was used, as opposed to HBP (22% vs. 16%, p < 0.05) when both techniques were applied to the same group of patients.
Another potential variable affecting MP prevalence is the instrument type used to record the BP. Most studies used a mercury sphygmomanometer to record office BP, whereas a few employed automatic oscillometric devices [Citation15]. One factor contributing to MP misdiagnosis is the doctors, nurses, or technicians recording the in-clinic BP, because they may cause an increase in BP records (WCHTN), affecting the detection and prevalence of MP. Additionally, the posture in which the BP records were taken, either supine or sitting, also influences the diagnosis and incidence of MP [Citation15]. The prevalence of MHTN is summarised in .
Table 1. Masked hypertension prevalence in general population and chronic kidney disease patients.
Factors affecting and mechanisms of masked hypertension
The underlying mechanism(s) of MHTN are not well established; however, lifestyle factors and a specific reaction to stresses that occur when recording BP outside an in-clinic setting are the underlying factors. To accurately diagnose MHTN, 24-hour ABPM or HBP recording outside a medical facility is necessary for most, if not all, patients, especially when MP is suspected. Diagnosing MHTN is crucial, as it is likely to progress to SHTN, which puts patients at risk for chronic diseases and increases mortality. Therefore, early identification and addressing of MHTN are essential for preventing complications [Citation28].
There are two possible underlying mechanisms of MHTN that may not be mutually exclusive [Citation29]. The first involves a situation in which the office BP record is lower than the ABPM records, although the exact reason remains unknown. It is plausible that anxiety or stress levels may increase outside the clinic, leading to higher BP records. In some instances, hypertensive individuals, especially older people, may experience lower BP record reductions after meals and standing, resulting in a misdiagnosis of MHTN instead of SHT [Citation30]. In addition, the elderly, especially males with increased BP variability, are more likely to have MHTN than those with sustained normotension or WCHTN. The second possible mechanism is selectively high ABPM records, which can be influenced by smoking, alcohol consumption, physical activity, interpersonal conflicts, mental anxiety, and job stress. A study found that NTs with exaggerated BP responses had a 41% prevalence of MHTN when measured via ABPM recording. Furthermore, the same study revealed that diastolic BP (DBP) measured during peak exercise was an independent predictor of MHTN among subjects with an exaggerated BP response [Citation31].
Mental distress at work or at home may elevate BP [Citation32,Citation33], increasing the MHTN diagnosis rate. Smoking and excessive alcohol consumption were associated with MHTN [Citation34]. Obese and sedentary individuals have poor exercise tolerance and their resting BP records in the doctor’s office are prehypertensive [Citation35]. The presence of metabolic syndrome, diabetes, CKD, diminished sleep duration, or obstructive sleep apnoea may predispose individuals to MHTN, primarily due to nocturnal HTN [Citation26, Citation36]. Elevated night-time BP and non-dipping or rising nocturnal BP patterns, with or without elevated daytime ABPM values, may be associated with normal in-clinic BP records and increasing MHTN diagnosis prevalence [Citation36,Citation37]. In short, 24-hour ABPM recording is required to evaluate both daytime and nocturnal BP to diagnose MP.
Masked Phenomenon screening and diagnosis
The European Society of Hypertension (ESH) defines HTN as in-clinic BP records of ≥ 140/90 mmHg, out-of-clinic BP as HBP records of ≥ 135/85 mmHg, daytime ABPM records of ≥ 135/85 mmHg, or 24-hour ABPM records of ≥ 130/80 mmHg [Citation38]. Based on available information, SHTN is identified if the in-clinic systolic BP (SBP) is > 130 mmHg or the office DBP is > 80 mmHg [Citation39]. Individuals with NT records during in-clinic assessments but have difficulty maintaining a consistent sleep schedule of ≥6 h, suffer from obstructive sleep apnoea, heavy stressful work, reside in high-stress environments, engage in daily heavy upper body isometric exercises, consume high daily salt intake or old age, and are advised to undergo both HBP and ABPM monitoring to exclude MHTN/MUHT. In addition, young people with normal or high in-clinic BP who have left ventricular hypertrophy (LVH), high BP history in parents, CV risk factors, diabetes, and obesity have a higher risk of MHTN [Citation38]. Approximately 10–30% of the population of the universe has MHTN [Citation38]. In 14% of cases, ABPM recording was used, and in 11% of cases, HBP recording was used to identify MHTN [Citation40]. When the in-clinic SBP is 120–129 mmHg or DBP is 75–79 mmHg, it is prudent to screen for MHTN with ABPM [Citation8, Citation40].
ABPM and HBP were used to assess BP records outside the clinic. However, the extent to which these two BP recording methods can be utilised interchangeably for this purpose remains uncertain. Several researchers have conducted comparative analyses of ABPM and HBP as diagnostic instruments for MHTN. A study revealed a lack of consensus on MHTN when comparing records obtained from HBP and ABPM recordings in approximately 20% to 30% of the participants, and the diagnosis of MHTN was concordant across both techniques in only 50% of the respondents [Citation41]. Viera et al. noted that the specificity and sensitivity for diagnosing MHTN were 23% and 67%, respectively, when HBP records were utilised instead of ABPM to evaluate out-of-clinic BP [Citation42]. Their results suggest that HBP may not be sufficient to accurately diagnose MHTN or may not potentially identify a distinct subtype of MHTN compared to ABPM.
The performance of daily life activities may have significant and diverse effects on BP [Citation43]. This phenomenon may lead to an overestimation of BP compared with BP records taken during rest periods. These variations may result in the underestimation of the MHTN frequency detected with HBP compared to that diagnosed through ABPM.
High BP during sleep has been identified as the most influential risk factor for end-organ damage, CVD morbidity, and mortality when considering the various components of BP over 24 h [Citation44,Citation45]. Additionally, isolated nocturnal HTN, defined as sleep BP ≥ 120/70 mmHg and daytime BP < 135/85 mmHg, has recently been recognised as a novel CV risk factor in the general population [Citation46]. An accurate evaluation of sleep BP can only be accomplished using the ABPM records. Nevertheless, there are several apprehensions regarding ABPM, including its substantial financial implications, challenges associated with data collection, disruption of regular sleep cycles caused by the device, and potential annoyance experienced by patients. However, significant data suggest that HBP monitoring, which utilises more cost-effective and replicable equipment compared to ABPM, may provide more accurate assessments of end-organ damage and prognosis compared to in-clinic BP recording. Consequently, the use of HBP as an outpatient BP recording technique has seen a significant surge [Citation46].
Many national and international recommendations for treating MHTN have advised the use of HBP in-clinic BP recording to diagnose and follow-up on the response to treatment. However, little information is available on exact evaluations of HBP measures and their predictive significance. Despite the variation in the diagnostic capabilities for MHTN, the prognostic significance of MHTN is unaffected by the diverse out-of-office BP recording techniques used to detect its presence [Citation9, Citation15]. Nevertheless, the prognosis of NTs with MHTNs when assessed using ABPM in preference to HBP remains uncertain. Therefore, when ABPM is unavailable or practical because of financial constraints or patient discomfort, NHTN diagnosis may be achieved using HBP [Citation47]. When using HBP monitoring to diagnose MHTN, it is vital to acknowledge that the confirmation of MHTN diagnosis necessitates the acquisition of three readings in the morning and three in the evening, spanning two consecutive days [Citation48]. If an individual’s BP falls within the prehypertensive range, namely between 125-135/75-85 mmHg, with evidence of end-organ damage, it is recommended to consider HBP or ABPM BP recording [Citation47]. Neglecting night-time BP recordings can lead to missing isolated nocturnal HTN detection, a prevalent condition in diabetes mellitus nephropathy and obstructive sleep apnoea. Furthermore, it disregards a significant predictor of CV event risk [Citation49,Citation50].
Masked hypertension end-organs complication
Complications of MHTN have been recognised for over two decades [Citation51]. The frequency of end-organ damage, such as CV events and CKD, in MHTN is minimal [Citation6, Citation52–54] because most studies used ABPM recordings in the general population. However, a few small studies involving patients with CKD have shown that MHTN may lead to higher incidences of LVH, proteinuria, and renal dysfunction [Citation12, Citation55,Citation56]. Other studies observed that CKD patients who experienced reduced estimated glomerular filtration rate (eGFR) and proteinuria tended to have higher BP during the night-time [Citation57,Citation58]. Although the exact cause of elevated night-time BP in CKD patients is not yet fully understood, it is believed to be due to factors including transient overload and fluid redistribution between intra-and extravascular compartments at night-time because of lying down, sodium handling alteration, and other conditions such as diabetes or autonomic insufficiency. However, it remains unclear whether increased night-time BP impacts the connection between MHTN and negative outcomes regarding renal complications. Furthermore, previous studies on cohorts with CKD have demonstrated a higher likelihood of MHTN in individuals with significant proteinuria, although these analyses were either unadjusted or were based on small cohorts [Citation10, Citation12, Citation56]. MHTN is associated with a higher rate of negative hypertensive renal outcomes in individuals with normal kidney function [Citation11].
Furthermore, ABPM recording characterises the link between BP and organ damage more than in-clinic BP recording in CKD [Citation11]. A study concluded that MHTN prevalence in nondialyzed CKD patients was high, and MHTN was associated with end-organs damage in CKD [Citation59]. In a randomised middle-aged Swedish population, MHTN was linked to CV event markers, suggesting that high HBP records are the best predictors of CV events [Citation60]. The same study reported that MHTN prevalence increased with abnormal blood glucose [Citation60], which might be an additional risk factor for CKD. It is significant to point out that the SPRINT CKD population had a minimal risk for CKD progression, mainly because the participants had mild CKD and low levels of proteinuria. In addition, when evaluated through 24-hour ABPM recordings, the group receiving intensive treatment showed a significantly hidden effect compared to the standard treatment group [Citation61]. These results demonstrate that MHTN may be of greater clinical significance for CKD onset and progression when more aggressive in-clinic BP targets are used.
Nevertheless, the impact of MHTN on CKD onset likelihood remains uncertain because previous research was either cross-sectional or limited to those diagnosed with CKD [Citation10,Citation11, Citation62]. Moreover, there is limited evidence on the correlation between MHTN and renal outcomes, particularly in the African-American population. A study conducted in 2018 reported that MHTN had an interplay with CKD onset in African-Americans [Citation62]. MHTN almost leads to a degree of end-organ damage similar to SHTN; therefore, clinicians must consider end-organ damage complications in MHTN patients [Citation63].
MHTN is linked to high proteinuria and a decline in eGFR in the general population and among CKD patients. Moreover, individuals with MHTN had lower eGFR and proteinuria levels than those with controlled clinical and ABPM records [Citation11]. Hence, it is hard to conclude whether MHTN causes renal damage or CKD leads to MHTN. Patients with declined eGFR and proteinuria are likely to have elevated night-time BP with a nondipping pattern [Citation64]. Patients with MUHTN and CKD had delayed recovery from exercise-induced HTN compared with healthy controls [Citation65]. Research has identified a correlation between MHTN and kidney impairment, as the kidneys are vulnerable targets. The incidence of MHTN in CKD is rising, and these patients are at a greater risk of CV events. Hence, future studies must prioritise or at the very least incorporate individuals with CKD in their assessments of the mechanism(s) driving MHTN and treatment plans.
In dialysis-dependent patients, MHTN was 15%. A median midweek intradialytic BP prevalence of 15% indicated MHTN, which was significantly higher than the 8.3% estimate reported in a meta-analysis [Citation13]. The threshold BP used for categorisation greatly impacts the prevalence estimates of various diseases, as demonstrated in a meta-analysis [Citation13]. Notably, patients with MHTN are often prescribed antihypertensive medications. This is surprising because if hypertension is truly concealed, doctors should not prescribe additional antihypertensive medications. Patients with MHTN had a much higher average predialysis BP (154.8/81.4 mmHg) than SN patients (135/72.6 mmHg) [Citation66]. It is possible that doctors base their treatment decisions on predialysis BP monitoring, which may have led to the increased use of antihypertensive medications in these individuals.
Among patients on dialysis, individuals with masked hypertension had a significantly higher mortality rate than those with maintained hypertension [Citation66]. It was anticipated that those with greater weight reduction during dialysis would have lower post-dialysis blood pressure, but higher interdialytic ambulatory blood pressure. The largest increase in interdialytic weight was observed in masked hypertensives. Although the risk of death remained high even after controlling for interdialytic weight increase, another mechanism may contribute to the increased mortality risk [Citation66]. Patients with masked hypertension may have higher arterial stiffness and interdialytic blood pressure than those with persistent normotension, for the same volume change. These findings may explain the higher mortality rate associated with masked hypertension, as arterial stiffness is closely linked to all-cause mortality [Citation67]. Treating patients on dialysis with masked hypertension is challenging, and post-dialysis blood pressure monitoring should guide blood pressure control interventions [Citation66].
A study of 61 MHTN and 234 NTs revealed that MHTNs had a greater LVMI than NTs. In addition, MHTNs had a significantly higher prevalence of carotid plaques than NTs did. In contrast, MHTN patients resemble sustained hypertensive individuals [Citation51]. Studies investigating untreated MHTN have confirmed the findings of Liu et al. and supported that MHTNs have more end-organ damage than NTs, and the levels of end-organ injury in MHTNs were comparable to those in SHTNs and were intermediate between those in NTs and SHTNs [Citation68–70]. An analysis of the PAMELA study revealed more LVMI prevalence among MHTN than NT individuals, albeit LVMI prevalence was less than that among SHTN subjects [Citation70]. Another study discovered that untreated MHTN individuals had, on average, more LVMI and thickness of carotid intima-media (cIMT) than NTs individuals [Citation66]. These observations are supported by a study of 282 untreated patients [Citation69]. Furthermore, MHTN patients have considerably greater pulse wave velocity than NTs (P0.001), which is almost equivalent to sustained hypertensives [Citation69].
Other studies failed to reveal a consistent link between end-organ damage and MHTN in untreated patients with MHTN [Citation71,Citation72]. In the Uppsala Longitudinal Study of Adult Men, the mean LVMI was comparable between MHTN and NT patients among untreated men. Nonetheless, the LV wall thickness of MHTN patients was greater than that of the NT and SHTN groups, indicating higher concentric remodelling in the MHTN group than in the other groups of elderly patients [Citation73]. In contrast, a study of 1,653 untreated MHTN individuals did not note considerable connections between MHTN and LVMI [Citation71]. Moreover, Ormezzano et al. reported no significant LVMI differences between WCHTNs, MHTNs, and NTs [Citation72]. However, among the 136 individuals in this cohort who underwent pulse wave velocity measurements, WCHTNs had significantly greater PWVs than NTs. In contrast, end-organ injury in MHTN-treated patients indicates insufficient therapy and poor BP control. Therefore, it is unsurprising that assorted studies have revealed more significant end-organ damage even in patients with poorly controlled MHTN than in those with well-controlled HTN. A significant LVMI reduction (p = 0.01), LVH prevalence in addition to concentric remodelling (p = 0.01), and microalbuminuria (p = 0.05) were observed in controlled-BP individuals on ABPM and clinical setting records in a prospective study involving 80 masked hypertensive nondiabetic patients. The MHTN group demonstrated non-significant cardiac structure changes, LVH concentric remodelling prevalence, or microalbuminuria improvement [Citation74]. Those with MHTN exhibited a greater LVMI than those with well-controlled HTN among diabetic hypertensive subjects with mild or moderate renal disease [Citation75]. Pierdomenico et al. claimed that individuals who received treatment for MHTN and whose BP was not controlled were more likely to develop LVH and experience CV events than those with well-controlled HTN, with the former being two-fold more likely to experience these health concerns [Citation76]. Tomiyama et al. observed that MHTN was a significant independent indicator of LVH (p = 0.01), carotid atherosclerosis (p = 0.003), and albuminuria (p = 0.042) in 332 HTN-treated Japanese patients [Citation75]. In the African American Study of Kidney Disease trial, Pogue et al. observed that MHTN patients had a greater LV mass prevalence than NTs patients (p = 0.01) [Citation12].
At 8.3 years of median follow-up, CV events in untreated HTN patients were 55% higher in MHTN and 2.13 times higher in SHTN. With managed HTN, CV events increased by 76% in MHTN and by 40% in uncontrolled HTN [Citation40]. People with MHTN had greater left ventricular mass index (LFVMI), maximum carotid intima-media thickness index (CIMi), and urine albumin levels than those with managed HTN, and were comparable to those with SHTN [Citation77]. At an 8-year follow-up, a meta-analysis of seven studies involving 11,502 participants with an average age of 63 years revealed that compared to NT individuals, people with SHNT experienced a 2-fold increase in first CV events, and people with SHTN experienced a 2.3-fold increase [Citation6]. According to eight trials that included 7,964 participants, those with MHTN experienced a 2.1-fold increase in CV events at follow-up compared to NT individuals. Simultaneously, those with SHTN experienced a 2.6-fold increase [Citation78]. Furthermore, People with MHTN had a 1.8 to 2- times higher rate of CV events at follow-up than those with NTs [Citation8]. Approximately 16.6% of the 1,332 Japanese people who had MHTN, ten years of follow-up, had a 1.9-times risk of CV disease mortality, a 2.2 times risk of stroke, and a 2.1-times risk of CV disease mortality and stroke compared to NTs [Citation79]. In contrast, people with SHTN had a 1.9-times higher rate of dying from CV disease, a 2.8-times higher stroke risk, and a 2.23-times higher rate of dying from CV disease and stroke [Citation79]. In the Dallas Heart Study, 17.8% of 3,027 participants, with an average age of 47 years and 50% of black ethnicity, had MHTN [Citation80]. After controlling for conventional risk factors, individuals with MHTN had a 2-times higher CV event rate than those with NT [Citation80].
In the Jackson Heart Study, which included 972 black people with a mean age of 60 years, MHTN was present in 25.9% of cases and 34.4% when the patients’ clinic BP records were normal [Citation81]. The LVMI and common carotid artery intima-media thicknesses were higher in the MHTN group than in the normal BP group. In the Jackson Heart Study, a lower frequency of MHTN was related to less modifiable risk factors, as per the American Heart Association Life’s Simple 7 [Citation50]. The 10-year predicted atherosclerotic disease risk in 644 Jackson Heart Study participants was increased by MHTN by the pooled cohort risk equation 1.4 times if the atherosclerotic disease risk was between 5% and 7.4%, 1.6-times if it was between 7.5% and 9.9%, and 1.9-times if it was above 10% [Citation82].
The MHTN patient study observed a cohort of 813 adults with a mean age of 45 years who did not receive treatment for HTN. In this group, 15.2% of the individuals had MHTN [Citation83]. The study found that patients with MHTN and pre-HTN had a higher echocardiographic LVMI [Citation83]. Among 588 Chinese individuals diagnosed with non-dialysis CKD, with an average age of 43 years, 20.6% had MHTN[Citation84]. After a median follow-up of 35 months, it was shown that MHTN was related to a significant increase in all-cause mortality by a factor of 8.9, renal dysfunction by a factor of 3.7, and major adverse cardiac and cerebrovascular events by a factor of 8.7 [Citation84].
Some reviews and studies have investigated end-organ damage and MHTN in children and teenagers [Citation85,Citation86]. A study investigating 234 adolescents (200 NTs and 34 MHTNs) reported that LVMI was greater in MHTNs than in NTs (p = 0.023). Furthermore, the same study reported that after a 12-month follow-up, 9% of participants with MHTN developed SHTN, while none of the participants with normal BP had [Citation87]. Research that included 85 children in Athens noted that MHTN children had substantially greater LV mass than NT children (p = 0.05), but no changes in CIMi [Citation86]. A meta-analysis review of young adults and children reported that MHTN is prevalent among the two patient groups and negatively affects their prognosis by increasing the risk of LVH. These complications made ABPM recording and echocardiography mandatory to evaluate their BP status [Citation88].
Furthermore, some evidence has linked MHTN to a higher likelihood of experiencing cerebrovascular events [Citation54,Citation73]. A 10-year follow-up study compared individuals with NTs to those with MHTNs and uncontrolled SHT. The results showed that the MHTN group had a 2.2 times higher stroke risk and a 2.1 times higher stroke-related mortality rate compared to the NT group. Similarly, the SHT group had a 1.94 times higher stroke risk and a 2.8 times risk of death from stroke-related complications compared to the NT group, with a 2.3 times higher stroke-related mortality risk [Citation79]. In another study, individuals who experienced partial WCHTN or MHTN faced a similar long-term stroke risk as those with complete MHTN or MHTN. To accurately assess stroke risk, it is essential to record HBP and ABPM [Citation89].
Treatment of masked hypertension
It is highly recommended that out-clinic BP be assessed to detect MHTN and MUHTN, according to the ACC-AHA guidelines [Citation55,Citation90]. Limited information is available on the appropriate management of MHTN [Citation91]. One potential strategy for managing MHTN involves recording 24-hour ABPM [Citation91]. In addition, it is imperative to adopt initiatives to address modifiable factors associated with MHTN. While it is essential to acknowledge the potential hazards associated with MHTN, such as a higher risk of CV complications and mortality, it is equally crucial to recognise that the shortage of clinical trial evidence supporting MHTN treatment could potentially lead to these events and increase the mortality rates. Given that MHTN has the potential to serve as a predictive indicator for unfavourable cerebrovascular events as well as mortality, it is essential to conduct clinical studies to explore the need to administer antihypertensive therapy for MHTN treatment.
MHTN can occur in hypertensive patients with normal clinic-based BP records. ABPM recording can detect this by providing out-of-office BP records during the day and night [Citation92]. The United States Preventative Services Task Force (USPSTF) and the 2017 and 2023 ACC-AHA guidelines recommend out-of-clinic BP recording to recognise HTN types before treatment [Citation93,Citation94]. The USPSTF recommendation regarding HTN focused solely on concerns about the overtreatment of WCHTN and did not address the potential risks associated with MHTN.
There is not much data on treating MHTN risks and benefits available. Ongoing trials are evaluating the safety and effectiveness of MHTN treatment. A prospective 4-year study of treated HTN patients (MASTER trial) evaluated the effects of in-clinic or ambulatory BP recording-based therapy on cardiac and renal damage. To assess outcomes, they included changes in LVM and microalbuminuria during the first year of follow-up, impact on CV events after four years, and any changes in variables related to BP [Citation95]. To our knowledge, MASTER study results have not yet been published.
There is strong evidence that MHTN increases end-organ damage risk and renal-associated morbidity [Citation16, Citation37, Citation96], with an overall risk approaching that of sustained hypertension [Citation37]. Therefore, one can make a compelling argument for initiating antihypertensive therapy when the diagnosis of MHTN has been substantiated. However, randomised controlled trials are lacking to assess the optimal daytime and night-time BP reduction levels to weigh therapeutic benefits versus risks. There are still some unanswered questions: 1) Is there a risk if in-office BP is further reduced in patients with MHTN, considering that it is already within the guidelines? 2) Will active therapy similarly reduce hypertensive end-organ damage in MHTN patients as in SHTN?
It is unclear whether the definition of MHTN should be limited to naive patients; the European Society of Hypertension-European Society of Cardiology guidelines for HTN management favour this approach [Citation16,Citation97]. However, the increase in the incidence of adverse events among patients with MHTN is similar between treated and untreated patients [Citation98]. Thus, the increase in adverse end-organ injury risk associated with MHTN is present regardless of the treatment status [Citation98], suggesting similarities between treated and untreated masked hypertensives. However, masked uncontrolled hypertension may exhibit different clinical characteristics.
It is generally acknowledged that there are three primary reasons for treating any HTN type, especially SHTN: reducing harm to end-organs, reducing risk, and preventing complications [Citation99]. Data indicate that MHTN patients have higher end-organ injury than NTs, and a risk profile similar to that of SHTN patients [Citation16,Citation99]. The hazards are primarily related to identifying patients at high risk for MHTN and MHTN diagnosis reproducibility and relative reliability [Citation99]. However, the central concern is whether the benefit of active medication treatment in reducing hypertensive target organ injury and events will be comparable in patients with MHTN [Citation99].
In MHTN, pharmacological treatment must be conducted according to European Guidelines [Citation16,Citation97]. When MHTN is identified, lifestyle modifications with/without antihypertensive medication therapy must be considered because MHTN has a risk similar to that of in-office and out-of-office SHTN [Citation16,Citation97]. Attention to dysmetabolic abnormalities in lipid and glucose levels, risk factors, and organ injury is mandatory during treatment decisions and follow-up, as these conditions are much more prevalent in MHTN than in NT individuals [Citation16,Citation97]. Treatment with antihypertensive agents is similar to that with SHT therapy regimens. The best-proposed algorithm for diagnosis and treatment of MHTN we found is the algorithm proposed by Ogedegbe et al. [Citation99]. The efficacy of antihypertensive therapy should be evaluated using ABPM and HBP recordings rather than in-clinic Bp measurement [Citation16,Citation97].
Treating MHTN with short or intermediate doses (atenolol, metoprolol, or clonidine) is preferable, while long-acting beta-blockers are not advisable, especially at night. This timing allows for the modulation of carotid baroreceptor function, aiding the management of BP while the patient is supine. When the patient assumes an upright position in the morning, the adverse effects of these medications diminish.
MHTN therapy provides a unique strategy based on a case series conducted at the American Society of Hypertension Comprehensive Centre [Citation100]. The mean age of the participants was 39 years, with a standard deviation of 5 years. Their average in-clinic BP records were 128 ± 11 mmHg systolic BP and 72 ± 6 mmHg diastolic BP, and they were not on antihypertensive medication. These individuals were diagnosed with symptomatic MHTN, which manifests as symptoms ranging from severe headaches to nosebleeds. These patients are better able to start their workdays with a precise dose of a short-acting calcium channel blocker to preserve their cognitive skills. The short-acting calcium channel blockers were either verapamil (80 mg) or diltiazem SR (180 mg), chosen for their selective impact on sympathetic tone. The therapies were highly tolerable, resulting in significant relief of patient symptoms. Additionally, a noticeable decrease in BP was observed within two weeks. Furthermore, it was observed that the first ABPM results showed a reduction in heart rate by an average of 12 ± 6 beats/min, which can reduce cardiac output and BP.
Potential alternatives to the practice of shorting non-dihydropyridine calcium channel blockers include the use of short- or intermediate-acting alpha-beta blockers (low dosage of carvedilol (12.5 mg) or atenolol (25-50 mg)) before exposure to established external environmental triggers. It is reasonable to refrain from using lipophilic beta-blockers because of their potential to induce sleeplessness. Long-acting antihypertensive medications should be avoided owing to the normalisation of BP in non-stressful environments, which may lead to a higher occurrence of undesirable effects and falls, especially in older individuals.
The therapy of an alternative aetiology of MHTN, namely those already on antihypertensive medication, yet with elevated BP records outside of clinical settings at various time intervals (day and night), necessitates a distinct therapeutic strategy. These individuals may have elevated BP during the day or night because of inadequate sleep. This group of patients exhibits higher BP fluctuations and requires the administration of medicines that suppress sympathetic tone and regulate volume. The treatment strategy for these individuals varies based on the patient’s characteristics and underlying aetiology of MHTN.
In several instances, the use of sleep aids or the promotion of uninterrupted sleep is crucial. To address these circumstances, it is necessary to provide increased dosages or implement add-on treatment using antihypertensive agents. Attention should be paid to sleep BP levels in individuals with CKD or sleep obstructive apnoea with the introduction of continuous positive airway pressure (CPAP), especially at night. The impact of CPAP therapy on patients with obstructive sleep apnoea had an average decrease in BP from 3 to 5 mmHg, with some cases reporting a decrease of 18 mmHg [Citation101].
The results of clinical studies showed that combination treatment, including calcium channel blockers and renin-angiotensin system inhibitors, is effective in decreasing sleep BP, BP variability, central BP, CKD progression, and CVD morbidity and mortality in hypertensive patients who are at high risk [Citation102,Citation103]. Antihypertensive medications at bedtime have shown efficacy in decreasing nocturnal BP and CV events in some individuals with early CKD [Citation104,Citation105]. The effectiveness of aldosterone blockers in obese individuals with or without obstructive sleep apnoea is evident in regularly reducing BP [Citation106]. The precise mechanism by which aldosterone blockers operate in this context remains unclear, but is likely associated with the promotion of aldosterone synthesis by adipocytes [Citation107,Citation108]. Given the high prevalence of obesity among individuals with sleep apnoea, it is logical to consider using these medicines to mitigate the effects of spironolactone [Citation109]. Diuretics play a significant role in managing MHTN, mainly when elevated salt levels contribute to increased BP during the day and night [Citation110].
Therefore, It is essential to highlight that there is presently insufficient evidence to recommend specific drug classes for use in treating MHTN in untreated subjects. Instead, treatment decisions should be based on the patients’ clinical characteristics, their 24-hour ambulatory BP profile, and any comorbidities. For MHTN in treated subjects, the approach may vary, with the current treatment regimen needing modification.
Masked uncontrolled hypertension
To achieve optimal BP control, healthcare professionals must have a deep understanding of MUHTN. MUHTN occurs when office BP is controlled (<130/80 mmHg), but out-of-clinic BP is uncontrolled (awake daytime ABP ≥130/80 mmHg) despite the use of antihypertensive medication use [Citation94,Citation111]. Prior studies have revealed that 30–50% of treated HTNs have MUHTN [Citation25,Citation49], and the According to the 2018 guidelines from ACC/AHA and ESH/ESC, MUCH prevalence of was estimated to be 66% [Citation112].
MUHTN is associated with dyslipidemia, a family history of stroke, and LVH. However, better outcomes can be achieved with diligent care and attention. Of the 14,840 individuals with treated HTN in a Spanish registry, 31.1% had MUHTN [Citation49]. The prevalence of this condition was higher in men, those with borderline in-clinic BP records, and those who smoked, had diabetes or were obese [Citation49]. Another study showed that of 803 individuals with an average age of 60 years who had high in-clinic managed BP, 32.1% exhibited MUHTN [Citation113]. In some individuals, MUHTN was attributed to elevated night-time BP in 22.3% of cases, whereas inadequate daytime BP elevation was observed in 10.1% of the cases. Furthermore, Agarwal et al. noted that MUHTN's prevalence is influenced by its definitions. By employing the conventional definition of MUCH, characterised by clinic BP <140/90 mmHg and daytime ambulatory BP ≥135/85 mmHg, 70 (27%) out of 260 HTN participants had MUCH. Upon further follow up visits, MUCH's prevalence was determined to be 28%, and that of well-controlled hypertension was 72% (n = 177) [Citation25]. In contrast to the above recalled definition, utilising the 24-hour ABPM threshold of ≥130/80 mmHg to classify hypertension resulted in a higher prevalence of MUCH, at 33% during the initial visit and 37% during the repeat visit. Additionally, applying the most lenient definition for daytime or night-time hypertension raised MUCH's prevalence to 56% and 57% at the baseline and repeat visits, respectively. When compared to ABPM-based definitions, home BP monitoring-based diagnosis revealed MUCH's prevalence to be roughly 46% on both occasions [Citation25]. A significant correlation was found between MUHTN and other factors, including male sex, longer HTN duration, obesity, smoking, and diabetes mellitus [Citation113]. The increased risk of MUHTN differed by ethnicity, with a lower risk in Asian-only cohorts and greater risk in predominantly black cohorts [Citation14]. MUHTN prevalence of MUCH is higher in the elderly [Citation114], Blacks [Citation81], and in CKD patients [Citation64].
MUCH increases the risk of sustained HTN development [Citation115], and CV complications. Compared with patients with controlled HTN, those with MUCH were at an increased risk of CV events and all-cause mortality. The results were consistent across subgroups by BP measurement method (ambulatory versus home), length of follow-up, and type of cardiovascular event [Citation116]. Prior research has shown a considerable increase in the potential for CV complications in individuals with uncontrolled HTN compared to those with well-managed HTN [Citation40,Citation111]. Although the significance of MUHTN as a prognostic indicator is yet to be determined, its relationship with MHTN suggests a heightened risk of complications.
Detection of MUHTN in outpatient in-clinic settings can be daunting without an efficient and widely used detection method. Therefore, developing a screening tool for MUHTN that relies on easily accessible clinical features would benefit the daily practice. A study analysed the clinical characteristics of MUHTN, identified significant predictors of its presence, and created a prediction model for MUHTN in patients undergoing antihypertensive treatment [Citation117]. A tool developed by a group of Korean researchers sheds light on patients taking antihypertensive medications. Their study found that over 50% of the patients who had controlled BP during the in-clinic recording had MUHTN. Patients at a higher risk of MUHTN included those with suboptimal in-clinic systolic and DBP records, those without HTN therapy, those with dyslipidemia, LVH, an elevated heart rate, or a family history of stroke. Kim and colleagues created a model that accurately predicted the presence of MUHTN in an outpatient clinic using these clinical predictors [Citation117]. Interestingly, 45.6% of HTN-treated individuals had MUHTN, which could be mistakenly perceived as reasonable control by masking. Additionally, borderline in-clinic systolic and DBP ranges were independently associated with a significantly higher risk of MUHTN. Ultimately, the proposed predictive model could serve as a quick screening tool for MUHTN before patients undergo 24-hour ABPM recording [Citation117].
It is worth remembering the 2017 AHA/ACC guidelines for the prevention, detection, evaluation, and treatment of adult HTN [Citation94]. To confirm the diagnosis of HTN, AHA/ACC strongly recommends (class I) the use of out-of-office BP records. They provided a moderate recommendation (IIa) for evaluating white-coat hypertension in both untreated and treated patients using out-of-office BP measurements. In untreated MHTNs, the task force recommended that the out-of-office BP record be screened for MHTN, which was moderate (IIa); however, in treated patients, it was feeble (IIb). As all the evidence supporting out-of-office BP records is based on observational data, a meta-analysis of the current issue of hypertension may suffice to upgrade the recommendation for out-of-office BP records in treated MHTNs with controlled in-clinic BP from mild to moderate. The second recommendation related to the meta-analysis findings is from the United States Preventive Services Task Force [Citation93]. The United States Preventative Services Task Force recommends obtaining BP records outside the clinical setting for diagnostic confirmation before commencing treatment and favours ABBP over HBP records. The recommendation is based exclusively on epidemiological data related to WCHTN and disregards the issue of MHTN and its elevated risk of adverse outcomes.
Masked and masked uncontrol hypertension reproducibility
MUCH diagnosis reproducibility was evaluated in 50 untreated participants with borderline HTN [Citation42]. They had daytime ABP ≥135/85 mmHg, which was utilised to define HTN. At their first visit, 54% of the participants had MHTN, which decreased to 53% one week later. These high rates may be attributed to the participants’ high baseline BP because most patients already had borderline HTN. Daytime ABP was successfully classified in 73% of the cases [Citation42]. A retrospective study involving 80% of participants who were being managed and 13% of whom had MUCH revealed a κ statistic of only 0.26 after a mean follow-up period of 1.5 years of follow up [Citation118]. It is important to note that the treatment for HTN could change, and the time passed between BP measurements was variable, with a standard deviation of 1.5 years. In contrast, among 503 untreated hypertensive Japanese participants, the κ coefficient for agreement was 0.58 at 6 months, when morning home BP was used as a standard for out-of-office BP measurement [Citation119].
Extensive research has been conducted to assess MUCH reproducibility after four weeks. There was a high participation rate of 295 individuals completing the initial visits and 274 completing eight weeks [Citation25]. The results of et al. indicated agreement ranging from 75% to 78% and a κ coefficient of 0.44-0.51, which was comparable to that of MHTN in a group of people who had untreated borderline HTN. They concluded that their findings suggest that concordance in diagnosing MUCH is not just a statistical phenomenon but may also have a biological basis [Citation25].
The reproducibility of MHTN is moderate when using various methods for measuring BP, such as 24-hour, sleeping, or office BP [Citation120]. The results were consistent regardless of whether office BP measurement was performed using an auscultatory method with a mercury sphygmomanometer or a validated oscillometric device. Moderate short-term reproducibility was found for MHTN diagnosis when using awake BP, as recommended by the 2017 American College of Cardiology/American Heart Association BP Hypertension Clinical Practice Guidelines, as well as when using 24-hour and asleep BP alone or in combination with awake BP. When diagnosing MHTN in individuals who do not have a high mean office BP throughout two office visits, clinicians must consider the clinical context while interpreting the single ABP monitoring session results in short term [Citation121].
In a systematic review, 15 studies were found to be appropriate for meta-analysis including 5729 patients [Citation120]. MHTN reproducibility was better with ABP than with HBP recording. The office-based HTN reproducibility of the two methods was low, suggesting slight concordance. Kappa’s reliability was marginally better with ABP than HBP recording. They also showed insignificant reproducibility of MHTN and office-based HTN assessed through HBP and ABP measurements. Given that office BP measurements may lead to poor reproducibility, an ABP/HBP measurement-based strategy should be implemented to assess and treat patients with MHTN or office-based HTN [Citation120].
Masked Phenomenon effects outcome
The prediction and persistence of MHTN are moderately accurate when patients with prehypertension are screened using a conventional in-clinic BP recording method [Citation42]. Researchers initially believed that MHTN was a precursor of SHTN [Citation122]. Nevertheless, a 5-year survey evaluated the persistence of MHTN and its progression to SHTN [Citation123]. Over 50% of patients with MHTN at baseline may develop SHTN at five years. One-third of these patients progressed to SHTN, the other third regressed to normotension, and one-fifth had MHTN > 5 years when untreated. The high prevalence of hypertensive target organ injury may be attributable to delayed MHTN diagnosis [Citation2,Citation124].
Several studies have demonstrated that MHTN is associated with CV, renal events, and all-cause mortality. In an analysis of 63,910 patients with ABPM recordings, the risk for all-cause death was higher in MHTN and MUHTN patients than in normotensive patients. Similar results concerning CV mortality were observed [Citation3]. In the Jackson Heart Study, MHTN was present in 53% of participants, was related to CKD onset [Citation62], and may lead to progression to ESRD. Few studies have evaluated the association between MHTN and adverse outcomes in CKD patients. Kushiro et al. investigated the association between morning home SBP and clinic SBP and CV risk in hypertensive patients with or without CKD receiving olmesartan-based antihypertensive therapy, using data from the Home Blood Pressure-Guided Antihypertensive Therapy Requires a Randomised Trial (HONEST) [Citation125]. CKD patients had a higher CV event rate than non-CKD patients. MHTN increases the risk of major CV events in patients with or without CKD [Citation125]. Two studies evaluated the association between MHTN and adverse clinical events in CKD patients. An association between MHTN and adverse clinical event evaluation in a cohort of 489 hypertensive patients with CKD showed that 15% had MHTN. Over a follow-up median of 5.2 years, MHTN patients had an increased CV risk for a composite of deadly and non-deadly myocardial infarction, stroke, congestive heart failure, peripheral vascular disease, and non-traumatic amputation [Citation56]. Patients with MHTN also have an increased risk of ESRD and death [Citation56]. Similar results were reported in patients with CKD in China [Citation84]. Comparing MHTN subjects with NTs, MHTN subjects were at an increased risk of death, renal events, and major adverse CV events [Citation84]. Home and clinic BPs were measured in a prospective cohort study of Veterans with CKD over a median follow-up of 3.5 years [Citation126]. One standard deviation rise in home SBP was accompanied by an increased risk for ESRD in a model adjusted for clinic SBP and other risk factors [Citation126].
Similarly, elevated ABPM records were accompanied by an increased ESRD risk after adjusting for systolic in-clinic BP, but the association was not significant after adjusting for proteinuria and the decline in eGFR [Citation126]. Moreover, the same study illustrated that 24-hour ABPM recording, but neither clinic nor HBP recording, was related to CV poor outcome risk [Citation126]. The AASK study linked systolic ABPM records, in-clinic SBP, and renal and CV events [Citation55]. However, after controlling for clinic BP, elevated ambulatory BP was only associated with poor renal outcomes in participants with clinic SBP < 130 mmHg (p < 0.05) [Citation55]. These studies noted that raised HBP and ABPM records cause renal and CV damage independent of clinical BP records in CKD. summarises the outcome of MHTN and MUHTN.
Table 2. Summary of comparison between masked/masked uncontrol hypertension outcomes.
Masked phenomenon treatment dilemma
Despite the recommendations of the AHA/ACC and United States Preventive Services Task Force, there are still areas of uncertainty. All the main HTN clinical trials have utilised in-clinic BP records to determine eligibility and monitor treatment effects. In standard and intensive treatment groups of treat-to-target investigations, in-clinic BP has guided therapy. In addition, patients with MHTN were excluded from HTN clinical trials, and patients with MUHTN did not have their antihypertensive medications intensified during the maintenance or treat-to-target phases of these trials. The meta-analysis included only observational studies and was therefore unable to ascertain whether treatment modified the MUHTN phenotype or reduced the risk of adverse outcomes in patients with MUHTN. As the categorisation was based on a single set of measurements, the current meta-analysis did not address the reproducibility of MUHTN. The meta-analysis also used ambulatory daytime BP to define MUHTN; categorisation using 24-hour or night-time BP may identify a higher-risk group of patients, given the significant association between elevated nocturnal BP and adverse outcomes. If out-of-office BP records are to be used to guide therapy, greater comprehension of the optimal approach to maximise adherence and minimise the burden on patients, providers, and the healthcare system is required.
Clinical trials evaluating therapies based on clinical versus ABPM or HBP records are urgently required. The ongoing MASTER trial (http://www.clinicaltrials.gov; identifier: NCT02804074) will enrol patients with MUHTN. This 12-month study will compare the effects of in-clinic versus ABPM antihypertensive therapy on LVMi and urine albumin excretion. This study will provide information on the feasibility, safety, and efficacy of treating patients with MHTN and inform the design of more extensive outcome trials necessary to determine the high risk of adverse outcomes identified by Pierdomenico et al. [Citation111]
A double-blind placebo-controlled RCT examined the effects of fixed-dose spironolactone (25 mg daily) in 115 untreated people without HTN but with a hypertensive response to exercise [Citation127]. The spironolactone group had significantly greater reductions in exercise-induced systolic BP (p < 0.01) and 24 h ambulatory pulse pressure (p < 0.05) among 40% of participants with MH by daytime ABPM (cut-off 135/85 mmHg). Three months of spironolactone and placebo showed no difference in the LVMI decrease.
In summary, in patients with MUHTN is modifiable. In the interim, it is reasonable but not required to base the use of antihypertensives on outpatient records. The fact that MP exists; however, treatment decisions are still controversial. Therefore, further discussion, recommendations, and trials are required to address this issue.
Conclusions
MHTN is an established phenotype of HTN. It is crucial to understand MHTN/MUHTN diagnosis, screening methods, factors, mechanisms, management, and end-organ effects. End-organ injuries are influenced by MHTN and MUHTN, inducing CKD onset and progression and increasing CV events. MHTN is a significant risk factor for SHTN, and relying solely on in-clinic recordings delays its detection. If in-clinic BP recordings are the only basis for treatment, 30% of patients with MHTN will be undertreated. Therefore, HBP and ABPM recordings are indicated in patients with suspected MP and in those who have associated risk factors, such as diabetes, smoking, and obesity. To improve MP control rates worldwide, it is necessary to support conventional in-clinic BP recording using the HBP and ABPM methods. The dilemma of treating or not treating MP is a primary concern and needs an answer. To address this issue, national and international guidelines, further discussions, and clinical trials should be conducted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fouad DA, Al Araby HH, Ashraf M, et al. Comparison between central and ambulatory blood pressure measurements in early detection of end-organ damage: a single-center prospective non-randomized controlled trial. Egypt Heart J. 2019;71(1):14. doi: 10.1186/s43044-019-0013-3.
- Hänninen MR, Niiranen TJ, Puukka PJ, et al. Target organ damage and masked hypertension in the general population: the Finn-Home study. J Hypertens. 2013;31(6):1136–1143. doi: 10.1097/HJH.0b013e32835fa5dc.
- Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. 2020;382(8):786–786. doi: 10.1056/NEJMc2001445.
- Pickering TG, Davidson K, Gerin W, et al. Masked hypertension. Hypertension. 2002;40(6):795–796. doi: 10.1161/01.hyp.0000038733.08436.98.
- Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29(1):102–111. doi: 10.1016/j.hlc.2019.08.006.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25(11):2193–2198. doi: 10.1097/HJH.0b013e3282ef6185.
- Aronow WS. Masked hypertension. Ann Transl Med. 2017;5(23):456–456. doi: 10.21037/atm.2017.09.24.
- Asayama K, Thijs L, Li Y, et al. Setting thresholds to varying blood pressure monitoring intervals differentially affects risk estimates associated with white-coat and masked hypertension in the population. Hypertension. 2014;64(5):935–942. doi: 10.1161/HYPERTENSIONAHA.114.03614.
- Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21(9):969–975. doi: 10.1038/ajh.2008.221.
- Iimuro S, Imai E, Watanabe T, et al. Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol. 2013;8(5):721–730. doi: 10.2215/CJN.06470612.
- Drawz PE, Alper AB, Anderson AH, et al. Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016;11(4):642–652. doi: 10.2215/CJN.08530815.
- Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20–27. doi: 10.1161/HYPERTENSIONAHA.108.
- Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2009;4(3):656–664. doi: 10.2215/CJN.05391008.
- Drawz PE, Brown R, De Nicola L, et al. Variations in 24-hour BP profiles in cohorts of patients with kidney disease around the World: the I-DARE study. Clin J Am Soc Nephrol. 2018;13(9):1348–1357. doi: 10.2215/CJN.13181117.
- Angeli F, Reboldi G, Verdecchia P. Masked hypertension: evaluation, prognosis, and treatment. Am J Hypertens. 2010;23(9):941–948. doi: 10.1038/ajh.2010.112.
- Mancia G, Kreutz R, Brunström M,G, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874–2071. doi: 10.1097/HJH.0000000000003480.
- Omboni S, Aristizabal D, De la Sierra A, et al. Hypertension types defined by clinic and ambulatory blood pressure in 14 143 patients referred to hypertension clinics worldwide. Data from the ARTEMIS study. J Hypertens. 2016;34(11):2187–2198. doi: 10.1097/HJH.0000000000001074.
- Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24(2):123–134. doi: 10.1038/ajh.2010.194.
- Fukuhara M, Arima H, Ninomiya T, et al. White-coat and masked hypertension are associated with carotid atherosclerosis in a general population: the Hisayama study. Stroke. 2013;44(6):1512–1517. doi: 10.1161/STROKEAHA.111.000704.
- Andalib A, Akhtari S, Rigal R, et al. Determinants of masked hypertension in hypertensive patients treated in a primary care setting. Intern Med J. 2012;42(3):260–266. doi: 10.1111/j.1445-5994.2010.02407.x.
- Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with nonelevated clinic blood pressure. Am J Epidemiol. 2017;185(3):194–202. doi: 10.1093/aje/kww237.
- Alwan H, Pruijm M, Ponte B, et al. Epidemiology of masked and white-coat hypertension: the family-based SKIPOGH study. PLoS One. 2014;9(3):e92522. doi: 10.1371/journal.pone.0092522.
- Palatini P. Masked hypertension: how can the condition be detected? Blood Press Monit. 2004;9(6):297–299. doi: 10.1097/00126097-200412000-00005.
- Verberk WJ, Thien T, de Leeuw PW. Masked hypertension, a review of the literature. Blood Press Monit. 2007;12(4):267–273. doi: 10.1097/mbp.0b013e3280fb2792.
- Agarwal R, Pappas MK, Sinha AD. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol. 2016;27(3):924–932. doi: 10.1681/ASN.2015030243.
- Franklin SS, Thijs L, Li Y, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61(5):964–971. doi: 10.1161/HYPERTENSIONAHA.111.00289.
- Hwang ES, Choi KJ, Kang DH, et al. Prevalence, predictive factor, and clinical significance of white-coat hypertension and masked hypertension in Korean hypertensive patients. Korean J Intern Med. 2007;22(4):256–262. doi: 10.3904/kjim.2007.22.4.256.
- Penmatsa KR, Biyani M, Gupta A. Masked hypertension: lessons for the future. Ulster Med J. 2020;89(2):77–82.
- Pickering TG, Eguchi K, Kario K. Masked hypertension: a review. Hypertens Res. 2007;30(6):479–488. doi: 10.1291/hypres.30.479.
- Tabara Y, Okada Y, Uetani E, et al. Postprandial hypotension as a risk marker for asymptomatic lacunar infarction. J Hypertens. 2014;32(5):1084–1090; discussion 1090. doi: 10.1097/HJH.0000000000000150.
- Kayrak M, Bacaksiz A, Vatankulu MA, et al. Exaggerated blood pressure response to exercise–a new portent of masked hypertension. Clin Exp Hypertens. 2010;32(8):560–568. doi: 10.3109/10641963.2010.503298.
- Trudel X, Brisson C, Milot A. Job strain and masked hypertension. Psychosom Med. 2010;72(8):786–793. doi: 10.1097/PSY.0b013e3181eaf327.
- Landsbergis PA, Dobson M, Koutsouras G, et al. Job strain and ambulatory blood pressure: a meta-analysis and systematic review. Am J Public Health. 2013;103(3):e61-71–e71. doi: 10.2105/AJPH.2012.301153.
- Seki M, Inoue R, Ohkubo T, et al. Association of environmental tobacco smoke exposure with elevated home blood pressure in Japanese women: the Ohasama study. J Hypertens. 2010;28(9):1814–1820. doi: 10.1097/HJH.0b013e32833a3911.
- Schultz MG, Hare JL, Marwick TH, et al. Masked hypertension is "unmasked" by low-intensity exercise blood pressure. Blood Press. 2011;20(5):284–289. doi: 10.3109/08037051.2011.566251.
- Li Y, Wang JG. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61(2):278–283. doi: 10.1161/HYPERTENSIONAHA.111.00217.
- Franklin SS, O'Brien E, Thijs L, et al. Masked hypertension: a phenomenon of measurement. Hypertension. 2015;65(1):16–20. doi: 10.1161/HYPERTENSIONAHA.114.04522.
- O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–1768. doi: 10.1097/HJH.0b013e328363e964.
- Schiffrin EL, Calhoun DA, Flack JM. Do we need a new definition of hypertension after SPRINT? Am J Hypertens. 2016;29(10):1127–1129. doi: 10.1093/ajh/hpw06.
- Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white-coat and masked hypertension. International database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2014;63(4):675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741.
- Stergiou GS, Salgami EV, Tzamouranis DG, et al. Masked hypertension assessed by ambulatory blood pressure versus home blood pressure monitoring: is it the same phenomenon? Am J Hypertens. 2005;18(6):772–778. doi: 10.1016/j.amjhyper.2005.01.003.
- Viera AJ, Hinderliter AL, Kshirsagar AV, et al. Reproducibility of masked hypertension in adults with untreated borderline office blood pressure: comparison of ambulatory and home monitoring. Am J Hypertens. 2010;23(11):1190–1197. doi: 10.1038/ajh.2010.158.
- Campbell NR, McKay DW. Accurate blood pressure measurement: why does it matter? CMAJ. 1999;161(3):277–278.
- Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35(7):695–701. doi: 10.1038/hr.2012.26.
- Yano Y, Hoshide S, Shimizu M, et al. Association of home and ambulatory blood pressure changes with changes in cardiovascular biomarkers during antihypertensive treatment. Am J Hypertens. 2012;25(3):306–312. doi: 10.1038/ajh.2011.229.
- Pickering TG, Miller NH, Ogedegbe G, et al. American Heart Association; American Society of Hypertension; Preventive Cardiovascular Nurses Association Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):1–9. doi: 10.1161/HYPERTENSIONAHA.107.189010.
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4(2):56–61. doi: 10.1016/j.jash.2010.03.003.
- Mallion JM, Genès N, Vaur L, et al. Detection of masked hypertension by home blood pressure measurement: is the number of measurements an important issue? Blood Press Monit. 2004;9(6):301–305. doi: 10.1097/00126097-200412000-00006.
- Banegas JR, Ruilope LM, de la Sierra A, et al. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35(46):3304–3312. doi: 10.1093/eurheartj/ehu016.
- Bromfield SG, Shimbo D, Booth JN, 3rd, et al. Cardiovascular risk factors and masked hypertension: the Jackson Heart Study. Hypertension. 2016;68(6):1475–1482. doi: 10.1161/HYPERTENSIONAHA.116.08308.
- Liu JE, Roman MJ, Pini R, et al. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131(8):564–572. doi: 10.7326/0003-4819-131-8-1999101.
- Babu M, Drawz P. Masked hpertension in CKD: increased prevalence and risk for cardiovascular and renal events. Curr Cardiol Rep. 2019;21(7):58. doi: 10.1007/s11886-019-1154-4.
- Wijkman M, Länne T, Engvall J, et al. Masked nocturnal hypertension–a novel marker of risk in type 2 diabetes. Diabetologia. 2009;52(7):1258–1264. doi: 10.1007/s00125-009-1369-9.
- Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of "masked hypertension" detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291(11):1342–1349. doi: 10.1001/jama.291.1.
- Gabbai FB, Rahman M, Hu B, et al. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7(11):1770–1776. doi: 10.2215/CJN.11301111.
- Minutolo R, Gabbai FB, Agarwal R, et al. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: a multicenter prospective cohort study. Am J Kidney Dis. 2014;64(5):744–752. doi: 10.1053/j.ajkd.2014.06.014.
- Fukuda M, Munemura M, Usami T, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65(2):621–625. doi: 10.1111/j.1523-1755.2004.00419.
- Paoletti E, Bellino D, Amidone M, et al. Relationship between arterial hypertension and renal damage in chronic kidney disease: insights from ABPM. J Nephrol. 2006;19(6):778–782.
- Li X, Lian R, Zhu Y, et al. Masked morning hypertension correlated with target organ damage in nondialysis patients with chronic kidney disease. J Hypertens. 2020;38(9):1794–1801. doi: 10.1097/HJH.0000000000002461.
- Af Geijerstam P, Engvall J, Östgren CJ, et al. Masked hypertension in a middle-aged population and its relation to manifestations of vascular disease. J Hypertens. 2023;41(7):1084–1091. doi: 10.1097/HJH.0000000000003431.
- Drawz PE, Pajewski NM, Bates JT, et al. Effect of intensive versus standard clinic-based hypertension management on ambulatory blood pressure: results from the SPRINT (Systolic Blood Pressure Intervention Trial) Ambulatory Blood Pressure Study. Hypertension. 2017;69(1):42–50. doi: 10.1161/HYPERTENSIONAHA.116.08076.
- Mwasongwe S, Min YI, Booth JN, 3rd, et al. Masked hypertension and kidney function decline: the Jackson Heart Study. J Hypertens. 2018;36(7):1524–1532. doi: 10.1097/hjh.0000000000001727.
- Wu Y, Zhang G, Hu R, et al. Risk of target organ damage in patients with masked hypertension versus sustained hypertension: a meta-analysis. CVIA. 2021;5(3):155–163. doi: 10.15212/CVIA.2019.1261.
- Agarwal R, Pappas MK. Delayed systolic blood pressure recovery following exercise as a mechanism of masked uncontrolled hypertension in chronic kidney disease. Nephrol Dial Transplant. 2017;32(10):1710–1717. doi: 10.1093/ndt/gfw266.
- Agarwal R, Light RP. GFR, proteinuria and circadian blood pressure. Nephrol Dial Transplant. 2009;24(8):2400–2406. doi: 10.1093/ndt/gfp074.
- Agarwal R, Sinha AD, Light RP. Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(8):2003–2008. doi: 10.2215/CJN.02700311.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061.
- Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in "white coat hypertension" and "masked hypertension. Am J Hypertens. 2008;21(4):393–399. doi: 10.1038/ajh.2008.15.
- Matsui Y, Eguchi K, Ishikawa J, et al. Subclinical arterial damage in untreated masked hypertensive subjects detected by home blood pressure measurement. Am J Hypertens. 2007;20(4):385–391. doi: 10.1016/j.amjhyper.2006.10.008.
- Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population [PAMELA] Study. Circulation. 2001;104(12):1385–1392. doi: 10.1161/hc3701.096100.
- Bombelli M, Sega R, Facchetti R, et al. Prevalence and clinical significance of a greater ambulatory versus office blood pressure ("reversed white coat" condition) in a general population. J Hypertens. 2005;23(3):513–520. doi: 10.1097/01.hjh.0000160206.58781.07.
- Ormezzano O, Baguet JP, François P, et al. Is there any real target organ damage associated with white-coat normotension? Clin Auton Res. 2004;14(3):160–166. doi: 10.1007/s10286-004-0174-2.
- Björklund K, Lind L, Zethelius B, et al. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107(9):1297–1302. doi: 10.1161/01.cir.0000054622.45012.12.
- Cuspidi C, Meani S, Fusi V, et al. Isolated ambulatory hypertension and changes in target organ damage in treated hypertensive patients. J Hum Hypertens. 2005;19(6):471–477. doi: 10.1038/sj.jhh.1001850.
- Kuriyama S, Otsuka Y, Iida R, et al. Morning blood pressure predicts hypertensive organ damage in patients with renal diseases: effect of intensive antihypertensive therapy in patients with diabetic nephropathy. Intern Med. 2005;44(12):1239–1246. doi: 10.2169/internalmedicine.44.1239.
- Pierdomenico SD, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18(11):1422–1428. doi: 10.1016/j.amjhyper.2005.05.014.
- Tomiyama M, Horio T, Yoshii M, et al. Masked hypertension and target organ damage in treated hypertensive patients. Am J Hypertens. 2006;19(9):880–886. doi: 10.1016/j.amjhyper.2006.03.006.
- Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24(1):52–58. doi: 10.1038/ajh.2010.203.
- Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of "masked" hypertension and "white-coat" hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46(3):508–515. doi: 10.1016/j.jacc.2005.03.070.
- Tientcheu D, Ayers C, Das SR, et al. Target organ complications and cardiovascular events associated with masked hypertension and white-coat hypertension: analysis from the Dallas Heart Study. J Am Coll Cardiol. 2015;66(20):2159–2169. doi: 10.1016/j.jacc.2015.09.007.
- Diaz KM, Veerabhadrappa P, Brown MD, et al. Prevalence, determinants, and clinical significance of masked hypertension in a population-based sample of African Americans: the Jackson Heart Study. Am J Hypertens. 2015;28(7):900–908. doi: 10.1093/ajh/hpu241.
- Anstey DE, Booth JN, 3rd, Abdalla M, et al. Predicted atherosclerotic cardiovascular disease risk and masked hypertension among Blacks in the Jackson Heart Study. Circ Cardiovasc Qual Outcomes. 2017;10(7):e003421. doi: 10.1161/CIRCOUTCOMES.116.003421.
- Shimbo D, Newman JD, Schwartz JE. Masked hypertension and prehypertension: diagnostic overlap and interrelationships with left ventricular mass: the Masked Hypertension Study. Am J Hypertens. 2012;25(6):664–671. doi: 10.1038/ajh.2012.15.
- Wang C, Zhang J, Li Y, et al. Masked hypertension, rather than white-coat hypertension, has a prognostic role in patients with nondialysis chronic kidney disease. Int J Cardiol. 2017;230:33–39. doi: 10.1016/j.ijcard.2016.12.105.
- Lurbe E. Masked hypertension in children and adolescents. Curr Hypertens Rep. 2008;10(3):165–166. doi: 10.1007/s11906-008-0030-1.
- Stabouli S, Kotsis V, Toumanidis S, et al. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol. 2005;20(8):1151–1155. doi: 10.1007/s00467-005-1979-5.
- Lurbe E, Torro I, Alvarez V, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45(4):493–498. doi: 10.1161/01.HYP.0000160320.39303.ab.
- Goulas I, Evripidou K, Doundoulakis I, et al. Prevalence of masked hypertension and its association with left ventricular hypertrophy in children and young adults with chronic kidney disease: a systematic review and meta-analysis. J Hypertens. 2023;41(5):699–707. doi: 10.1097/HJH.0000000000003402.
- Satoh M, Asayama K, Kikuya M, et al. Long-term stroke risk due to partial white-coat or masked hypertension based on home and ambulatory blood pressure measurements: the Ohasama Study. Hypertension. 2016;67(1):48–55. doi: 10.1161/HYPERTENSIONAHA.
- Reboussin DM, Allen NB, Griswold ME, et al. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e116–e135. doi: 10.1161/HYP.0000000000000067.
- Peacock J, Diaz KM, Viera AJ, et al. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens. 2014;28(9):521–528. doi: 10.1038/jhh.2014.9.
- Drawz PE, Abdalla M, Rahman M. Blood pressure measurement: clinic, home, ambulatory, and beyond. Am J Kidney Dis. 2012;60(3):449–462. doi: 10.1053/j.ajkd.2012.01.026.
- Siu AL, U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–786. doi: 10.7326/M15-2223.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: executive summary: A Report of the American College of Cardiology/American Heart Association Task F. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066.
- Parati G, Agabiti-Rosei E, Bakris GL, et al. Masked-uncontrolled hypertension management based on office BP or on ambulatory blood pressure measurement (MASTER) Study: a randomised controlled trial protocol. BMJ Open. 2018;8(12):e021038. doi: 10.1136/bmjopen-2017-021038.
- Minutolo R, Agarwal R, Borrelli S, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171(12):1090–1098. doi: 10.1001/archinternmed.2011.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339.
- Kario K, Thijs L, Staessen JA. Blood pressure measurement and treatment decisions. Circ Res. 2019;124(7):990–1008. doi: 10.1161/CIRCRESAHA.118.313219.
- Ogedegbe G, Agyemang C, Ravenell JE. Masked hypertension: evidence of the need to treat. Curr Hypertens Rep. 2010;12(5):349–355. doi: 10.1007/s11906-010-0140-4.
- Yano Y, Bakris GL. Recognition and management of masked hypertension: a review and novel approach. J Am Soc Hypertens. 2013;7(3):244–252. doi: 10.1016/j.jash.2013.02.002.
- Guralnick A, Bakris GL. Approaches for targeting blood pressure control in sleep disorders. Curr Opin Nephrol Hypertens. 2012;21(5):469–474. doi: 10.1097/MNH.0b013e32835623f5.
- Palatini P, Winnicki M, Santonastaso M, et al. Prevalence and clinical significance of isolated ambulatory hypertension in young subjects screened for stage 1 hypertension. Hypertension. 2004;44(2):170–174. doi: 10.1161/01.HYP.00001352.
- Bakris GL, Sarafidis PA, Weir MR, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a.
- Bidani AK, Griffin KA, Epstein M. Hypertension and chronic kidney disease progression: why the suboptimal outcomes? Am J Med. 2012;125(11):1057–1062. doi: 10.1016/j.amjmed.2012.04.008.
- Rahman M, Greene T, Phillips RA, et al. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension. 2013; Jan61(1):82–88. doi: 10.1161/HYPERTENSIONAHA.112.200477.
- Kario K. Obstructive sleep apnea syndrome and hypertension: mechanism of the linkage and 24-h blood pressure control. Hypertens Res. 2009;32(7):537–541. doi: 10.1038/hr.2009.73.
- Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100(24):14211–14216. doi: 10.1073/pnas.2336140100.
- Dall’Asta C, Vedani P, Manunta P, et al. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis. 2009;19(2):110–114. doi: 10.1016/j.numecd.2008.06.001.
- Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52(22):1749–1757. doi: 10.1016/j.jacc.2008.08.036.
- Raheja P, Price A, Wang Z, et al. Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension. 2012;60(2):319–325. doi: 10.1161/HYPERTENSIONAHA.112.1947.
- Pierdomenico SD, Pierdomenico AM, Coccina F, et al. Prognostic value of masked uncontrolled hypertension. Hypertension. 2018;72(4):862–869. doi: 10.1161/HYPERTENSIONAHA.118.11499.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940.
- Naser N, Dzubur A, Durak A, et al. Blood pressure control in hypertensive patients, cardiovascular risk profile and the prevalence of Masked Uncontrolled Hypertension (MUCH). Med Arch. 2016;70(4):274–279. doi: 10.5455/medarh.2016.70.274-279.
- Drawz PE. Masked uncontrolled hypertension. Hypertension. 2018;72(4):843–845. doi: 10.1161/HYPERTENSIONAHA.118.11640.
- Lurbe E, Thijs L, Torro MI, et al. Sexual dimorphism in the transition from masked to sustained hypertension in healthy youths. Hypertension. 2013;62(2):410–414. doi: 10.1161/HYPERTENSIONAHA.113.01549.
- Cacciolati C, Tzourio C, Hanon O. Blood pressure variability in elderly persons with white-coat and masked hypertension compared to those with normotension and sustained hypertension. Am J Hypertens. 2013;26(3):367–372. doi: 10.1093/ajh/hps054.
- Kim HJ, Shin JH, Lee Y, et al. Clinical features and predictors of masked uncontrolled hypertension from the Korean Ambulatory Blood Pressure Monitoring Registry. Korean J Intern Med. 2021;36(5):1102–1114. doi: 10.3904/kjim.2020.
- Ben-Dov IZ, Ben-Arie L, Mekler J, et al. Reproducibility of white-coat and masked hypertension in ambulatory BP monitoring. Int J Cardiol. 2007;117(3):355–359. doi: 10.1016/j.ijcard.2006.04.088.
- Kawabe H, Saito I. Reproducibility of masked hypertension determined from morning and evening home blood pressure measurements over a 6-month period. Hypertens Res. 2007;30(9):845–851. doi: 10.1291/hypres.30.845.
- Antza C, Farmakis I, Doundoulakis I, et al. Reproducibility of masked hypertension and office-based hypertension: a systematic review and meta-analysis. J Hypertens. 2022;40(6):1053–1059. doi: 10.1097/HJH.0000000000003111.
- Cohen LP, Schwartz JE, Pugliese DN, et al. Short-term reproducibility of masked hypertension among adults without office hypertension. Hypertension. 2020;76(4):1169–1175. doi: 10.1161/HYPERTENSIONAHA.120.15287.
- Pickering TG, Gerin W, Schwartz JE, et al. Should doctors still measure blood pressure? The missing patients with masked hypertension. J Hypertens. 2008;26(12):2259–2267. doi: 10.1097/HJH.0b013e32831313c4.
- Trudel X, Milot A, Brisson C. Persistence and progression of masked hypertension: a 5-year prospective study. Int J Hypertens. 2013;2013:836387–836387. doi: 10.1155/2013/836387.
- de la Sierra A, Gorostidi M, Banegas JR, et al. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27(5):680–687. doi: 10.1093/ajh/hpt175.
- Kushiro T, Kario K, Saito I, et al. Increased cardiovascular risk of treated white coat and masked hypertension in patients with diabetes and chronic kidney disease: the HONEST study. Hypertens Res. 2017;40(1):87–95. doi: 10.1038/hr.2016.87.
- Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(2):406–411. doi: 10.1038/sj.ki.5000081.
- Hare JL, Sharman JE, Leano R, et al. Impact of spironolactone on vascular, myocardial, and functional parameters in untreated patients with a hypertensive response to exercise. Am J Hypertens. 2013;26(5):691–699. doi: 10.1093/ajh/hpt008.
