Abstract
Objectives. To investigate the efficacy and tolerability of valsartan (Val) 320 mg once daily (o.d.), Val/hydrochlorothiazide (HCTZ) 320/12.5 mg o.d. and Val/HCTZ 320/25 mg o.d. in patients with hypertension not adequately controlled by Val monotherapy. Methods. This double‐blind, active‐controlled, parallel‐group, randomized trial recruited patients ⩾18 years with mild‐to‐moderate essential hypertension, defined as mean sitting diastolic blood pressure (MSDBP) of ⩾95 mmHg and <110 mmHg without treatment. After washout, 3805 eligible patients received Val 320 mg o.d. single‐blind for 4 weeks. Subsequently, patients with MSDBP ⩾90 and <110 mmHg (n = 2702) were randomized to double‐blind treatment with Val 320 mg, Val/HCTZ 320/12.5 mg or Val/HCTZ 320/25 mg for 8 weeks. Mean changes in MSDBP and mean sitting systolic BP (MSSBP) from the start of the single‐blind period were analysed, as well as the proportion of responders (MSDBP <90 mmHg or ⩾10 mmHg decrease from the start of the double‐blind period). Tolerability and safety were also assessed. Results. Reductions in MSDBP and MSSBP were observed in all groups. Both combinations were associated with significantly greater reductions than monotherapy for MSDBP and MSSBP at Weeks 8 and 12 (all p<0.0001). Both combinations also resulted in significantly greater proportion of responders at study end (74.9% and 68.8% for Val/HCTZ 320/25 mg and Val/HCTZ 320/12.5 mg, respectively) than monotherapy (52.7%; both p<0.0001). In addition, a dose–response was observed with increasing dose of HCTZ with respect to MSSBP. All treatments were well tolerated. Conclusions. The combination of Val and HCTZ at doses of 320/12.5 mg and 320/25 mg increases antihypertensive efficacy in patients with mild‐to‐moderate hypertension inadequately controlled with Val 320 mg monotherapy, without compromising tolerability.
Introduction
Since the 1970s, data from population‐based, randomized, controlled clinical trials have demonstrated unequivocally the clinical benefits of blood pressure (BP) lowering for reducing overall cardiovascular (CV) risk in patients with hypertension Citation[1]. International guidelines recommend a target BP of <140/90 mmHg for most patients, with a lower target (<130/80 mmHg) in high‐risk patient populations, such as those with target organ damage, diabetes or renal disease Citation[2,3]. Aggressive treatment of hypertension to achieve these BP targets is crucial, given that each 20‐mmHg increment of systolic BP (SBP) and 10‐mmHg in diastolic BP (DBP) doubles the risk of CV disease across the range of 115/75 mg to 185/115 mmHg Citation[4].
Despite these guideline recommendations, BP targets are reached in less than 35% of patients with hypertension Citation[5,6]. Therefore, there is a clear need to optimize the use of effective and well‐tolerated antihypertensive regimens. To meet target BP, a more aggressive treatment approach to antihypertensive therapy is often warranted, involving high starting doses of monotherapy and the use of combinations in the management of those individuals with inadequately controlled hypertension Citation[2,3],Citation[7]. Combinations of antihypertensive drugs have been used since the importance of hypertension has been understood and widely treated. It is well established that many hypertensive patients require two or more antihypertensive drugs to control their BP adequately, and that combination therapy leads to a better control of hypertension Citation[8,9]. Antihypertensive combinations should ideally provide powerful BP‐lowering efficacy combined with additional cardioprotective benefits, without compromising tolerability. For example, the combination of an angiotensin II type 1 receptor blocker (ARB) with a diuretic combines reduction of the angiotensin II‐mediated sodium retention and vasoconstriction with the volume‐reducing benefits of the diuretic.
Valsartan (Val) is an orally active ARB used worldwide for the treatment of hypertension. Studies have shown that Val monotherapy effectively reduces SBP and DBP in a dose‐dependent manner without concomitant worsening of tolerability Citation[10]. Patients whose BP is not adequately controlled by Val monotherapy at doses up to 320 mg may be treated by adding a diuretic, such as hydrochlorothiazide (HCTZ). The combinations of Val at doses of 80 mg and 160 mg with HCTZ at doses of 12.5 mg and 25 mg have been shown to have an additive effect on lowering BP compared with each of the respective monotherapies Citation[11], including in patients inadequately controlled on Val monotherapy Citation[12].
This study was a pivotal, registration study done according to the “Note for guidance on clinical investigation of medical products in the treatment of hypertension” from the EMEA for second‐line therapy. The study was done to investigate whether there was a clinically relevant and statistically significant superiority of Val/HCTZ combination therapy compared with Val monotherapy in patients not responding to monotherapy. The highest dose of Val available (320 mg) was used, as in some patients further BP reductions may be required in addition to those achieved from the highest dose of monotherapy.
In this study, the antihypertensive efficacy and tolerability of high doses of Val 320 mg once daily (o.d.), Val/HCTZ 320/12.5 mg o.d. and Val/HCTZ 320/25 mg o.d. was assessed in patients with mild‐to‐moderate hypertension not adequately controlled by 4 weeks' monotherapy with Val 320 mg o.d.
Patients and methods
Study population
Patients were recruited at 237 centres in 17 countries across Europe, North America, South America and South Africa. Patients screened were 18–80 years old, with mild‐to‐moderate essential hypertension, defined as mean sitting DBP (MSDBP) of ⩾95 mmHg and <110 mmHg without treatment. Patients with severe or secondary hypertension, hypertensive retinopathy, significant cardiac, renal or hepatic disease, type 1 diabetes or poorly controlled type 2 diabetes, or malignancy, were excluded, as were pregnant or lactating women and women of childbearing age not using effective contraceptive methods. Hormonal contraception was not allowed. Use of any antihypertensive medication, other than study medication, was prohibited throughout the study.
The study was conducted in accordance with good clinical practice and the Declaration of Helsinki. The study protocol and written subject information were reviewed and approved by the Ethics Review Committees for each site. All patients provided written informed consent prior to entering the study.
Study design
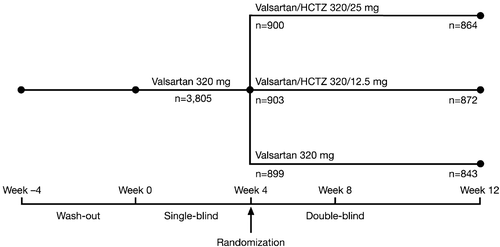
This was a double‐blind, active‐controlled, parallel‐group, randomized trial involving patients with mild‐to‐moderate essential hypertension. The study design is shown in . During the 1–4‐week washout period, all antihypertensive medication was withdrawn. After the washout period, all eligible patients received Val 320 mg o.d. monotherapy during a 4‐week single‐blind period. At the end of this period, patients with MSDBP ⩾90 and <110 mmHg were considered inadequately controlled and were subsequently randomized in a double‐blind fashion to receive Val 320 mg o.d., Val/HCTZ 320/12.5 mg o.d. or Val/HCTZ 320/25 mg o.d. for 8 weeks. Adequately controlled patients discontinued the study. Study visits took place at the start of washout, at the start of the single‐blind period, and every 4 weeks during the double‐blind period.
Efficacy and safety evaluations
BP measurements
BP assessments, using a calibrated oscillometric electronic BP measuring device (Omron 705 IT) and appropriately sized cuff, were made according to the recommendations of the European Society of Hypertension Citation[13]. All BP measurements were performed at trough, i.e. 24±3 h after the last dose of study medication. At each study visit, three BP measurements were taken at 1–2‐min intervals; the mean of these measurements was used for analysis.
Other assessments
Adverse events (AEs) and serious AEs (SAEs) were monitored and recorded during physical examinations and by questioning the patient. Haematology, blood chemistry tests and urinalysis were performed at the start of single‐blind monotherapy, and levels monitored during the clinical visits throughout the study. All laboratory analyses were performed centrally in one certified laboratory.
Efficacy and safety variables
The primary efficacy variable was the change in MSDBP during the active treatment period. The secondary efficacy variables were the change in mean sitting SBP (MSSBP) during the active treatment period, the proportion of patients with adequate response for DBP (defined as MSDBP <90 mmHg at the end of the study and/or decrease in MSDBP of ⩾10 mmHg from the start of the double‐blind period), the proportion of patients with adequate response for SBP (defined as MSSBP <140 mmHg at the end of the study and/or decrease in MSSBP of ⩾20 mmHg from the start of the double‐blind period), the proportion of patients with adequate control for both SBP and DBP (defined as MSDBP <90 mmHg and MSSBP <140 mmHg at the end of the study, respectively).
Assessment of safety was based on the frequency of AEs, SAEs and laboratory abnormalities. The frequency of AEs was summarized by treatment group, body system and preferred term (e.g. headache, nasopharyngitis, dizziness). SAEs were narrated. In addition, summaries were presented for deaths, SAEs, AEs leading to discontinuation and AEs suspected to be related to study medication. The safety population was defined as all patients who received at least one dose of active treatment.
Statistical analyses
Covariance analysis was used for the primary efficacy variables with treatment and centre (pooled) as fixed factors and MSDBP at the start of the single‐blind period as covariate. The same statistical method was used to analyse changes in MSSBP. The proportion of responders was analysed by logistic regression, with treatment and centre (pooled) as factors. Primary and secondary efficacy analyses were performed on the intent‐to‐treat (ITT) population, defined as all randomized patients who had one efficacy assessment at week 4 and at least one efficacy measurement during the double‐blind period. The safety population included all randomized patients who received at least one dose of study drug during the double‐blind treatment period. A subgroup analysis was conducted on patients with MSDBP ⩾100 mmHg after 4 weeks on Val 320 mg monotherapy in the single‐blind period. All statistical tests were two‐sided and a p‐value less than 0.05 was considered statistically significant.
Results
Patients
Patient disposition is shown in . After the single‐blind period, 2702 patients with inadequate response to Val 320 mg o.d. monotherapy were randomized in the double‐blind period to Val 320 mg o.d. (n = 899), Val/HCTZ 320/12.5 mg o.d. (n = 903) or Val/HCTZ 320/25 mg o.d. (n = 900). All randomized patients were part of the safety population. Of these, 2,675 patients were included in the ITT population with n = 891 in Val 320 mg o.d., n = 895 in Val/HCTZ 320/12.5 mg o.d. and n = 889 in Val/HCTZ 320/25 mg o.d.
Of the 326 patients who did not complete the single‐blind period, the most common reasons for discontinuation were protocol violations (3.1%), AEs (1.5%) and abnormal laboratory values (1.2%). Discontinuation rates from the double‐blind period were similar in all three groups, with an overall rate of 4.6% (123 patients). The main reasons for discontinuation were AEs (1.7%), withdrawal of consent (0.7%) and protocol violations (0.6%).
The treatment groups were generally comparable with respect to demographics and clinical characteristics, including MSDBP and MSSBP (). The majority of patients were Caucasian and the proportions of men and women were similar across the three groups. Overall, more than 65% of the patients took antihypertensive medication prior to the start of the single‐blind run‐in period, and the most frequently used were angiotensin II‐converting enzyme (ACE) inhibitors (22.9%), selective beta‐blocking agents (15.9%), dihydropyridine derivatives (13.6%), ARBs (11.3%), ARBs and diuretics (7.9%), sulfonamides (5.2%), and ACE inhibitors and diuretics (5.1%).
Table I. Patient demographics and characteristics at baseline (randomized population).
Efficacy
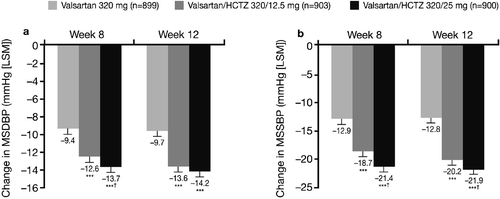
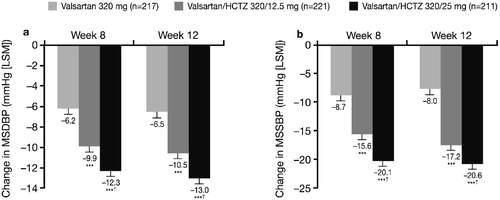
Initial monotherapy with Val 320 mg (4 weeks single‐blind) led to MSDBP reductions of 3.93, 4.02 and 3.77 mmHg in the patients subsequently randomized to the Val 320mg, Val/HCTZ 320/12.5 mg and Val/HCTZ 320/25 mg treatment groups, respectively. Additional MSDBP and MSSBP reductions were observed for all treatment groups during the double‐blind active treatment period. Overall reductions in MSDBP and MSSBP from the start of the single‐blind period (Week 0) to Weeks 8 and 12 are shown in . Significantly greater BP reductions were observed with both combination treatments versus monotherapy for MSDBP and MSSBP at Weeks 8 and 12 (all p<0.0001; ). Adjusted MSDBP reductions from the start of the single‐blind period to Week 12 were 9.7, 13.6 and 14.2 mmHg in the Val 320 mg, Val/HCTZ 320/12.5 mg and Val/HCTZ 320/25 mg groups, respectively. Adjusted MSSBP reductions from the start of the single‐blind period to Week 12 were 12.8, 20.2 and 21.9 mmHg in the respective groups. Val/HCTZ 320/25 mg was associated with significantly greater reductions than Val/HCTZ 320/12.5 mg in MSDBP from the start of the single‐blind period to Week 8 (), and in MSSBP from the start of the single‐blind period to Weeks 8 and 12 ().
Figure 2. Mean sitting diastolic blood pressure(MSDBP) (a) and mean sitting systolic blood pressure (MSSBP) (b) changes from the start of the single‐blind period (Week 0) to Week 8 and Week 12 (intent‐to‐treat population on randomized treatment). ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. LSM, least squares means.
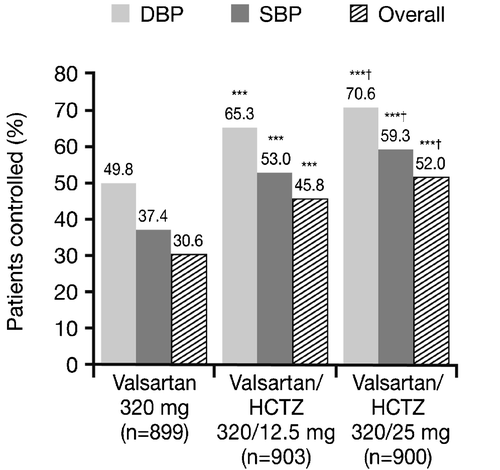
The efficacy differences between monotherapy and the combinations were further reflected in the proportion of responders at the end of the study, where both combination treatments were superior to Val 320 mg monotherapy (52.7% diastolic responders for Val 320 mg; 68.8% for Val/HCTZ 320/12.5 mg; 74.9% for Val/HCTZ 320/25 mg; p<0.0001 for both combinations versus monotherapy; ). In addition, the proportion of responders in the Val/HCTZ 320/25 mg group was significantly greater than that seen in the Val/HCTZ 320/12.5 mg group. Similar results were observed for the response in SBP. At the end of the study 49.8%, 65.3% and 70.6% of patients reached adequate DBP control; 37.4%, 53.0% and 59.3% reached adequate SBP control and 30.6%, 45.8% and 52.0% reached adequate overall BP control in the Val 320 mg, Val/HCTZ 320/12.5 mg and Val/HCTZ 320/25 mg groups, respectively; ). As with the proportion of BP response, the proportion of patients whose BP was adequately controlled was significantly greater for both combination treatments versus monotherapy (all p<0.0001) and Val/HCTZ 320/25 mg was superior to Val/HCTZ 320/12.5 mg.
Figure 3. Proportion of responders at the end of the study(Week 12) (intent‐to‐treat population). ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. Diastolic blood pressure (DBP) responders defined as patients with mean sitting DBP (MSDBP) <90 mmHg at endpoint and/or decrease in MSDBP of 10 mmHg from start of double‐blind period; systolic blood pressure (SBP) responders defined as patients with mean sitting SBP (MSSBP) <140 mmHg at endpoint and/or decrease in MSSBP of 20 mmHg from start of double‐blind period.
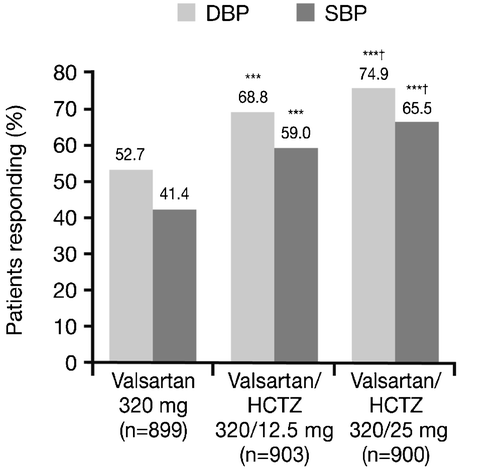
Figure 4. Proportion of patients with adequately controlled blood pressure(BP) at the end of the study (Week 12) (intent‐to‐treat population). ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. Diastolic blood pressure (DBP) control defined as patients with mean sitting DBP (MSDBP) <90 mmHg from the start of the double‐blind period to study endpoint; systolic blood pressure (SBP) control defined as patients with mean sitting SBP (MSSBP) <140 mmHg from the start of the double‐blind period to study endpoint; overall control defined as MSSBP <140 mmHg and MSDBP <90 mmHg.
Subgroup analysis
A subgroup analysis was performed on 649 patients with MSDBP ⩾100 mmHg after 4 weeks on Val 320 mg monotherapy in the single‐blind period. All treatments produced reductions in MSDBP () and MSSBP () from the start of the single‐blind period to Weeks 8 and 12 in this group of patients (). Both combinations produced significantly greater reductions in MSDBP and MSSBP than monotherapy (p<0.0001) at Weeks 8 and 12. In addition, the Val/HCTZ 320/25 mg group was associated with significantly greater reductions in MSDBP and MSSBP than the Val/HCTZ 320/12.5 mg group at Weeks 8 and 12 (p<0.05). Both combinations were associated with a greater proportion of patients with adequate response for DBP than monotherapy at the end of the study, with significantly more patients responding adequately in the Val/HCTZ 320/25 mg group (69.2%) than in the Val/HCTZ 320/12.5 mg group (56.1%).
Figure 5. Mean sitting diastolic blood pressure(MSDBP) (a) and mean sitting systolic blood pressure (MSSBP) (b) changes from the start of the single‐blind period (Week 0) to Week 8 and Week 12 in ITT patients on randomized treatment with MSDBP ⩾100 mmHg after receiving the initial 4 weeks valsartan 320 mg monotherapy. ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. LSM, least squares means.
Tolerability and safety
All treatments were generally well tolerated and the overall frequency of AEs during the active treatment period, regardless of study drug relationship, ranged from 33.0% in the Val/HCTZ groups to 33.7% in the Val 320 mg group (). Headache was the most frequently reported AE, occurring at a frequency of 3.9–4.9% across treatment groups (). The next most frequently reported AEs were nasopharyngitis, dizziness, vertigo and diarrhoea.
Table II. Incidence of most common adverse events (⩾1% in any treatment group) during the active treatment period (safety population).
There were no significant differences in the proportion of patients discontinuing the study due to AEs between groups during the active treatment period; 102 patients withdrew from the study because of AEs. There were 37 SAEs during the active treatment period, none of which was considered treatment related. Three deaths occurred during the study, none of which was considered related to study treatment.
Few clinically relevant changes in laboratory parameters were observed, and there were no discontinuations as a result of such changes. The only notable change in haematology in the double‐blind safety population was a >50% increase from randomization in white blood cell (WBC) count. The incidence of this change ranged from 1.8% in the Val 320 mg group to 3.0% in the Val/HCTZ 320/12.5 mg group; however, for the majority of these patients WBC counts remained within the normal range. Changes in biochemical parameters during the double‐blind safety population were also clinically unremarkable (). Shifts from normal to low potassium levels were more frequent with Val/HCTZ 320/25 mg (2.0%) than with Val/HCTZ 320/12.5 mg (0.6%) or Val 320 mg monotherapy (0.1%). For blood urea nitrogen (BUN) the frequency of patients with values >50% increased with higher doses of HCTZ and was lowest in the Val 320 mg monotherapy treatment group. Similarly, increases of uric acid of >50% were more frequent in the Val/HCTZ 320/25 mg treatment group compared with the other treatment groups, whereas no dose–response was observed for increases of creatinine >50%. For all of these variables, however, the majority of patients meeting the criteria for notable changes presented above had values that remained within normal ranges. Notable changes in all other biochemical parameters were infrequent and were not dose related.
Table III. Number (%) of patients with abnormal biochemistry values during the double‐blind treatment period (double‐blind safety population).
For all the haematology and biochemistry variables, the observed changes did not generally result in values that were outside the normal ranges and were consistent with the known dose‐related effects of component therapies.
Discussion
This study investigated the antihypertensive efficacy of Val/HCTZ 320/12.5 mg and Val/HCTZ 320/25 mg combination therapies and Val 320 mg monotherapy in patients with hypertension inadequately controlled after 4 weeks of Val 320 mg monotherapy. It should be noted that, although patients were selected for inadequate response to Val monotherapy at Week 4, all three treatment regimens significantly lowered BP during monotherapy and the double‐blind active treatment phase. The two combination therapies demonstrated additional efficacy in reducing BP compared with Val monotherapy. Furthermore, a significant dose‐related increase in efficacy was observed between Val/HCTZ 320/12.5 mg and Val/HCTZ 320/25 mg for changes in MSSBP. Previous studies have shown a dose–response following the addition of 12.5 mg or 25 mg HCTZ to 320 mg Val Citation[14], and the combinations of Val/HCTZ 160/12.5 mg and Val/HCTZ 160/25 mg were shown to be superior to Val 160 mg monotherapy in patients not responding to Val 160 mg monotherapy Citation[12]. This study shows that similar increases in efficacy are achievable with Val 320 mg‐based combination therapy in patients not adequately controlled on monotherapy.
In the monotherapy group, BP reductions observed during the first 4 weeks of single‐blind treatment were greater following a further 4 weeks monotherapy (Week 8) and were subsequently maintained to the end of the study, with only small additional reductions in BP between Weeks 8 and 12. Similarly, in a population non‐responsive to 4 weeks monotherapy (Val 160 mg), Mallion et al. Citation[12] reported additional BP reductions following a further 8 weeks' therapy. Although the combination therapies were associated with the largest BP reductions, the improvement obtained from remaining on monotherapy for an additional 4 weeks was reflected in the proportion of patients achieving BP control in the current study; 45.0% of patients whose MSDBP was ⩾90 and <110 mmHg after 4 weeks of monotherapy achieved MSDBP control (<90 mmHg) following a further 4 weeks of monotherapy (Week 8, data not shown). These data suggest that although the onset of action of Val is rapid, the maximum BP‐lowering effect is achieved over several weeks. The reductions in BP seen with combination therapy were mirrored in proportions of patients with adequate BP response and control. Approximately 75% of patients who were inadequately controlled with 4 weeks' Val monotherapy achieved adequate DBP response (defined as patients with MSDBP <90 mmHg at study end and/or decrease in MSDBP of ⩾10 mmHg from the start of the double‐blind period) after 8 weeks of therapy with Val/HCTZ 320/25 mg. Up to 70% of patients who were inadequately controlled with 4 weeks' Val monotherapy achieved adequate DBP control (defined as MSDBP <90 mmHg) after 8 weeks of therapy with Val/HCTZ 320/25 mg and 52% achieved overall BP control (defined as MSSBP <140 mmHg and MSDBP <90 mmHg).
These results reflect current treatment guidelines for hypertension, which suggest that, dependent on baseline BP and the presence of complications, treatment of hypertension should be started with either monotherapy or low doses of two or more agents. In patients non‐responsive to monotherapy, or in those with additional CV risk factors such as diabetes, combination therapy to achieve BP targets should be considered Citation[2,3]. Mallion et al. reported that a large percentage of patients inadequately controlled by Val 160 mg o.d. monotherapy were controlled by combination treatment with HCTZ 12.5 mg or 25 mg; findings which support the guideline treatment recommendations Citation[12]. Although low‐dose combination therapy has been advocated for the effective treatment of hypertension, some patients do not achieve BP control with such treatment regimens, and, as this study shows, there is potential to achieve higher proportions of control using higher‐dose combination therapy. In the study reported here, higher proportions of responders and patients achieving BP control were observed with the highest dose of Val/HCTZ (320/25 mg). In addition, a significant dose−response between Val/HCTZ 320/12.5 mg and Val/HCTZ 320/25 mg was observed for both MSDBP and MSSBP, suggesting that a more aggressive strategy can be employed with success in those patients with continuing uncontrolled DBP, and demonstrating the utility of Val/HCTZ 320/25 mg in such patients.
Although all patients in this study were selected on the basis of inadequate control on Val monotherapy, a subgroup analysis was performed to determine the longer‐term benefits of continuing on monotherapy or switching to combination therapy in patients with uncontrolled diastolic hypertension. The patients selected for subgroup analysis represented those whose hypertension was most severe and difficult to treat. In these patients, all three treatment regimens reduced MSDBP and MSSBP; however, combination therapy was significantly more effective than monotherapy by all efficacy measures.
The good tolerability seen in this trial confirms that reported previously with Val and HCTZ alone and in combination at doses up to 320/25 mg Citation[10–12],Citation[14,15]. There were no differences in the incidence or types of AEs across the three treatment strategies, suggesting that the additional efficacy benefits seen with more aggressive titration to combination therapy is not at the cost of reduced tolerability. In addition, all haematology and biochemistry values remained within the normal ranges for the duration of the trial. The decreased serum potassium and increased BUN seen with the HCTZ combination therapy in this trial are consistent with the known dose‐related effects of thiazide diuretics.
In conclusion, this study demonstrated that Val and HCTZ combinations provide statistically and clinically significant BP‐lowering benefits without compromising tolerability in patients not controlled on Val monotherapy.
Acknowledgement
This study was funded by Novartis. The sponsor was involved in the design of the study and collection of the data. Novartis Biostatistics and Statistical Reporting group provided the analysis of the data. All authors had access to study data and were responsible for interpretation of the data, development and approval of the finished manuscript. Professional medical writing support in drafting and editing this manuscript was provided by Christine Arris (ACUMED, Tytherington, UK) and Joanne Jess (contracted to ACUMED), with financial support from Novartis Pharmaceuticals Corporation. The authors would like to acknowledge Philippe Brudi's contribution to this study.
References
- Sica D. A. Are current strategies for treating hypertension effective?. J Clin Hypertens (Greenwich) 2003; 5((3 Suppl 2))23–32
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L., Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252
- Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., et al. 2007 guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–1187
- Lewington S., Clarke R., Qizilbash N., Peto R., Collins R., Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: A meta analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913
- Marques‐Vidal P., Tuomilehto J. Hypertension awareness, treatment and control in the community: Is the “rule of halves” still valid?. J Hum Hypertens 1997; 11: 213–220
- Antikainen R. L., Moltchanov V. A., Chukwuma C., Sr., Kuulasmaa K. A., Marques‐Vidal P. M., Sans S., et al. Trends in the prevalence, awareness, treatment and control of hypertension: The WHO MONICA Project. Eur J Cardiovasc Prev Rehabil 2006; 13: 13–29
- Neutel J. M., Smith D. H., Weber M. A. Low‐dose combination therapy: An important first‐line treatment in the management of hypertension. Am J Hypertens 2001; 14: 286–292
- Bakris G. L. The importance of blood pressure control in the patient with diabetes. Am J Med 2004; 30S–38S
- Sica D. A. Rationale for fixed‐dose combinations in the treatment of hypertension. The cycle repeats. Drugs 2002; 62: 443–462
- Pool J., Oparil S., Hedner T., Glazer R., Oddou‐Stock P., Hester A. Dose‐responsive antihypertensive efficacy of Valsartan, a new angiotensin II‐receptor blocker. Clin Ther 1998; 20: 1106–1114
- Benz J. R., Black H. R., Graff A., Reed A., Fitzsimmons S., Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension: A multiple dose, double‐blind, placebo‐controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12: 861–866
- Mallion J. M., Carretta R., Trenkwalder P., Martinez J‐F., Tykarski A., Teitelbaum I., et al. Valsartan/hydrochlorothiazide is effective in hypertensive patients inadequately controlled by valsartan monotherapy. Blood Press 2003; 12((Suppl 1))36–43
- O'Brien E., Waeber B., Parati G., Staessen J., Myers M. G. Blood pressure measuring devices: Recommendations of the European Society of Hypertension. BMJ 2001; 322: 531–536
- Pool J., Glazer R., Weinberger M., Alvarado R., Huang J., Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: A double‐blind, placebo‐controlled study followed by long‐term combination therapy in hypertensive adults. Clin Ther 2007; 29: 61–73
- Ruilope L. M., Malacco E., Khder Y., Kandra A., Bonner G., Heintz D. Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: The VAST study. Clin Ther 2005; 27: 578–587