Abstract
It is well documented that reducing blood pressure (BP) in hypertensive individuals reduces the risk of cardiovascular (CV) events. Despite this, many patients with hypertension remain untreated or inadequately treated, and fail to reach the recommended BP goals. Suboptimal BP control, whilst arising from multiple causes, is often due to poor patient compliance and/or persistence, and results in a significant health and economic burden on society. The use of fixed‐dose combinations (FDCs) for the treatment of hypertension has the potential to increase patient compliance and persistence. When compared with antihypertensive monotherapies, FDCs may also offer equivalent or better efficacy, and the same or improved tolerability. As a result, FDCs have the potential to reduce both the CV event rates and the non‐drug healthcare costs associated with hypertension. When FDCs are adopted for the treatment of hypertension, issues relating to co‐payment, formulary restrictions and therapeutic reference pricing must be addressed.
Introduction
Fixed‐dose combinations (FDCs) consist of two or more active drugs combined in a single tablet or capsule. FDCs have been shown to be valuable tools for the management of conditions such as asthma Citation[1] and diabetes Citation[2], representing effective, convenient alternatives to multiple‐drug therapy with the individual components. Hypertension represents another field where FDCs are available, with the potential to improve compliance, and therefore outcomes, and reduce overall costs compared with multi‐drug regimens.
Hypertension is a major risk factor for cardiovascular disease (CVD) and death. Conversely, it is well documented that reducing blood pressure (BP) in hypertensive individuals reduces the risk of cardiovascular (CV) events Citation[3]. Despite this, many patients with hypertension remain inadequately treated and fail to reach the recommended BP goals Citation[4]. Here we explore the underlying reasons for this and review the health and economic impact of under‐treatment of subjects with hypertension. In particular, we examine the potential public health value of FDCs in this context and the hurdles for their adoption in the current reimbursement environment in Europe.
Health and economic burden of hypertension
In 2000, it was estimated that 972 million adults worldwide had hypertension, and this figure was predicted to increase by about 60% by 2025 Citation[5]. In industrialized countries, at least a quarter of the population of adults aged 35–64 years are hypertensive, if BP>140/90 mmHg is taken as the threshold level () Citation[6]. Hypertension is a largely asymptomatic, but potentially lethal condition. It represents one of the major treatable risk factors of CVD, which is, in turn, the leading cause of premature death globally Citation[7]. There is strong evidence from meta‐analyses of clinical trials that the higher BP levels are, the greater the risk of CV complications such as stroke, heart attacks, heart failure and kidney disease Citation[3]. In fact, for individuals aged 40–70 years, each increment of 20 mmHg in systolic BP doubles the risk of CV death Citation[3]. Second only to tobacco, high BP is responsible for nearly 11% of the total loss in disability‐adjusted life‐years in developed countries Citation[8]. The consequences of uncontrolled hypertension place a substantial health and economic burden on society Citation[9,10].
Figure 1. Hypertension prevalence and its pharmacological treatment: North America and Europe Citation[6].
![Figure 1. Hypertension prevalence and its pharmacological treatment: North America and Europe Citation[6].](/cms/asset/a096a88a-d646-47ec-bd63-13051369d316/iblo_a_11151264_f0001_b.gif)
Need for combination therapy
Clinical trials have demonstrated that the control of BP reduces the number of strokes, heart attacks and heart failures Citation[11–13]. European and US guidelines currently recommend a target BP level of <140/90 mmHg for subjects with uncomplicated hypertension and a more stringent target of <130/80 mmHg for those with additional risk factors, such as diabetes or renal disease Citation[14,15]. For patients with hypertension who require pharmacological treatment, these guidelines indicate that a combination of two or more different classes of antihypertensive agents is often more effective in reaching BP goals than increasing the dose of a single agent. The safety and tolerability profiles of antihypertensive drugs should also be considered. While the risk of adverse events may increase with the use of additional agents, certain combinations may actually attenuate the risk of some class‐specific side‐effects Citation[16] (see Potential value of FDCs).
Ultimately, most individuals have been found to require a combination of antihypertensive drugs for effective BP control Citation[17]. In the Antihypertensive and Lipid‐lowering Treatment to Prevent Heart Attack Trial (ALLHAT), the average number of drugs per patient increased from 1.4 in the first year up to 2.0 by the fifth year, when the majority of patients had reached their goals Citation[17]. In the Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) trial, the majority of patients also needed at least two drugs to achieve their BP goals Citation[18]. In the Hypertension Optimal Treatment (HOT) trial, an average of 3.3 medications were needed, with over 70% of patients requiring combination therapy to achieve the stringent BP targets in this high‐risk group Citation[13].
Prevalence and consequences of poor BP control
Despite the high prevalence of hypertension, and the well documented association between hypertension and CV risk, there is evidence from the USA and Europe that it is not always treated nor well controlled. Wolf‐Maier and colleagues reviewed national surveys of hypertension among adults aged 35–64 years and found that 68–75% of hypertensive subjects in the five European countries studied were untreated Citation[4]. Furthermore, of those who were treated, 60–81% were not reaching their BP targets (). The results of a retrospective, observational study using data from a GP database in the UK indicated even poorer BP control, with only 14.2% of patients reaching guideline‐determined BP targets at 1 year Citation[19].
Figure 2. Proportion of treated hypertensive patients reaching BP goal* in five European countries Citation[4]. *Target BP goal <140/90 mmHg.
![Figure 2. Proportion of treated hypertensive patients reaching BP goal* in five European countries Citation[4]. *Target BP goal <140/90 mmHg.](/cms/asset/6b37f74b-7e95-4dfc-aceb-1677754b470d/iblo_a_11151264_f0002_b.gif)
Failure to control BP adequately has significant consequences for healthcare expenditures as well as health outcomes. In 2003, it was estimated that 5.7 million adults in the UK (12% of the population aged over 16 years) had BP above 160/95 mmHg and that a further 10.3 million (21% of the population) had BP in the range 140/90–160/95 mmHg Citation[20]. The authors determined that, if all patients had BP treated to target, the cost to the NHS of managing major CV events would fall by £97.2 (€160) million per year at 2000/01 prices. Similarly, a US study published in 2002 estimated that inadequate BP control resulted in nearly 40,000 CV events, nearly 8,400 CVD‐related deaths and $964 million in direct healthcare expenditure Citation[21]. The incremental cost of failure to reach BP goals within the medicated population was estimated at approximately $467 million. In a European review, Degli Esposti & Valpiani found that patients not at their BP goal had an increased number of hospital admissions for hypertension and associated diseases, resulting in higher healthcare costs Citation[22].
Inadequate treatment or poor compliance?
Evidence from clinical trials indicates that it is possible for the majority of patients to attain BP goals, including the elderly and those with multiple risk factors and diabetes Citation[17],Citation[23]. In the ALLHAT study, for example, which involved approximately 42,000 patients with hypertension, drug dosages were escalated and/or other drugs added as needed based upon level of BP control Citation[17]. At 5 years, 66% of patients had reached the BP goal of 140/90 mmHg. In another prospective study (VALUE), conducted in a regular clinical setting, over 12,000 patients with hypertension and high CV risk (92% of whom were already on antihypertensive therapy) were randomized to valsartan or amlodipine and followed for 30 months Citation[18]. Physicians were encouraged to monitor patients and use a step‐wise approach to increase the dose or add a diuretic throughout the trial. At 30 months, the percentage of patients with systolic BP control had increased to 62.2% from a baseline level of 21.9%. These results demonstrate that, with the use of appropriate monitoring, treatment adjustment and guidance, physicians can help patients achieve better control.
Nevertheless, many patients are still failing to reach their BP targets. Even if physicians prescribe appropriate therapy, it is ultimately the patient who is responsible for following the treatment regimen. In over half of treatment failures, the problem can be attributed to suboptimal patient compliance rather than an inadequate regimen Citation[24]. Compliance, or adherence, has been defined as “the extent to which a person's behaviour corresponds with agreed recommendations from a healthcare provider” Citation[25,26] and involves taking medication as prescribed, on time, and at the correct dose, and following any recommended lifestyle modifications, such as diet or exercise. Persistence in this context is defined as continuing use of the prescribed therapies Citation[27]. A retrospective database analysis conducted in the Netherlands on 8,988 newly diagnosed and treated hypertensive patients found that fully adherent patients had a higher probability of reaching BP goals compared with non‐adherent patients, but that less than 50% of the patients were persistent after 1 year and only 30% of them were fully adherent (80% or more days with pill possession) Citation[28].
There are many potential reasons for non‐compliance and lack of persistence with treatment, including financial constraints, scepticism about treatment benefits Citation[29], poor drug tolerability Citation[30], and the need for multiple agents or complex treatment regimens Citation[29],Citation[31]. Many hypertensive patients have co‐morbidities that also require medications, such as hypercholesterolaemia and diabetes, thereby further increasing the patient's pill burden. A high pill burden is not only inconvenient for the patient but can increase problems related to health literacy, such as obtaining, processing and understanding dosing regimens and self‐management Citation[32]. It has also been shown that changes in therapeutic regimen can reduce persistence with therapy Citation[33,34].
Impact of non‐compliance and non‐persistence
A recent review found that poor compliance with therapeutic regimens accounts for “substantial worsening of disease, death and increased healthcare costs in the US” Citation[35]. Conversely, Halpern and colleagues reported that increased compliance and persistence after reaching BP goals resulted in reduced risks for adverse CV outcomes Citation[36] (). Similarly, Breekveldt and co‐workers found that patients with hypertension who were treatment‐persistent have a significantly lower risk of heart attacks and stroke Citation[37]. These studies in the USA and Europe, in addition to others Citation[38], make a strong connection between adherence and/or persistence with antihypertensive treatment and improved long‐term outcomes.
Figure 3. Increased compliance and persistence to antihypertensive therapy reduces risk of adverse cardiovascular outcomesCitation[36]. †Medication‐possession ratio ⩾80%. ‡Remaining on therapy for 12 months.
![Figure 3. Increased compliance and persistence to antihypertensive therapy reduces risk of adverse cardiovascular outcomesCitation[36]. †Medication‐possession ratio ⩾80%. ‡Remaining on therapy for 12 months.](/cms/asset/36371875-4040-4460-8cb2-3571d3a113ea/iblo_a_11151264_f0003_b.gif)
There is considerable evidence suggesting that poor compliance increases healthcare costs Citation[39]. In 1994, a US study calculated that each additional day of uninterrupted antihypertensive drug therapy in the Medicaid population saved approximately $3/day in healthcare costs Citation[40]. The higher drug costs for patients with continuous treatment ($281 per patient, p = 0.0001) were offset by savings in hospital care ($637 per patient, p<0.0002) and ambulatory care ($175 per patient, p = 0.0228). A retrospective cohort analysis of US patients with hypertension found that higher levels of compliance were associated with reduced hypertension‐related non‐drug healthcare costs (including outpatient services, ER services and hospitalization), again offsetting higher drug costs, and a lower risk of all‐cause hospital admissions (admissions associated with any condition during the trial period) () Citation[41]. In another US study, Levy and colleagues calculated that 5% of hospital admissions are due to non‐compliance with medication in general, accounting for an estimated $100 billion annually in direct and indirect costs for all types of diseases Citation[42].
Figure 4. (A) All‐cause hospitalization risk according to different levels of compliance. Reproduced with permission from Citation[41]. (B) Hypertension‐related healthcare costs according to different levels of compliance Citation[41]. *p<0.05 vs 80–100% compliant group.
![Figure 4. (A) All‐cause hospitalization risk according to different levels of compliance. Reproduced with permission from Citation[41]. (B) Hypertension‐related healthcare costs according to different levels of compliance Citation[41]. *p<0.05 vs 80–100% compliant group.](/cms/asset/29ecc98a-7c21-4cb7-a5b5-04469c0ca57f/iblo_a_11151264_f0004_b.gif)
Inevitably, productivity is affected by non‐compliance because of the increased number of days missed from work due to disease or treatment. Rizzo et al. in the US calculated that the net benefit to employers of covering the costs of prescription medications for workers in 1987 was $286 per employee with hypertension. This benefit was attributed to an increase in compliance, resulting in a substantially lower level of absenteeism Citation[43].
Changes in drug regimens – including alteration of drug dosages, switching between drugs and discontinuation of therapy, all of which can reduce compliance and/or persistence, have been estimated to account for almost one‐third of the total treatment cost of patients with hypertension Citation[34]. Applying this approximation to the total treatment costs for hypertension in the US (estimated by the American Heart Association), it is predicted that in 2005 an excess of around $14.6 billion may have been spent on switching. Hughes & McGuire suggested that the excess costs incurred as a result of drug switching and discontinuation might be reduced if first‐line therapy provided sufficient efficacy and tolerability to encourage patients to continue treatment Citation[34].
Potential value of FDCs
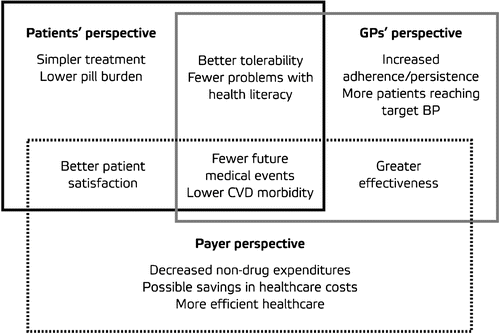
FDCs of different drug classes can reduce pill burden and increase compliance and persistence, thereby reducing CV event rates and lowering non‐drug healthcare costs for hypertension Citation[31] (). They may also offer equivalent or better efficacy, and the same or improved tolerability, compared with monotherapies for hypertension. Recently, 6‐month data from the Avoiding Cardiovascular Events Through Combination Therapy In Patients Living With Systolic Hypertension (ACCOMPLISH) trial showed that initial therapy with FDCs resulted in BP control rates that were higher than those achieved in other multinational trials Citation[44,45].
Many of the current FDCs for hypertension consist of a diuretic plus another antihypertensive agent, but increasingly there are other combinations that may have specific advantages. For example, although monotherapy with a calcium‐channel blocker (CCB) is often the preferred treatment for moderate‐to‐severe hypertension, the occurrence of peripheral oedema limits its titration Citation[46]. Combination therapy (fixed or free) comprising a renin–angiotensin system blocker, such as an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker, with a CCB has been shown not only to improve BP‐lowering efficacy, often at lower doses of the component agents, but also to reduce the rate of peripheral oedema, due to complementary mechanisms of action Citation[16],Citation[47,48].
In terms of compliance, once‐daily FDC regimens have been shown to be superior to once‐daily co‐administration of two‐pill regimens, regardless of the number of concomitant medications Citation[49]. A US retrospective study found that patients receiving FDC amlodipine besylate/benazepril HCI had significantly higher compliance rates over a 1‐year period than those receiving separate pills (80.8% vs 73.8%; p<0.001) Citation[31]. As a result, the total annual CV‐related healthcare cost per patient was significantly smaller in the FDC group. This difference in cost was consistent for most of the patient population when matched for severity (). A similar study estimated that patients receiving FDC amlodipine besylate/benazepril HCl were more compliant than those receiving separate pills, resulting in almost 50% lower healthcare costs comprising ambulatory, drug and hospital expenditure ($4,604 vs $8,532) Citation[50].
Figure 6. Average total annual healthcare cost per patient for fixed‐dose combination (FDC) and separate pills by severity score. Reproduced with permission Citation[31]. *Difference is significant at p⩽0.05 level for severity scores: 0–3, 5 and total. Abbreviations: HCI, hydrochlorothiazide; DHP CCB, dihydropyridine calcium‐channel blocker; ACE, angiotensin‐converting enzyme.
![Figure 6. Average total annual healthcare cost per patient for fixed‐dose combination (FDC) and separate pills by severity score. Reproduced with permission Citation[31]. *Difference is significant at p⩽0.05 level for severity scores: 0–3, 5 and total. Abbreviations: HCI, hydrochlorothiazide; DHP CCB, dihydropyridine calcium‐channel blocker; ACE, angiotensin‐converting enzyme.](/cms/asset/2f8c529f-3ce0-4b3d-81b6-931855929221/iblo_a_11151264_f0006_b.gif)
Cost savings have been demonstrated simply by switching to FDCs. One study looked at patients with moderate‐to‐severe hypertension who maintained BP control (88% of the study population) after switching from high‐dose CCBs to amlodipine/benazepril FDC Citation[51]. Overall, a $214 per patient per year saving was realised with the FDC, the primary cost advantage being a lower acquisition price. Even greater savings of $1,080 per patient per year (based on 1997 prices) were calculated for an African‐American population if they switched from a free combination with an ACE inhibitor and a CCB to fixed‐dose amlodipine/benazepril Citation[52]. Whist studies have demonstrated the cost savings associated with FDCs, their true public health value will only be determined by long‐term follow‐up of their use.
Persistence with treatment may be improved with FDCs. In a study in newly diagnosed patients, the proportion of patients persisting with treatment at 12 months was significantly higher in those receiving FDC lisinopril/hydrochorothiazide (HCTZ) compared with those receiving lisinopril and a separate diuretic (68.7% vs 57.8%; 18.8% difference; p<0.05) Citation[53]. Similarly, the persistence rate at 12 months was significantly higher with FDC enalapril/HCTZ compared with enalapril and a separate diuretic (70.0% vs 57.5%; 21.7% difference; p<0.05) Citation[53]. Although this study did not take therapeutic switches into consideration (e.g. switches to a different ACE inhibitor), the authors referred to a previous study they had conducted in which 95% of treatment regimen modifications involved the addition of a new drug from another class rather than a switch, supporting the likelihood that little switching occurred in the present study. In another study, persistence at 12 months was significantly better with FDC valsartan/HCTZ compared with the individual components (54% vs 19%; p<0.0001) in an antihypertensive‐naïve population in the USA Citation[54]. In the Netherlands, the persistence rate with separate administration of an ACE inhibitor and diuretic was 12% lower than with the same drugs given as FDC, with 2.09 odds ratio of being non‐adherent Citation[28]. In patients who were non‐persistent in this study, a larger proportion of patients co‐administered two pills continued with only one of the components compared with those treated with the FDC (60.0% vs 39.9%, respectively).
Healthcare environment impacts on FDCs
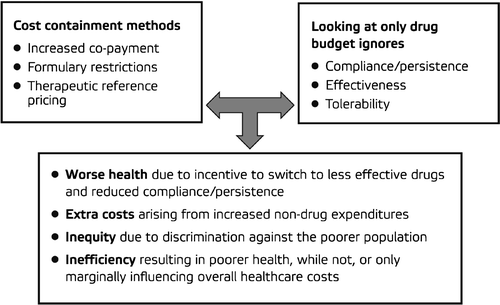
In theory, the three main goals for healthcare systems are efficiency, equity and cost‐containment. In recent decades, cost‐containment has tended to become a priority for decision‐makers Citation[55]. Assessing new programs, interventions and technologies only according to their immediate impact on the healthcare budget can create serious efficiency and equity problems. For example, the reimbursement of cheaper, but less effective medicines can lead to poorer health and increased non‐drug expenditure. As the separation of healthcare budgets by resource type (e.g. inpatient, GP, pharmaceutical) and cost‐containment are becoming more and more widespread, other important aspects of treatment, such as compliance, persistence and effectiveness, are ignored, creating a potentially negative approach to newer, more effective drugs, such as FDCs, which are playing an increasingly important role in the day‐to‐day management strategy for many disease areas.
In terms of the current healthcare environment, certain issues need to be addressed for the adoption of FDCs. These include co‐payment (patient and/or employer contributions towards the cost of drugs), formulary restrictions and therapeutic reference pricing (the practice of setting a maximum reimbursement price for a particular type of agent), all of which may ultimately lead to suboptimal treatments, poorer health, inequity of care, inefficiency and increased overall costs ().
Co‐payment and reimbursement restrictions
In situations where patients have prescription insurance, co‐payments may be reduced with a combination drug compared with the separate components each time the prescriptions are refilled Citation[56]. However, higher co‐payment for FDCs compared with free combinations can be a barrier to their use; lower reimbursement and thus higher co‐payment for FDCs compared with free combinations may influence physicians to prescribe the latter in order to avoid higher out‐of‐pocket payments for their patients. This may result in increasing healthcare expenditure Citation[31],Citation[50],Citation[57,58]. Co‐payment may also increase inequalities in access to healthcare, potentially reducing the health status of those already disadvantaged in socio‐economic terms Citation[59].
Evidence of the effects of co‐payments on drug and healthcare expenditure is contradictory and depends on the healthcare system context. For example, in Sweden, increased co‐payment in the 1990s reduced the use of essential drugs, leading to worsening health without influencing the overall costs Citation[60]. In Italy and the UK, higher out‐of‐pocket expenditures tended to force patients with hypertension and dyspepsia to stop their medication, select fewer drugs, or lower their drug dosages Citation[59]. In order to counteract this inefficiency, a variety of measures are used in different countries. Possible options include lower co‐payment/prescription charge for lower income groups (e.g. in the UK), or a positive list of drugs exempt from co‐payment in low income populations (e.g. in Italy and Hungary).
Formulary restrictions
Formulary lists need to be updated regularly to ensure that FDCs are included in the list of covered drugs. Formulary restrictions, such as exclusion of FDCs from listings, or placing them as second‐ or third‐line therapies, are intended to reduce utilization and costs. As with co‐payment, however, basing prescribing decisions on drug price alone can lead to higher overall healthcare expenditure, equity problems and inefficiency.
Therapeutic reference pricing
Therapeutic reference pricing, or clustering, can affect FDCs by decreasing reimbursement for their prescription if it is based upon therapeutic classes including both generic and patent‐protected drugs, as in the Netherlands, Canada, Germany, Hungary and Australia Citation[57,58],Citation[61]. Generally, drugs with the lowest price per daily dose are chosen as reference products. This system offers a higher level of reimbursement, and thereby smaller co‐payment, to older and cheaper drugs, and a lower level of reimbursement and greater co‐payment to newer, more expensive drugs. Clustering can put FDCs at a disadvantage by including them in the same therapeutic group as the monotherapies.
In order to minimize co‐payment costs for the patient, physicians have an incentive to choose the reference product(s) over a potentially more effective or convenient product with lower reimbursement. Reference pricing takes no account of the potential advantages of FDCs over their monocomponents in terms of efficacy, compliance and persistence. Reducing reimbursement for FDCs in hypertension may therefore lead to fewer patients reaching target BP, increased medical event rates and increased non‐drug healthcare costs.
Furthermore, experiences with reference pricing have not all been favourable. Norway abandoned reference pricing because no cost savings emerged, and it is likely also to be abandoned in the Netherlands Citation[58],Citation[62]. In Hungary, contrary to expectations, reference pricing actually increased drug expenditure Citation[57], while in Spain it resulted in insignificant savings and even those savings were debated Citation[63]. Reference pricing raises even more pronounced equity problems than across‐the‐board co‐payment increases, since the system discriminates against those unable to afford higher co‐payments.
Finally, reference pricing in most countries is based on price per daily defined dose (DDD) Citation[57]. The DDD, established by the WHO, is “the assumed average maintenance dose per day for a drug used for its main indication in adults” Citation[64]. It is linked to the Anatomical Therapeutic Chemical classification, where different groups of drugs are determined according to the organ or system on which they act and their chemical, pharmacological and therapeutic properties. Thus, while DDDs are useful for drug utilization studies and international treatment comparisons, they do not reflect comparative efficacy. An assumption of equal efficacy across the DDDs and their use for reimbursement purposes is an obvious misuse of that system and is not recommended by the WHO Citation[64]. For FDCs in particular, DDD calculations are incomparable to those of the monocomponents Citation[64].
Policy recommendations
In concert with the more positive attitude of decision‐makers towards FDCs in other therapeutic areas, such as infectious and respiratory diseases, all the advantages of antihypertensive FDCs should be taken into account for reimbursement:
Effectiveness, taking into account compliance and persistence;
The positive effects on healthcare costs and efficiency;
Reduced pill burden in a condition with high co‐morbidities and concomitant medication;
Improved tolerability with some FDCs;
Where available, information on cost‐effectiveness.
FDCs should not be included in the same therapeutic reference pricing group as the monocomponents, due to the difference in effectiveness.
The level of co‐payment should not be a barrier to FDC use compared with free combinations or influence physicians to prescribe the latter in order to avoid higher out‐of‐pocket payments for their patients.
Formulary lists and reimbursement plans should be reviewed and updated regularly to ensure that FDCs are included in the list of covered drugs.
Guidance on clinical practice should take into account the greater effectiveness of FDCs.
Conclusions
Uncontrolled hypertension imposes a major health and financial burden on society. More effective and efficient treatments, such as FDCs, are therefore required. Through a simplified therapeutic regimen, the use of FDCs can increase compliance and persistence, thus delivering enhanced effectiveness compared with their equivalent monocomponents administered as separate pills.
As a result, FDCs can help more patients reach target BP, thereby reducing CV morbidity and mortality. This may in turn translate into reduced numbers of physician visits and hospital admissions, shortened hospital stays, reduced non‐drug and overall healthcare expenditure, and improved productivity.
Reimbursement hurdles introduced into drug budgets because of emphasis on cost‐containment can ultimately lead to inefficiency, equity problems and higher healthcare costs, and can represent a major barrier to the use of FDCs in hypertension.
To encourage the use of FDCs we recommend that compared with their monocomponents, they have similar or lower co‐payments depending on their added value, and should not be included in the same therapeutic reference pricing group. Formulary lists, reimbursement plans and clinical guidelines should be reviewed and updated regularly to ensure that they include FDCs.
Acknowledgements
Preparation of this manuscript was supported by Novartis Pharma AG. Medical writing and editorial services were provided by professional medical writers Sharon Smalley and Joanne Bentley (ACUMED, Tytherington, UK) and contracted medical writer Liz Anfield. Luis M Ruilope has served as an advisor and speaker for Novartis. Michel Burnier has served as a speaker for Novartis. Noemi Muszbek and Ruth E Brown have received partial support for this review from Novartis and work for numerous pharmaceutical companies. Abdulkadir Keskinaslan, Philippe Ferber and Günter Harms are employees of Novartis AG.
References
- Corren J. Evaluation and treatment of asthma: An overview. Am J Manag Care 2005; 11((14 Suppl))S408–S415
- Leichter S. B., Thomas S. Combination medications in diabetes care: An opportunity that merits more attention. Clin Diabetes 2003; 21: 175–178
- Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913
- Wolf‐Maier K., Cooper R. S., Kramer H., Banegas J. R., Giampaoli S., Joffres M. R., et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension 2004; 43: 10–17
- Kearney P. M., Whelton M., Reynolds K., Muntner P., Whelton P. K., He J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005; 365: 217–223
- Wolf‐Maier K., Cooper R. S., Banegas J. R., Giampaoli S., Hense H‐W., Joffres M., et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003; 289: 2363–2369
- Ezzati M., Lopez A. D., Rodgers A., Vander Hoom S., Murray C. J. L., the Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–1360
- World Health Organization. The World Health Report 2002 – Reducing risks, promoting healthy life. 83, Chapter 4
- Mullins C. D., Sikirica M., Seneviratne V., Ahn J., Akras K. S. Comparisons of hypertension‐related costs from multinational clinical studies. Pharmacoeconomics 2004; 22: 1001–1014
- Chockalingam A., Campbell N. R., Fodor J. G. Worldwide epidemic of hypertension. Can J Cardiol 2006; 22: 553–555
- Neal B., MacMahon S., Chapman N., Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood‐pressure‐lowering drugs: Results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet 2000; 356: 1955–1964
- Klungel O. H., Kaplan R. C., Heckbert S. R., Smith N. L., Lemaitre R. N., Longstreth W. T., Jr., et al. Control of BP and risk of stroke among pharmacologically treated hypertensive patients. Stroke 2000; 31: 420–424
- Hansson L., Zanchetti A., Carruthers S. G., Dahlöf B., Elmfeldt D., Julius S., et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998; 351: 1755–1762
- ESH–ESC Guidelines Committee. 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension (ESH 2003). J Hypertens 2003; 21: 1011–1053
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. I., Jr., et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA 2003; 289: 2560–2572
- Jamerson K. A., Nwose O., Jean‐Louis L., Schofield L., Purkayastha D., Baron M. Initial angiotensin‐converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens 2004; 17: 495–501
- Cushman W. C., Ford C. E., Cutler J. A., Margolis K. L., Davis B. R., Grimm R. H., et al. Success and predictors of blood pressure control in diverse North American settings: The antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4: 393–404
- Julius S., Kjeldsen S. E., Brunner H., Hansson L., Platt F., Ekman S., et al. VALUE trial: Long‐term blood pressure trends in 13,449 patients with hypertension and high cardiovascular risk. Am J Hypertens 2003; 16: 544–548
- Walley T., Duggan A. K., Haycox A. R., Niziol C. J. Treatment for newly diagnosed hypertension: Patterns of prescribing and antihypertensive effectiveness in the UK. J Royal Soc Med 2003; 96: 525–531
- Lloyd A., Schmieder C., Marchant N. Financial and health costs of uncontrolled blood pressure in the United Kingdom. Pharmacoeconomics 2003; 21(Suppl 1)33–41
- Flack J. M., Casciano R., Casciano J., Doyle J., Arikian S., Tang S., et al. Cardiovascular disease costs associated with uncontrolled hypertension. Manag Care Interface 2002; 15: 28–36
- Degli Esposti L., Valpiani G. Pharmacoeconomic burden of undertreating hypertension. Pharmacoeconomics 2004; 22: 907–928
- Black H. R., Elliott W. J., Grandits G., Grambsch P., Lucente T., White W. B., et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA 2003; 289: 2073–2082
- Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens 2006; 19: 1190–1196
- World Health Organization (WHO). Adherence to long term therapies: Evidence for action. 2003, WHO
- International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Pharmacoeconomic guidelines around the word. http://www.ispor.org/PEguidelines/index.asp (accessed on 25 August 2006).
- Berger M. L., Bingefors K., Hedblom E. C., , editors, et al. Health care cost, quality and outcomes: ISPOR book of terms. 2003, ISPOR
- Sturkenboom M. C. J. M., Picelli G., Dieleman J. P., Mozaffari E., Pompen M., van der Lei J. Patient adherence and persistence with antihypertensive therapy: One‐versus two‐pill combinations. J Hypertens 2005; 23(Suppl 2)S326
- DiMatteo M. R., Giordani P. J., Lepper H. S., Croghan T. W. Patient adherence and medical treatment outcomes: A meta‐analysis. Med Care 2002; 40: 794–811
- Dezii C. M. Medication noncompliance: What is the problem?. Manag Care 2000; 9((9 Suppl))S7–S12
- Taylor A. A., Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benazepril HCl versus comparable component‐based therapy. Congest Heart Fail 2003; 9: 324–332
- Davis T. C., Wolf M. S. Health literacy: Implications for family medicine. Fam Med 2004; 36: 595–598
- Caro J. J., Speckman J. L., Salas M., Raggio G., Jackson J. D. Effect of initial drug choice on persistence with antihypertensive therapy: The importance of actual practice data. CMAJ 1999; 160: 41–46
- Hughes D., McGuire A. The direct costs to the NHS of discontinuing and switching prescriptions for hypertension. J Hum Hypertens 1998; 12: 533–537
- Osterberg L., Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497
- Halpern M. T., Vincze G., Stewart W. F., Khan Z. M. Persistence with hypertension therapy and long‐term cardiovascular outcomes. J Hypertens 2006; 24(Suppl 4)S182–S183, (P11.86)
- Breekveldt‐Postma N. S., Siiskonen S. J., Penning‐Van Beest F. J. A., et al. Non‐persistent use of antihypertensive drugs leads to increased risk of hospitalizations for acute myocardial infarction or stroke. Value Health 2006; 9(Suppl)A339
- Granger B. B., Swedberg K., Ekman I., Granger C. B., Olofsson B., McMurray J. J., et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double‐blind, randomised, controlled trial. Lancet 2005; 366: 2005–2011
- Cleemput I., Kesteloot K., DeGeest S. A review of the literature on the economics of non‐complicance. Room for methodological improvement. Health Policy 2002; 59: 65–94
- McCombs J. S., Nichol M. B., Newman C. M., Sclar D. A. The costs of interrupting antihypertensive drug therapy in a Medicaid population. Med Care 1994; 32: 214–226
- Sokol M. C., McGuigan K. A., Verbrugge R. R., Epstein R. S. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005; 43: 521–530
- Levy G., Zamacona M. K., Jusko W. J. Developing compliance instructions for drug labeling. Clin Pharmacol Ther 2000; 68: 586–591
- Rizzo J. A., Abbott T. A., 3rd., Pashko S. Labour productivity effects of prescribed medicines for chronically ill workers. Health Econ 1996; 5: 249–265
- Jamerson K. A., Bakris G. L., Wun C. C., Dahlöf B., Lefkowitz M., Manfreda S., et al. Rationale and design of the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) trial: The first randomized controlled trial to compare the clinical outcome effects of first‐line combination therapies in hypertension. Am J Hypertens 2004; 17: 793–801
- Jamerson K., Bakris G. L., Dahlöf B., Pitt B., Velazquez E., Gupte J., et al. ACCOMPLISH Investigators. Exceptional early blood pressure control rates: The ACCOMPLISH trial. Blood Press 2007; 16: 80–86
- Ruilope L. M., Malacco E., Khder Y., Kandra A., Bönner G., Heintz D. Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: The VAST study. Clin Ther 2005; 27: 578–587
- Andreadis E. A., Tsourous G. I., Marakomichelakis G. E., Katsanou P. M., Fotia M. E., Vassilopoulos C. V., Diamantopoulos E. J. High‐dose monotherapy vs low‐dose combination therapy of calcium channel blockers and angiotensin receptor blockers in mild to moderate hypertension. J Hum Hypertens 2005; 19: 491–496
- Neutel J., Smith D. H. Evaluation of angiotensin II receptor blockers for 24‐hour blood pressure control: Meta‐analysis of a clinical database. J Clin Hypertens (Greenwich) 2003; 5: 58–63
- Wanovich R., Kerrish P., Gerbino, Shoheiber O. Compliance patterns of patients treated with two separate antihypertensive agents versus fixed‐dose combination therapy. Am J Hypertens 2004; 17: 223A
- Dickson M., Plauschinat C. A. Antihypertensive therapy compliance and total cost of care in a Medicaid population: Fixed‐dose combination vs free‐combination treatment. Am J Health Syst Pharm 2005; 62: P71E
- Hilleman D. E., Reyes A. P., Wurdeman R. L., Faulkner M. Efficacy and safety of a therapeutic interchange from high‐dose calcium channel blockers to a fixed‐dose comfobination of amlodipine/benazepril in patients with moderate‐to‐severe hypertension. J Hum Hypertens 2001; 15: 559–565
- Kountz D. S. Cost containment for treating hypertension in African Americans: Impact of a combined ACE inhibitor‐calcium channel blocker. J Natl Med Assoc 1997; 89: 457–460
- Dezii C. M. A retrospective study of persistence with single‐pill combination therapy vs. concurrent two‐pill therapy in patients with hypertension. Manag Care 2000b; 9(Suppl)S2–S6
- Jackson K., Brixner D., Oderda G., et al. Persistence of fixed versus free combination therapy with valsartan and HCTZ for patients with hypertension. Value Health 2006; 9(Suppl)A363
- Drummond M., Jonsson B. Moving beyond the drug budget silo mentality in Europe. Value Health 2003; 6(Suppl 1)S74–S77
- Wertheimer A., Santella T. M., Finestone A. J., Levy R. Clinical and economic advantages of modern dosage forms: Improving medication adherence. 2006, Center for Pharmaceutical Health Services Research
- Kalo Z., Muszbek N., Bodrogi J., Bidlo J. Does therapeutic reference pricing always result in cost‐containment? The Hungarian evidence. Health Policy 2006; 89: 401–412
- Lopez‐Casasnovas G., Puig‐Junoy J. Review of the literature on reference pricing. Health Policy 2000; 54: 87–123
- Atella V., Peracchi F., Depalo D., Rossetti C. Drug compliance, co‐payment and health outcomes: Evidence from a panel of Italian patients. Health Economics 2006; 15: 875–892
- Andersson K., Petzold M. G., Sonesson C., Lonnroth K., Caristen A. Do policy changes in the pharmaceutical reimbursement schedule affect drug expenditures? Interrupted time series analysis of cost, volume and cost per volume trends in Sweden 1986–2002. Health Policy 2006; 79: 231–243
- Kanavos P., Reinhardt U. Reference pricing for drugs: Is it compatible with US health care?. Health Affairs (Millwood) 2003; 22: 16–30
- Koopmanschap M. A., Rutten F. F. H. The drug budget silo mentality: The Dutch case. Value Health 2003; 6(Suppl 1)S46–S51
- Puig‐Junoy J. Incentives and pharmaceutical reimbursement reforms in Spain. Health Policy 2004; 67: 149–165
- World Health Organization Collaborating Centre for Drug Statistics Methodology (WHO), About the ATC/DDD system: http://www.whocc.no/atcddd/6 (Last accessed 25 August 2006).