Abstract
Background. To compare the tolerability and safety of telmisartan±hydrochlorothiazide (HCTZ). Methods. This retrospective analysis was performed on all hypertensive patients that were enrolled in telmisartan studies. A total of 30 double‐blind (n = 8023) and 20 open‐label (n = 8393) studies were available at the time of this analysis, and were included. Treatments investigated were placebo, telmisartan 10–160 mg, or telmisartan 10–160 mg plus HCTZ 6.25–25 mg. The incidence and causality of all adverse events (AEs) and laboratory abnormalities occurring during treatment were recorded. Results. The incidences of all‐cause AEs in the double‐blind studies were: 2.73 per patient‐year (PY) (36.1%; placebo); 2.03/PY (37.4%; telmisartan monotherapy) and 2.09/PY (44.8%; telmisartan plus HCTZ). The respective numbers in the open‐label studies were: 0.65/PY (49.6%; telmisartan monotherapy) and 0.70/PY (40.3%; telmisartan plus HCTZ). The most frequent suspected adverse reactions were dizziness and headache, which were comparable across groups and studies. The overall incidence of drug‐related laboratory abnormalities was low in all treatment groups. Treatment‐related hyperuricaemia and hypokalaemia occurred in less than 0.1% of patients, respectively, treated with telmisartan plus HCTZ. Incidences of discontinuation due to an AE were 4.6%, 4.5% and 4.7%, respectively, for the placebo, telmisartan and telmisartan plus HCTZ treatment groups. Conclusion. The consolidated data show that telmisartan±HCTZ are well tolerated in patients of all ages and have placebo‐like tolerabilities.
Introduction
Hypertension is often asymptomatic, with the patient only discovering that their blood pressure is elevated after consulting a physician with an unrelated condition. Even after treatment, blood pressure may remain high, as poor compliance is a common problem in hypertensive patients Citation[1], who often cite side‐effects and hence reduced quality of life as the reasons for them not taking their medication Citation[2]. It has been reported that more than 50% of hypertensive patients discontinue the first prescribed treatment after 1 year Citation[3], and it is widely accepted that this is the main reason why blood pressure control in the hypertensive population is low Citation[4].
It has been established that the angiotensin receptor blocker (ARB) telmisartan has high selectivity for the AT1 receptor, and a terminal half‐life of about 24 h Citation[5–8]. Clinical studies have demonstrated the long‐term efficacy of telmisartan either alone or in combination with other agents in hypertensive patients, including those with comorbid conditions Citation[9]. Recently published studies have shown that telmisartan when used in combination with hydrochlorothiazide (HCTZ) 12.5−25 mg provides significantly greater blood pressure reductions than valsartan plus HCTZ, and both treatment combinations were well tolerated Citation[10,11]. In the Study of Micardis (telmisartan) in Overweight/Obese patients with Type 2 diabetes and Hypertension (SMOOTH), 840 patients were randomized to treatment for up to 10 weeks Citation[11]. Telmisartan plus HCTZ provided significantly greater reductions in 24‐h mean ambulatory blood pressure, and during the last 6 h of the dosing interval, compared with valsartan plus HCTZ.
Telmisartan has also been shown to have a favourable safety profile Citation[9],Citation[12]. In a large‐scale, open‐label, post‐marketing study, the safety of telmisartan over a 6‐month period was documented using data from 19 870 hypertensive patients, including those with hypercholesterolaemia, diabetes, congestive heart failure and renal insufficiency Citation[12]. In this study, the tolerability of telmisartan was rated as very good in 74.7% of all patients irrespective of gender, age, comorbidities, previous or current antihypertensive drug use.
The aim of the current survey was to analyse the tolerability and safety profile of telmisartan either alone or in combination with HCTZ using data from 50 Phase IIIb/IV, double‐blind or open‐label studies. The findings of this analysis are reported in this paper.
Patients and methods
Study designs
Safety data were collected from 50 clinical studies conducted in hypertensive patients. These comprised all studies conducted with telmisartan and available in the MICARDIS® project database by May 2006. The studies were all performed in adults (⩾18 years of age). In 47 studies, the patients had mild‐to‐moderate hypertension (usually defined as a supine or seated diastolic blood pressure [DBP] of ⩾95 mmHg). In two studies, only patients with severe hypertension (supine DBP ⩾115 mmHg) were eligible, and one study was conducted in patients in the age range 35–84 years with isolated systolic hypertension (DBP <90 mmHg, systolic blood pressure [SBP] ⩾150 mmHg). All patients provided informed consent prior to enrolment, and the studies had received local ethics committee approval.
The studies were either double‐blind (n = 30) or open‐label (n = 20). In four open‐label studies, patients had participated in a double‐blind study before, whereas in the other 16 open‐label studies, the patients had not previously participated in a study. In patients who had been in receipt of antihypertensive therapy prior to enrolment in the study, the randomized study phase was preceded by a washout period, usually of 4 weeks' duration, during which the patients received placebo.
Patients were randomized or allocated to treatment with placebo, telmisartan 10–160 mg or telmisartan 10–160 mg plus HCTZ 6.25–25 mg. The majority of patients receiving telmisartan were treated with a dose of 40–80 mg alone and/or in combination with HCTZ 12.5−25 mg; all treatments were administered once daily in the morning. The planned duration of treatment varied in different studies between 7 days and 2 years. Dose titration was performed in some studies, with an increase in the monotherapy dose and/or the addition of HCTZ to telmisartan monotherapy. Some patients received two courses of telmisartan in two separate studies, whereas others were randomized or up‐titrated to telmisartan plus HCTZ therapy. Other patients, who in the initial study had received a comparator, were assigned either to telmisartan monotherapy or telmisartan plus HCTZ in the follow‐up study. Concurrent treatment with other antihypertensive agents was permitted in most of the studies.
Safety evaluation
The primary purpose of all studies was to evaluate the antihypertensive efficacy of the treatments. In addition, each study monitored the tolerability and safety of the study medications. All adverse events (AEs) whether spontaneously reported by the patient or detected by the investigator, which occurred during the double‐blind or open‐label treatment phase of each study, or in the 14 days after the discontinuation of therapy, were recorded. In the pre‐registration Phase III studies, all AEs within this period were allocated to the treatment given before, and in the Phase IV studies post‐registration‐only AEs occurring on the first day after discontinuation were allocated to treatment.
An AE was defined as any untoward medical occurrence reported by a patient or established during a clinical evaluation. Serious AEs were defined as any untoward medical occurrences that resulted in death, were life‐threatening, or required hospitalization of the patient or extension of the period of hospitalization. The investigators also recorded the intensity and causality of these AEs. AEs were coded according to MedDRA Version 8.1. Suspected adverse reactions were defined as those events for which a causal relationship to the treatment had been suspected by the reporting or reviewing healthcare professional (usually the investigator or study monitor). Patients who had multiple occurrences of a specific AE, for analysis purposes, were counted only once. If a patient experienced more than one AE of different types, each event was included in the analysis.
Blood and urine samples were collected prior to receipt of the first dose of study drug, at intervals during the administration of the study drug, and after completion of the study in Phase II/III studies. Standard laboratory tests (blood chemistry, haematology and urinalysis) were performed on all patients. Any finding that fell outside the normal ranges was recorded if considered a clinically meaningful drug‐related event (i.e. requiring therapy and/or discontinuation of study drug).
Statistical methods
The statistics in this survey were purely descriptive. Given that there are differences in the reporting of AEs between double‐blind and open‐label studies, the AE frequencies were calculated separately for both study types and/or phases within a study. AE frequencies are given as raw percentages and, to reflect the differences in exposure time, as occurrences per patient‐year (PY); this reflects a standardized number of events having been observed in a patient treated for 1 year. Expressing data as per PY has a number of benefits: it not only enables physicians to judge the typical long‐term (i.e. 12 months) AEs associated with a particular treatment, but it also enables a comparison between studies of different length.
Results
Patient demographics
The baseline characteristics of the 8023 patients treated with placebo (n = 1167), telmisartan monotherapy (n = 5013) or a combination of telmisartan plus HCTZ (n = 1843) in the double‐blind studies, and the baseline characteristics of the 8393 patients treated with telmisartan monotherapy (n = 5907), or a combination of telmisartan plus HCTZ (n = 2486) in the open‐label studies are summarized in Tables and , respectively. Overall, the proportion of patients who were ⩾65 years was >20% in the double‐blind studies and >25% in the open‐label studies. Around 4% in the double‐blind studies and 5% in the open‐label studies were ⩾75 years. A higher percentage of black patients were treated with telmisartan plus HCTZ in both double‐blind and open‐label studies compared with non‐black patients.
Table Ia. Baseline characteristics of patientsa (n = 8023) in the double‐blind studies.
Table Ib. Baseline characteristics of patientsa (n = 8393) in the open‐label studies.
Duration of treatment
The mean duration of exposure to study drugs in the double‐blind and open‐label studies is shown in Tables and , respectively. In the double‐blind studies, drug exposures in PYs were: 154 (placebo), 923 (telmisartan monotherapy) and 395 (telmisartan plus HCTZ). The respective values in the open‐label studies were 3670 (telmisartan monotherapy) and 1424 (telmisartan plus HCTZ). The majority of patients receiving combination therapy were given a HCTZ dose of 12.5 mg (>60% in double‐blind studies, >95% in open‐label studies); smaller proportions of patients received HCTZ doses of 6.25 mg and 25 mg. Roughly 10% of the patients received treatment with telmisartan alone and/or in combination with HCTZ for more than 2 years in the open‐label studies.
Table IIa. Duration of exposure to study drug in double‐blind studies.
Table IIb. Duration of exposure to study drug in open‐label studies.
Clinical AEs
In the double‐blind studies, the incidences of all‐causality AEs/PY were: 2.73 (36.1%; placebo), 2.03 (37.4%; telmisartan monotherapy) and 2.09 (44.8%; telmisartan plus HCTZ). In the open‐label studies, the respective numbers were 0.65 (49.6%; telmisartan monotherapy) and 0.70 (40.3%; telmisartan plus HCTZ). During the double‐blind studies, the incidences of suspected adverse reactions/PY were: 0.67 (6.6%; placebo), 0.55 (10.1%; telmisartan monotherapy) and 0.58 (12.3%; telmisartan plus HCTZ). In the open‐label studies, the corresponding numbers were 0.15 (9.1%; telmisartan monotherapy) and 0.12 (6.8%; telmisartan plus HCTZ). In general, the incidences of all‐causality AEs per PY were lower in patients ⩾65 years than younger patients <65 years (Tables and ).
Table IIIa. The overall incidence of AEs according to patient age in double‐blind studies.
Table IIIb. The overall incidence of AEs according to patient age in open‐label studies.
The suspected adverse reactions occurring at a frequency of >1% in any treatment group are listed in (a) and (b). The most frequent individual suspected adverse reactions were dizziness, headache and fatigue. The incidences of cough, erectile dysfunction and peripheral oedema were <1% across all treatment groups (). Cardiac and renal abnormalities were very rare.
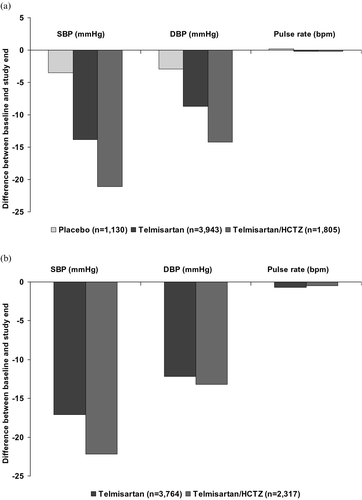
Figure 1. Incidence(%) of the most frequently (>1% in any treatment group) occurring suspected adverse reactions in (a) the double‐blind and (b) open‐label studies. HCTZ, hydrochlorothiazide.
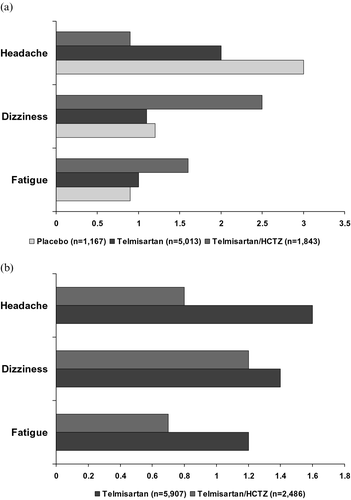
Table IV. The incidence (%) of suspected adverse reactions relating to cough, peripheral oedema and erectile dysfunction in any treatment group.
In the double‐blind studies, the incidences of serious AEs (per PY) were: 0.09 (1.2%; placebo) 0.07 (1.2%; telmisartan monotherapy) and 0.06 (1.2%; telmisartan plus HCTZ). The corresponding numbers in the open‐label studies were 0.07 (4.4%; telmisartan monotherapy) and 0.08 (4.5%; (telmisartan plus HCTZ). There were no differences in the incidence of specific serious AEs between the active treatment groups in the double‐blind studies, and none occurred at an incidence >0.2%. In the open‐label studies, there were 13 myocardial infarctions (MIs) (0.004/PY; 0.3%) in the telmisartan monotherapy group and 10 MIs (0.007/PY; 0.4%) in the telmisartan plus HCTZ group.
Overall, there were 17 deaths (0.0037/PY) in the telmisartan monotherapy group and seven deaths (0.0038/PY) in the telmisartan plus HCTZ group. Assuming the incidence/PY in the placebo group is the same as in the telmisartan monotherapy or telmisartan plus HCTZ groups, then the expected number of deaths for placebo (based on 153.9 PYs) is less than 1 (0.570).
Laboratory abnormalities
The overall incidence of drug‐related laboratory abnormalities was low in all treatment groups. Hepatobiliary system disorders only occurred in the double‐blind studies with very low incidences: none for placebo, <0.05% for telmisartan monotherapy and 0.1% for telmisartan plus HCTZ. In the double‐blind studies, metabolic and nutrition disorders occurred in 0.2% of patients receiving placebo, 0.2% of those in receipt of telmisartan monotherapy, and in 0.5% of patients treated with telmisartan plus HCTZ. In the open‐label studies, the corresponding numbers were: 0.2% for telmisartan monotherapy and 0.2% for telmisartan plus HCTZ. No single laboratory abnormality occurred with an incidence >1% in any treatment arm. The incidence of blood and lymphatic system disorders was low (⩽0.1%) in all groups, irrespective of study design. Drug‐related changes in liver enzymes, hyperuricaemia and hypokalaemia were noted in <0.1% of patients in any of the treatment groups.
Treatment discontinuations
In the double‐blind studies, treatment discontinuations/PY due to AEs were as follows: 0.33 (4.4%; placebo), 0.14 (2.6%; telmisartan monotherapy) and 0.16 (3.4%; telmisartan plus HCTZ). In the open‐label studies, the respective numbers were 0.07 (4.0%) in the telmisartan monotherapy group and 0.05 (2.6%) in the telmisartan plus HCTZ group.
Antihypertensive efficacy
The effects of study drugs on blood pressure and pulse rate were assessed using the last treatment given during the observation periods. In the double‐blind studies (), the mean SBP/DBP was little changed from 157.8/97.3 mmHg at baseline to 154.2/94.4 mmHg at study end in placebo‐treated patients (n = 1130 with available data). In patients in receipt of telmisartan monotherapy at study end (n = 3943), the mean SBP/DBP was reduced from 158.0/97.8 mmHg to 144.2/89.1 mmHg. In patients being treated with telmisartan plus HCTZ at study end (n = 1805), the mean SBP/DBP was reduced from 157.5/101.4 mmHg to 136.4/87.2 mmHg. In open‐label studies (), the mean SBP/DBP was reduced from 158.4/100.0 mmHg to 141.3/87.8 mmHg in patients (n = 3764) in receipt of telmisartan monotherapy at study end. In patients being treated with telmisartan plus HCTZ at study end (n = 2317), the mean SBP/DBP was reduced from 160.3/98.7 mmHg to 138.1/85.5 mmHg. Mean pulse rate was not influenced by any of the study drugs.
Discussion
These data from 50 studies confirm that telmisartan is well tolerated. The results from double‐blind studies show that the safety profiles of telmisartan monotherapy and telmisartan plus HCTZ were similar to that of placebo. The data also demonstrate that the excellent tolerability of telmisartan is not jeopardized by the concomitant administration of HCTZ.
Despite the mean duration of double‐blind active treatment being longer than for placebo (with more than 10% of patients being treated with telmisartan alone or in combination for more than 3 months), incidences of AEs were similar in patients receiving active treatment to that recorded in patients receiving placebo. When the incidence was expressed in terms of the number of events/PY, placebo was even more likely to result in a treatment‐related AE.
Cough and impotence are among the side‐effects of antihypertensive agents that can reduce the hypertensive patients' quality of life Citation[15,16] and may compromise patient compliance (i.e. persistence with treatment). Cough and peripheral oedema can be a problem with ACE inhibitors Citation[16,17]. Erectile dysfunction is often associated with the use of diuretics, especially at high doses, and beta‐blockers Citation[15],Citation[18]. The data reported here show that these side‐effects, which often only become apparent after long‐term treatment, were very infrequent (cough, peripheral oedema, erectile dysfunction: <1%) in patients treated with telmisartan alone or in combination with HCTZ, despite the treatment being administered for relatively long periods compared with many clinical studies. In another meta‐analysis in which four ACE inhibitors (captopril, enalapril, ramipril and trandolapril) and three ARBs (candesartan, losartan and valsartan) were examined, the ARBs also had a number of advantages over ACE inhibitors, particularly with regard to lower incidences of cough Citation[17]. In VALIANT, for example, there were 0.6% withdrawals due to cough in the valsartan treatment group compared with 2.5% in the captopril treatment group Citation[17,18]. In OPTIMAAL, the cough‐related withdrawals were also lower in the losartan‐treatment group compared with captopril (1% vs 4.1%; p<0.0001) Citation[17],Citation[19]. Taken together, these findings indicate that the incidence of cough may be comparable across different ARBs.
One of the more frequent treatment‐related side‐effects of any antihypertensive therapy is dizziness; it demonstrates the agents' ability to lower blood pressure. This was also true for treatment with telmisartan, with or without HCTZ. Dizziness is a particular problem in the elderly, as it can result in falls and can lead to fractures that require hospitalization. In the telmisartan database, there were only two reports of a serious fall. Given the relative high proportion of elderly patients evaluated, this is probably below the normal incidence of falls in this age group. Approximately 30% of people >65 years old fall each year, with about one in 10 of these falls resulting in a fracture and one in five requiring some medical attention Citation[20].
A pharmacokinetic study in healthy subjects suggests that, in the presence of telmisartan, absorption of digoxin is more rapid, with an increase in the maximum plasma concentration, but without any significant effect on the trough plasma digoxin concentration Citation[21]. During the clinical studies in this survey, there was generally no restriction on the concomitant use of digoxin; however, serious ventricular arrhythmia, which is suggestive of digoxin toxicity, occurred in only one patient while being treated with telmisartan. The very low incidence of treatment‐related ventricular arrhythmia would indicate that the relatively transient effect of telmisartan on plasma digoxin concentrations, with no change in trough levels in plasma, is unlikely to be of clinical significance.
Thiazide diuretics are relatively frequently implicated in hyperuricaemia and hypokalaemia Citation[22]. Dose‐dependent HCTZ‐induced diuresis produces a reactive rise in plasma renin activity Citation[23]. This promotes urinary potassium excretion and results in hypokalaemia. The low incidence of hypokalaemia detected in patients treated with a combination of telmisartan and HCTZ may be explained by the relatively low dose of HCTZ of 12.5 mg usually required to achieve blood pressure control if used with another class of antihypertensive agent that acts on the RAS Citation[24]. This is also an explanation for the low incidence of hyperuricaemia detected in patients receiving telmisartan plus HCTZ.
Elevated uric acid has been shown to be a risk factor for both cardiovascular and renal disease progression Citation[25,26]. There is evidence to suggest that some ARBs, such as losartan and irbesartan can reduce serum uric acid Citation[27,28]; this could have clinical implications for both efficacy (particularly in terms of renoprotection) and for safety (in terms of hyperuricaemia‐lowering). Furthermore, some ARBs may be more effective at lowering uric acid than others. Losartan, pratosartan and telmisartan have all been shown to inhibit the uptake of uric acid by renal uric acid transporters (URAT1); however, only telmisartan exhibited this property at higher doses Citation[23]. In contrast, valsartan and olmesartan were not inhibitory. Although the clinical implications of these differential effects of ARBs on URAT1 have yet to be fully evaluated, it may be suggestive of differing safety profiles between ARBs. This could also have an implication on which ARB to use in combination therapy with HCTZ. The lack of an inhibitory effect of valsartan on URAT1 could, in part, also offer an explanation as to why valsartan withdrawals due to renal failure in the ValHeFT trial were significantly higher compared with placebo (1.1% vs 0.2%; p<0.001, respectively) Citation[17],Citation[29]. Similarly, in VALIANT, there were more withdrawals due to renal disorders with valsartan plus captopril (1.3%) than captopril alone (0.8%) Citation[17,18].
In conclusion, safety data from 50 studies that included patients randomized to placebo, telmisartan monotherapy or telmisartan plus HCTZ demonstrate that telmisartan is a well tolerated antihypertensive agent. Whether used alone or in combination with HCTZ, the side‐effect profile was at least comparable to that of placebo, with a very low incidence of laboratory abnormalities. Also, there is no evidence of serious drug–drug interactions. The low incidence of side‐effects with telmisartan, whether given alone or in combination with HCTZ, may help to enhance patient compliance and make telmisartan an appropriate choice for patients who have previously experienced unacceptable side‐effects while being treated with other antihypertensive agents.
Acknowledgements
We would like to thank Ms Rita Müller who managed the database retrieval and ran the analysis programs.
References
- Costa F. V. Compliance with antihypertensive treatment. Clin Exp Hypertens 1996; 18: 463–472
- Nunes M. I. The relationship between quality of life and adherence to treatment. Curr Hypertens Rep 2001; 3: 462–465
- Bloom B. S. Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther 1998; 20: 671–681
- Marques‐Vidal P., Tuomilehto J. Hypertension awareness, treatment and control in the community: Is the rule of halves still valid?. J Hum Hypertens 1997; 11: 213–220
- Wienen W., Entzeroth M., van Meel J. C. A., Stangier J., Busch U., Ebner T., et al. A review on telmisartan: A novel, long‐acting angiotensin II‐receptor antagonist. Cardiovasc Drug Rev 2000; 18: 127–156
- Stangier J., Su C. A. P. F., Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res 2000; 28: 149–167
- Sharpe M., Jarvis B., Goa K. L. Telmisartan: A review of its use in hypertension. Drugs 2001; 61: 1501–1529
- Stangier J., Su C. A., van Heiningen P. N., Meinick T., van Lier J. J., de Bruin H., et al. Inhibitory effect of telmisartan on the blood pressure response to angiotensin II challenge. J Cardiovasc Pharmacol 2001; 38: 672–685
- Battershill A. J., Scott L. J. Telmisartan: A review of its use in the management of hypertension. Drugs 2006; 66: 51–83
- White W. B., Punzi H. A., Murwin D., Koval S. E., Davidai G., Neutel J. M. Effects of the angiotensin II receptor blockers telmisartan vs valsartan in combination with hydrochlorothiazide 25 mg once daily for the treatment of hypertension. J Clin Hypertens 2006; 8: 626–633
- Sharma A. M., Davidson J., Koval S., Lacourciere Y. Telmisartan/hydrochlorothiazide versus valsartan/hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: The SMOOTH study. Cardiovasc Diabetol 2007; 6: 28
- Michel M. C., Bohner H., Köster J., Schäfers R., Heemann U. Safety of telmisartan in patients with arterial hypertension: An open‐label observational study. Drug Saf 2004; 27: 335–344
- Keene L. C., Davies P. H. Drug‐related erectile dysfunction. Adverse Drug React Toxicol Rev 1999; 18: 5–24
- Overlack A. ACE inhibitor‐induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf 1996; 15: 72–78
- Vleeming W., van Amsterdam J. G., Stricker B. H., de Wildt D. J. ACE inhibitor‐induced angioedema. Incidence, prevention and management. Drug Saf 1998; 18: 171–188
- Prichard B. N., Owens C. W., Woolf A. S. Adverse reactions to diuretics. Eur Heart J 1992; 13(Suppl G)96–103
- No authors listed. Angiotensin II receptor antagonists and heart failure: Angiotensin‐converting‐enzyme inhibitors remain the first‐line option. Prescrire Int 2005; 14: 180–186
- Pfeffer M. A., McMurray J. J., Velazquez E. J., Rouleau J. L., Kober L., Maggioni A. P., et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1906, Erratum in N Engl J Med 2004; 350: 203
- Dickstein K., Kjekshus J., OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Optimal Tril in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002; 360: 752–760
- Gillespie L. D., Gillespie W. J., Robertson M. C., Lamb S. E., Cumming R. G., Rowe B. H. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev 2001; CD000340
- Papademetriou V. Diuretics in hypertension: Clinical experiences. Eur Heart J 1992; 13(Suppl G)92–95
- Stangier J., Su C. A., Hendricks M. G., van Lier J. J., Sollie F. A., Oosterhuis B., et al. The effect of telmisartan on the steady‐state pharmacokinetics of digoxin in healthy male volunteers. J Clin Pharmacol 2000; 40: 1373–1379
- Weinberger M. H. Diuretics and their side effects. Dilemma in the treatment of hypertension. Hypertension 1988; 11: 16–20
- Weinberger M. H. Angiotensin converting enzyme inhibitors enhance the antihypertensive efficacy of diuretics and blunt or prevent adverse metabolic effects. J Cardiovasc Pharmacol 1989; 13: S1–S4
- Iwanaga T., Sato M., Maeda T., Ogihara T., Tamai I. Concentration‐dependent mode of interaction of angiotensin II receptor blockers with uric acid transporter. J Pharmacol Exp Ther 2007; 320: 211–217
- Viazzi F., Leoncini G., Ratto E., Falqui V., Parodi A., Conti N., et al. Mild hyperuricemia and subclinical renal damage in untreated primary hypertension. Am J Hypertens 2007; 20: 1276–1282
- Coronel F., Cigarran S., Garcia‐Mena M., Herrero J. A., Calvo N., Perez‐Flores I. Irbesartan in hypertensive non‐diabetic advanced chronic kidney disease. Comparative study with ACEI. Nefrologia 2008; 28: 56–60
- Reid J. L. Molecular‐specific effects of angiotensin II antagonists: Clinical relevance to treating hypertension?. J Renin Angiotensin Aldosterone Syst 2005; 6: 15–24
- Cohn J. N., Tognoni G., Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675