Abstract
Background: Different treatment approaches aimed at reducing attention-deficit/hyperactivity disorder (ADHD) core symptoms are available. However, factors such as intolerance, side-effects, lack of efficacy, high new technology costs, and placebo effect have spurred on an increasing interest in alternative or complementary treatment.
Aim: The aim of this study is to explore efficacy of multimodal treatment consisting of standard stimulant medication (methylphenidate) and neurofeedback (NF) in combination, and to compare it with the single treatment in 6-month follow-up in ADHD children and adolescents.
Methods: This randomized controlled trial with 6-month follow-up comprised three treatment arms: multimodal treatment (NF + MED), MED alone, and NF alone. A total of 130 ADHD children/adolescents participated, and 62% completed the study. ADHD core symptoms were recorded pre-/post-treatment, using parents’ and teachers’ forms taken from Barkley’s Defiant Children: A Clinician’s Manual for Assessment and Parent Training, and a self-report questionnaire.
Results: Significant ADHD core symptom improvements were reported 6 months after treatment completion by parents, teachers, and participants in all three groups, with marked improvement in inattention in all groups. However, no significant improvements in hyperactivity or academic performance were reported by teachers or self-reported by children/adolescents, respectively, in the three groups. Changes obtained with multimodal treatment at 6-month follow-up were comparable to those with single medication treatment, as reported by all participants.
Conclusions: Multimodal treatment using combined stimulant medication and NF showed 6-month efficacy in ADHD treatment. More research is needed to explore whether multimodal treatment is suitable for ADHD children and adolescents who showed a poor response to single medication treatment, and for those who want to reduce the use of stimulant medication.
Background
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by primary core symptoms of inattention, impulsivity, and hyperactivity (Citation1). ADHD represents a considerable burden for the individual, family, school, and society, and the Norwegian primary healthcare provides relief and support for children with ADHD as well as their families (Citation2,Citation3).
Various treatment approaches aiming to reduce ADHD core symptoms have been evaluated, with some possibly causing adverse reactions, and others not being optimal (Citation4–9). Despite the beneficial effects of most treatment approaches, factors such as intolerance, side-effects, lack of efficacy, time-limitation, high new technology costs, and placebo effect call for alternative or complementary treatments (Citation9).
A number of specific and general behavioural programmes are recommended for ADHD treatment (Citation4), including behavioural training for the child/adolescent, as well as their parents and teachers, to address social and academic poor performance associated with ADHD (Citation5).
The use of cognitive behavioural therapy (CBT) has been shown to improve ADHD symptoms (Citation6,Citation10,Citation11). However, CBT has several limitations concerning its effects on individuals with lower cognitive skills, and it is time-limited and resource-demanding (Citation5,Citation6).
Moreover, psycho-education of children and parents improves their understanding of ADHD and the challenges it brings to daily living (Citation12). Although educational programmes have had relatively beneficial effects in treating ADHD, there is only limited evidence of such effects from blinded behavioural assessments (Citation9).
Since the introduction of methylphenidate (MPH) in the 1960s, MPH has been the drug of choice for ADHD worldwide (Citation13). Pharmacological interventions are considered to address ADHD core symptoms. However, there are many challenges in optimizing pharmacological treatment, including careful adjustments in dosing and risk of drug abuse during treatment (Citation14–16). Although MPH treatment is effective in reducing ADHD core symptoms, side-effects are frequently reported (Citation17–21). Thus, long-term studies are needed to evaluate the pharmacological effects of MPH on function and impairment (Citation22).
Alternative medicine refers to treatments including herbal medicine, homeopathy, and nutrition treatments. Use of various dietary restrictions, such as restricted elimination diets, artificial food colour exclusions and free fatty acid supplementation (i.e. low-carbohydrate, high-protein, and ketogenic diets), have been described in ADHD treatment (Citation9,Citation23–25). However, a meta-analysis reported limited support for the efficacy of non-pharmacological treatments for core symptoms of ADHD, including dietary and psychological treatments (Citation9).
Neurofeedback (NF) is a behavioural therapy that aims at developing the capacity of cortical activity self-regulation (Citation26,Citation27). It is a type of biofeedback that uses real-time electroencephalography (EEG) displays to improve skills that regulate brain activity (Citation27–29). Theoretically, patients with ADHD have an under-aroused brain with insufficient communication among the neurons. EEG in ADHD patients has been described as showing brain activity with increased (excess) theta and decreased alpha/beta frequencies (Citation28,Citation30–33). NF aims to normalize the EEG by improving cortical functioning (Citation33). Thus, the patient learns to enhance the desired EEG frequencies and suppress the unwanted ones in the form of a reward system (Citation34), which, in the process, could possibly affect attention or other neurocognitive activities (Citation17).
Significant treatment effects of NF have been confirmed in meta-analyses by Sonuga-Barke et al. (Citation9) and Arns et al. (Citation35). Most of the trials, however, have been criticized for their methodological weaknesses, particularly the lack of appropriate control conditions and the number of patients included. The meta-analysis by Sonuga-Barke et al. (Citation9) included eight randomized controlled trials and three well-controlled trials of NF, and found the effects of NF were stronger for unblinded measures and no effects for the best blinded ADHD outcomes (Citation36).
In addition, randomized controlled studies of NF effects in ADHD demonstrated a lower effect size (ES) for hyperactivity, suggesting that hyperactivity is probably most sensitive to non-specific treatment factors used in short-term studies (e.g. time spent with a therapist), which is not the case in non-randomized controlled studies (Citation17,Citation18,Citation35,Citation37–40). A review by Moriyama et al. (Citation28) concluded that non-randomized controlled trials found medium-to-large ES, whereas the evidence for NF effects was less robust when considering only randomized controlled studies. Still, there are no available data on optimal short- and long-term treatment effects of NF.
Unlike medication, NF effect has not been tested in many well-designed, double-blind studies, which makes it difficult to ascertain whether any positive results are due to the treatment itself or other confounding factors like the placebo effect. In addition, evidence of long-term outcomes from the combined use of stimulant medication and NF is scarce. A few studies with 6- or 12-month follow-up have reported consistently positive effects of NF on ADHD core symptoms (Citation17,Citation41,Citation42), while the long-term effects of NF, even after treatment, have also been supported by other studies (Citation43,Citation44).
To date, most studies have not investigated the effects of multimodal treatment consisting of pharmacologic therapy and NF in ADHD. Some studies evaluating multimodal pharmacologic and non-pharmacologic treatments (i.e. NF, cognitive therapy, parental consulting) found multimodal treatment was not superior, but comparable, to single treatments (Citation45,Citation46). In contrast, Li et al. (Citation47) found pharmacologic treatment combined with NF to be more effective than pharmacologic treatment and NF alone, and to be particularly suitable for children/adolescents with ADHD who showed a poor response to pharmacologic treatment alone or who experienced side-effects. This study focused on evaluating possible effect of multimodal pharmacological/non-pharmacological treatment with ADHD children and adolescents.
Aims
The present study was a RCT with 6-month follow-up exploring multimodal treatment of methylphenidate combined with NF on core symptoms in children and adolescents with ADHD and compared the effects of pharmacologic treatment using methylphenidate and NF alone.
Materials and methods
Study design
This is an RCT with a 6-month follow-up period, comprising three treatment arms: NF treatment alone (NF), standard pharmacologic treatment alone using methylphenidate (MED), and multimodal treatment (NF + MED).
Participants
A total of 285 children and adolescents with ADHD were referred to the Child and Adolescent Mental Health Clinic (CAMHC) at Haugesund Hospital in Norway, over a period of 3 years. Of these, 275 number met the inclusion criteria (see below) and were invited to participate, of whom 130 (54% of invited participants) were included in this study after exclusion and confirmed participation. Only 62% (81/130) completed the entire study, including the 6-month follow-up.
Assessment of the referred ADHD study participants was as described previously (Citation13,Citation48,Citation49). Briefly, all children and adolescents referred to CAMHC underwent clinical assessment by a child psychiatrist, and a diagnosis of hyperkinetic disorder was made according to the ICD-10 diagnostic criteria. A physical and mental examination was also performed to exclude any underlying somatic conditions that could be the cause of ADHD symptoms.
The mean age of ADHD-referred children was 11.6 years (SD = 3.0) compared to non-ADHD-referred children, 10.3 years of age (SD = 3.7), (difference of mean: –1.3; 95% CI = –2.3 to –0.3). More boys were found in the ADHD referred group compared to the non-ADHD group.
Clinical ADHD assessment at referral
Diagnosis of ADHD was made using the ICD-10 research diagnostic criteria (The ICD-10 Classification of Mental and Behavioural Disorders, World Health Organization, 1992), conducted as described previously (Citation48,Citation50,Citation51). Assessment of symptoms of hyperactivity and inattention was made using the Disruptive Behaviour Disorders Rating Scale–Parent Form (Form 4), Disruptive Behaviour Disorders Rating Scale–Teacher Form (Form 5), and Clinical Interview–Parent Report Form (Form 6) taken from Barkley’s Defiant Children: A Clinician’s Manual for Assessment and Parent Training (Citation52).
Inclusion criteria
All children who met the following criteria were invited to participate: (1) symptomatology consistent with Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for the diagnosis of ADHD; (2) age 6–18 years; and (3) cognitive function above an intelligence quotient (IQ) of 70. The children were evaluated using the Wechsler Intelligence Scale for Children (WISC-IV) (Citation53).
Exclusion criteria
Children who met the following criteria were excluded from the study: (1) involvement in another intervention group, including CBT and Stop Now And Plan (SNAP); (2) the presence of co-morbid disorders other than ODD or anxiety disorder; and (3) the presence of a neurological and/or cardiovascular condition.
Sample size
Due to a lack of reference values when starting the study, the sample size was determined by access to the patient during the study period, as previously described in detail (Citation54).
Randomization
Unstratified randomization was carried out using a random list with arbitrary numbers (0–1–2) to set up three intervention groups (MED, NF + MED, NF), as previously described (Citation48,Citation50).
Interventions
NF group
NF depresses theta activity and increases the production of beta waves. Unipolar sensors were placed on the patient’s scalp to process signals as brainwaves or computer frequencies, while measuring brain activity. Brain activities were then shown to the subject through a video game or a film, so they could attempt to change their activity level. The child was allowed to play the video game to produce the desired brainwaves, which helps shape the brainwaves to a more regulated performance.
NF treatment was conducted using Infinity software and equipment (Thought Technology, Montreal, Canada), as previously described (Citation55). Electrode placement was monopolar in the central area (CZ). The treatment protocol for ADHD of beta/theta was used for the assessment of theta depression (EEG frequency range of 4–7 Hz) and beta enhancement (EEG frequency range of 12–15 Hz), as previously described (Citation56,Citation57).
All study participants underwent NF treatment three times a week, with a total of 30 sessions comprising beta/theta suppression and alpha stimulation training. Treatment effects were defined as SMR/beta activity enhancement of 13–20 Hz, theta activity reduction of 4–7 Hz, and a decrease in electromyography (EMG) activity (Citation48).
Medication
All MED-randomized participants were treated with MPH at a dosage of 1 mg/kg/day in the form of long-acting MPH capsules. The total dose of MPH was between 20–60 mg. Compliance and side-effects were recorded.
Multimodal treatment
Multimodal treatment comprised a combination treatment of NF and MPH, with each treatment given as per their respective protocols already described.
Procedures
After the randomization process and group assignments (at T1), the participants were treated according to the group to which they were assigned. All three intervention groups received treatment for 3 months administrated of the child and adolescent psychiatrist (i.e. until treatment study completion, T2). All treatments evaluation were made at three time points: 1 week before treatment initiation (T1), at treatment completion (T2), and at 6-month (T3). At the 6-month follow-up period defined as the time from T2 to T3, only children assigned to the MED group continued with medication treatment. No NF treatment was given during this follow-up period.
Outcomes
Parents and teachers report
The two main core symptoms of ADHD—attention deficit and hyperactivity—were evaluated using Barkley’s Defiant Children: A Clinician’s Manual for Assessment and Parent Training (Citation58). The rating scale is divided into three rating sub-scales for inattention and hyperactivity (Forms 4, 5, and 6), as well as a total score. Parents and teachers were asked to complete these rating forms at three study time points: at treatment initiation (T1), at the end of the 3-month study treatment period (T2), and at the end of the 6-month follow-up period (T3).
Self-report
To ensure the child participant is able to complete the entire self-report questionnaire (SRQ), the number of questions in the questionnaire was limited only to those related to ADHD. Thus, a specialized questionnaire was designed based on the Self-rated Scale of Self-regulating Function and the Piers-Harris Children’s Scale of Self-Concept (Citation59). The SRQ comprised five separate items, two of which were relevant to the ADHD symptoms of inattention and hyperactivity, while three were related to the academic performance and skill levels of the child in reading, writing techniques, and math. The total of the individual scores to these three latter questions defined the school performance. The children rated themselves from 1–10, based on their perception of their attention deficiency, school performance, and hyperactivity.
Treatment evaluation
Treatment evaluation was conducted at three specific study time points (T1, T2, T3). A baseline assessment (T1) was completed at 1 week prior to treatment initiation. Treatment effectiveness in terms of changes in core symptoms from baseline was then evaluated at 1 week after study treatment completion (T2), and then also at 6-month follow-up post-treatment completion (T3). Results on treatment evaluation at T2 were published previously (Citation48,Citation49).
Statistical analysis
To investigate the baseline data, basic descriptive methods were used. A generalized linear-model repeated measure was used to investigate treatment effects on outcomes. For each outcome, we fitted a model depending on the treatment groups (overall treatment effect), time (overall time effect), and the interaction between time and treatment (time-dependent treatment effect). We used simple contrasts, i.e. changes from baseline, in the model to avoid the assumption of linearity in the time domain. The marginal means from the model and their 95% confidence intervals (CIs) were used to illustrate the effects graphically.
The level of significance was set at 0.05. All calculations were carried out using SPSS22 (Citation52). Graphical illustrations were done using MATLAB 2010 (Citation60).
Ethics
Written consent was obtained from all participating parents and children. The study design and protocol were approved by the Regional Ethics Committee on Medical Research.
Results
Participants
Of all randomized patients who participated, 62% completed the study, including the follow-up period (). At baseline (T1), there was no significant difference in ADHD core symptoms and socio-demographic status, including factors such as family constellation, siblings, parent’s education, and economic and other support, between the treatment groups. There was also no significant difference in academic skills (IQ mean difference = −9.8, 95% CI = −7.9–5.9) between the treatment groups at baseline (T1) ().
Figure 1. Clinical population of children and adolescents with Attention Deficit/Hyperactivity Disorder invited to this 6-month follow-up randomized trial.
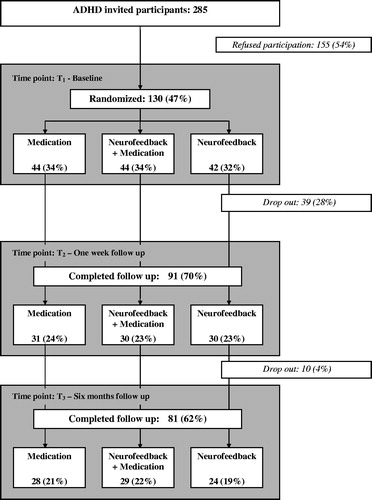
Table 1. A clinical randomized controlled study with 91 participants. Demographic and ADHD symptoms in the pre-treatment period (T1).
Ninety-one children and adolescents successfully completed treatment (T2). A total of 39 (30%) participants in the three treatment groups dropped out before (n = 29) and during (n = 10) the treatment period due to either parental or participants’ lack of interest and motivation or other practical reasons (). No significant difference in socio-demographic status was found at T3 between participants and dropouts. During the 6-month follow-up period, 10 more participants dropped out and 81 (62%) completed the study until the end of follow-up (T3).
Outcome comparison within treatment groups
Results of treatment outcomes within each treatment groups are shown in and .
Figure 2. Effectiveness patterns towards treatment. 0 = baseline, 3 = completed treatment, 9 = completed follow-up period.
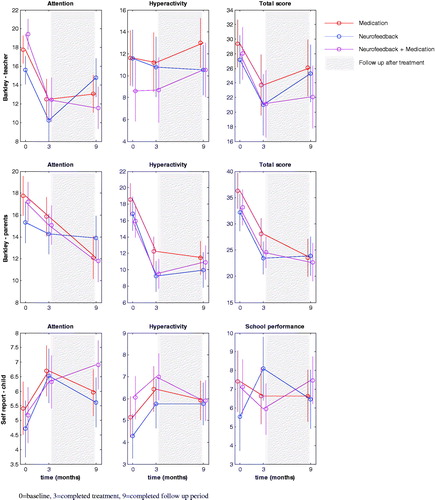
Table 2. ADHD core symptoms reported from teacher, parent, and self-report from baseline to 6 months follow-up.
Within each treatment group, there was significant treatment improvement (p = 0.01) on all three rating sub-scales of the Barkley scores for inattention, as reported by parents and teachers, and patient improvement was evident during the treatment period, while the scores remained stable during the follow-up period, as shown in . On the other hand, hyperactivity was reported by teachers as remaining stable throughout the entire study.
Significant improvement in inattention (p = .01) and hyperactivity (p = .01) was self-reported by children and adolescents. Patient improvement was also evident here during treatment. No changes were self-reported in school performance.
Outcome comparison between treatment groups
Parents and teachers from all treatment groups reported significant treatment effects on inattention (teachers: p = 0.01; parents: p = .02) in all groups. However, the most marked changes in all three rating sub-scales of the Barkley scores were obtained in the NF + MED (multimodal) and MED (medication) groups.
In contrast, no significant treatment-dependent changes in self-reported attention and hyperactivity were observed. During the treatment period, significant improvements in school performance were self-reported (p = .02), although there was no significant change during the follow-up period.
Discussion
In this RCT with 6-month follow-up, improvements in ADHD core symptoms were reported by parents and teachers, as well as self-reported by participants. However, there were no changes in hyperactivity reported by teachers throughout the entire study. In addition, treatment-dependent changes were observed in the multimodal treatment group (NF + MED), which was comparable to the findings from the MED group at 6-month follow-up.
Various treatments are available for ADHD treatment that is applicable in clinical practice. However, no consistent evidence has been found for any one particular therapy that would provide meaningful benefits in the long-term for children and adolescents with ADHD. Evidence for the effects of multimodal treatment (combined medication and NF) has also been poorly described in previous studies.
In our study, the effects of multimodal treatment after treatment completion (T2) were previously shown to be non-significant with a low ES for ADHD core symptoms (Citation48). However, the effectiveness of multimodal treatment at 6 months post-treatment reflects a different trend. Our results support previous findings of Monastra et al. (Citation17), which showed the best ADHD core symptom improvements with the combination treatment of NF and MPH. In addition, Li et al. (Citation47) reported multimodal treatment with NF and MPH to be effective, and also suggested that dose reduction of MPH could be possible.
Combination treatment has its advantages, including reduction in medication dosage and less time required for parents and teachers to spend on behaviour monitoring. However, for sustained long-term improvements aimed at improving the quality-of-life, longer follow-up periods of more than 6 months are required. On the other hand, the impact of non-specific factors (such as parental support and/or cognitive training) during NF treatment could also contribute to a positive behavioural effect later reported in NF treatment, as shown in previous studies (Citation35,Citation61). Moreover, parental support, including simply providing transportation for participants to and from the treatment centre, is also of importance when evaluating the effects of NF and multimodal treatments.
In this study, the disparity of treatment given during the 6-month follow-up period, i.e. continuing pharmacologic treatment but not NF, was because the medication group acted as controls and we considered it appropriate to compare the effects of the gold standard pharmacologic treatment and multimodal treatment. The first RCT with 6-month follow-up was by Meisel et al. (Citation62) in 2013, comparing NF and a stimulant medication in ADHD. Our study is the first randomized controlled study addressing not only the separate effects of NF and medication, but also the effects of combined NF and pharmacologic (MED) treatments. Overall, no significant difference in treatment effects between NF, MED, and NF + MED was found. The likely explanation is that, although NF did not enhance the effects of MED alone or surpass the positive effects of MED alone, NF treatment alone was equivalent to MED treatment alone and did not show any negative treatment effects, either alone or in combination with MED.
In the present study, the randomization process required a longer study period of 3 years due to the number of participants and the three-arm study design. We included approximately half of all ADHD children referred to our clinic (Citation48). It was not possible to investigate whether there were any significant differences in clinical symptoms, academic skills, and socio-demographic status between non-participants and participants. However, our dropout analysis did not demonstrate any significant differences in clinical symptoms, socio-demographic status, or academic abilities between the dropout population and those who completed the study (Citation48).
The three-arm design of our study compared the change(s) elicited by a multimodal treatment (NF + MED) with those by a single treatment (either NF or MED) to explore the effects of NF as an alternative treatment regimen that could eventually help to reduce medication dosages. We found that NF + MED treatment was superior, as expected. Interestingly, other studies evaluating combined pharmacologic and non-pharmacologic treatments (NF, cognitive therapy, parental consulting, or education) showed combined/multimodal treatment was not superior, but rather comparable, to single treatments (e.g. either MED or NF alone) (Citation45,Citation46).
Study strengths and limitations
The strengths of this study include the effective randomization of subject groups as well as comprehensive assessments of children with respect to age, sex, IQs, and distribution of ADHD core symptoms. Although the randomized group sizes were comparatively smaller, results obtained seem to be important in shedding light on the approaches used to treat ADHD. Despite the dropout rate encountered in the study, we consider the study design to be sufficiently robust for our RCT on an ADHD population, considering a few thousand NF treatments and reports from participants, and their parents and teachers were successfully given and assessed.
A limitation to the present study is the small sample size (approximately two in three participants) that completed the study in each treatment group. The high number of dropouts (one in four) compromised the established sample size, although dropout rates were similar in all three treatment groups. The majority of dropouts were observed at baseline, which could indicate that insufficient information was given to parents and children/adolescents about the treatments at the start of the study. Another limitation is the lack of an untreated control group in the study design. This was not possible for ethical reasons. In addition, the impact of non-specific factors, such as parental support and/or cognitive training, during NF treatment has been evaluated (Citation35,Citation61), e.g. patient–therapist interactions and the time spent with the therapist in a structured learning environment could contribute to a positive behavioural effect reported in NF treatment (Citation61). NF sessions are thought to be a form of ‘cognitive training’, as the therapist and patient interact, meet regularly, and spend time together. Evaluation of cognitive training activities could have positive implications on NF treatment results, as they may support the learning process, thereby enhancing the treatment effects.
Conclusions
Multimodal treatment using combined stimulant medication and NF showed 6-month efficacy in ADHD treatment. More research is needed to explore whether multimodal treatment is suitable for ADHD children and adolescents who showed poor response to single stimulant medication treatment.
Acknowledgements
We are grateful to all participants and their families who took part in this clinical study. We thank the Child and Adolescent Psychiatry Department of Helse Fonna Hospital Haugesund, Helse Fonna Trust Haugesund, Norway for its support in completing this study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The authors received no financial support for the research or authorship of this article. Trial registration: NCT01252446 Current Controlled Trials.
Additional information
Funding
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
- Langberg JM, Brinkman WB, Lichtenstein PK, Epstein JN. Interventions to promote the evidence-based care of children with ADHD in primary-care settings. Expert Rev Neurother 2009;9:477–87.
- Langberg JM, Froehlich TE, Loren RE, Martin JE, Epstein JN. Assessing children with ADHD in primary care settings. Expert Rev Neurother 2008;8:627–41.
- Norsk barne- og ungdomspsykiatriskforening DNL. Veileder i Barne- og Ungdomspsykiatri. 2010.
- Zwi M, Jones H, Thorgaard C, York A, Dennis JA. Parent training interventions for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev 2011;12:CD003018.
- Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA 2010;304:875–80.
- Webster-Stratton C. Combining parent and child training for young children with ADHD. J Clin Child Adolesc Psychol 2011;40:191–203.
- Costin J, Chambers SM. Parent management training as a treatment for children with oppositional defiant disorder referred to a mental health clinic. Clin Child Psychol Psychiatry 2007;12:511–24.
- Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 2013;170:275–89.
- Fredriksen A, Aune T, Aarseth J. Kognitiv atferdsterapi med små barn. Tidsskrift Norsk Psykologforen 2011;48.
- Berge T, Repål A. Kognitiv Terapi for Barn Og Unge. I. Håndbok i Kognitiv Terapi. Oslo: Gyldendal Akademisk; 2009.
- Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux PY, Secnik K. Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. J Adolesc Health 2004;35:346 e1–9.
- (NICE) LNIfHaCE. Developing NICE Guidelines: The Manual. Process Meth Guides NICE 2015;20.
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456–63.
- Methylphenidate: abuse in Europe. Prescrire Int 2013;22:47.
- Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood – a naturalistic long-term follow-up study. Addict Behav 2013.
- Monastra VJ, Monastra DM, George S. The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Appl Psychophysiol Biofeedback 2002;27:231–49.
- Thompson L, Thompson M. Neurofeedback combined with training in metacognitive strategies: effectiveness in students with ADD. Appl Psychophysiol Biofeedback 1998;23:243–63.
- Breggin PR, Talking back to Ritalin. Monroe, ME: Common Courage Press. Breggin, P R (1998) Talking back to Ritalin Monroe, ME: Common Courage Press; 1998.
- Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry 1999;38:503–12.
- Spencer TJ, Wilens TE, Biederman J, Weisler RH, Read SC, Pratt R. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of attention-deficit/hyperactivity disorder in adolescent patients: a 4-week, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006;28:266–79.
- Weiss MD, Gadow K, Wasdell MB. Effectiveness outcomes in attention-deficit/hyperactivity disorder. J Clin Psychiatry 2006;67(Suppl 8):38–45.
- Conners CK, Blouin AG. Nutritional effects on behavior of children. J Psychiatr Res 1982;17:193–201.
- Millichap JG, Yee MM. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics 2011.
- Murphy P, Likhodii SS, Hatamian M, McIntyre Burnham W. Effect of the ketogenic diet on the activity level of Wistar rats. Pediatr Res 2005;57:353–7.
- Arns M, Heinrich H, Strehl U. Evaluation of neurofeedback in ADHD: The long and winding road. Biol Psychol 2014;95:108–15.
- Heinrich H, Gevensleben H, Strehl U. Annotation: neurofeedback - train your brain to train behaviour. J Child Psychol Psychiatry 2007;48:3–16.
- Moriyama TS, Polanczyk G, Caye A, Banaschewski T, Brandeis D, Rohde LA. Evidence-based information on the clinical use of neurofeedback for ADHD. Neurotherapeutics 2012;9:588–98.
- Gevensleben H, Rothenberger A, Moll GH, Heinrich H. Neurofeedback in children with ADHD: validation and challenges. Expert Rev Neurother 2012;12:447–60.
- Arns M, Drinkenburg W, Leon Kenemans J. The effects of QEEG-informed neurofeedback in ADHD: an open-label pilot study. Appl Psychophysiol Biofeedback 2012;37:171–80.
- Surmeli T, Ertem A. Post WISC-R and TOVA improvement with QEEG guided neurofeedback training in mentally retarded: a clinical case series of behavioral problems. Clin EEG Neurosci 2010;41:32–41.
- Monastra VJ. Quantitative electroencephalography and attention-deficit/hyperactivity disorder: implications for clinical practice. Curr Psychiatry Rep 2008;10:432–8.
- Butnik SM. Neurofeedback in adolescents and adults with attention deficit hyperactivity disorder. J Clin Psychol 2005;61:621–5.
- Friel PN. EEG biofeedback in the treatment of attention deficit/hyperactivity. Alter Med Rev 2007;12.
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci 2009;40:180–9.
- Holtmann M, Sonuga-Barke E, Cortese S,DB. Neurofeedback for ADHD: a review of current evidence. Child Adolesc Psychiatr Clin N Am 2014;23:789–806.
- Bakhshayesh AR, Die Wirksamkeit von Neurofeedback im Vergleich zum EMGBiofeedback bei der Behandlung von ADHS-Kindern. PhD thesis, Universität Potsdam, Germany 2007.
- Gevensleben H, Holl B, Albrecht B, Vogel C, Schlamp D, Kratz O, et al. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J Child Psychol Psychiatry 2009;50:780–9.
- Lubar JF, Lubar JO. Neurofeedback assessment and treatment for attention deficit/hyperactivity disorders introduction to quantitative EEG and neurofeedback New York: Academic Press; 1999, pp. 103–41.
- Lubar JF, Swartwood MO, Swartwood IN, O'Donnell P. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul 1995;20:83–99.
- Gevensleben H, Holl B, Albrecht B, Schlamp D, Kratz O, Studer P, et al. Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. Eur Child Adolesc Psychiatry 2010;19:715–24.
- Leins U, Goth G, Hinterberger T, Klinger C, Rumpf N, Strehl U. Neurofeedback for children with ADHD: a comparison of SCP and Theta/Beta protocols. Appl Psychophysiol Biofeedback 2007;32:73–88.
- Fuchs T, Birbaumer N, Lutzenberger W, Gruzelier JH, Kaiser J. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: a comparison with methylphenidate. Appl Psychophysiol Biofeedback 2003;28:1–12.
- Strehl U, Leins U, Goth G, Klinger C, Hinterberger T, Birbaumer N. Self-regulation of slow cortical potentials: a new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics 2006;118:e1530–40.
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Bio Psychiatry 2007;61:1361–9.
- Lofthouse N, Arnold LE, Hersch S, Hurt E, DeBeus R. A review of neurofeedback treatment for pediatric ADHD. J Atten Disord 2012;16:351–72.
- Li L, Yang L, Zhuo CJ, Wang YF. A randomised controlled trial of combined EEG feedback and methylphenidate therapy for the treatment of ADHD. Swiss Med Week 2013;143:w13838.
- Duric NS, Assmus J, Gundersen D, Elgen IB. Neurofeedback for the treatment of children and adolescents with ADHD: a randomized and controlled clinical trial using parental reports. BMC Psychiatry 2012;12:107.
- Duric NS, Assmus J, Elgen IB. Self-reported efficacy of neurofeedback treatment in a clinical randomized controlled study of ADHD children and adolescents. Neuropsychiatric Disease and Treatment 2014;10:1645–54.
- Duric NS, Elgen IB. Characteristics of Norwegian children suffering from ADHD symptoms: ADHD and primary health care. Psychiatry Res 2011;3:402–5.
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision; 1992.
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0.
- Wechsler D. The Wechsler Intelligence Scale for Children (WISC). 1949.
- Duric NS, Children and Adolescents with Attention Deficit Hyperactivity Disorder;Characteristics and Treatment with Neurofeedback [doctoral thesis]. www.uib.no2014.
- Thought Technology Ltd.
- Friel PN. EEG biofeedback in the treatment of attention deficit hyperactivity disorder. Altern Med Rev 2007;12:146–51.
- Lubar JF. Quantitative Electroencephalographic Analysis (QEEG) databases for neurotherapy: description, validation, and application. CRC Press; 2004. p. 200.
- Barkley RA, Defiant children. 2nd ed. A clinician's manual for assessment and parent training: Guilford Publications, Inc.; 1997.
- Linden M, Habib T, Radojevic V. A controlled study of the effects of EEG biofeedback on cognition and behavior of children with attention deficit disorder and learning disabilities. Biofeedback Self Regul 1996;21:35–49.
- MathWorks. MATLAB; 2010.
- Bakhshayesh AR, Hansch S, Wyschkon A, Rezai MJ, Esser G. Neurofeedback in ADHD: a single-blind randomized controlled trial. Eur Child Adolesc Psychiatry 2011.
- Meisel V, Servera M, Garcia-Banda G, Cardo E, Moreno I. Neurofeedback and standard pharmacological intervention in ADHD: a randomized controlled trial with six-month follow-up. Biol Psychol 2013;94:12–21.
