Abstract
Background: Accurate prevalence rates of the neurodevelopmental disorders (ND) and comorbid conditions in child and adolescent mental health services (CAMHS) are essential for treatment planning and organization of health care. However, valid and reliable prevalence estimates from Nordic CAMHS populations are scarce, and the published findings vary.
Aims: To report prevalence rates of ND (attention-deficit hyperactivity disorder: ADHD, tic disorder: TD or autism spectrum disorder: ASD) and comorbid disorders by a validated diagnostic instrument in children referred to CAMHS outpatient clinics.
Methods: Parents of 407 consecutively referred children aged 7–13 years were interviewed with the semistructured interview schedule for affective disorders and schizophrenia, present and lifetime version (Kiddie-SADS-PL) at time of admittance.
Results: One or more ND was diagnosed in 226 children (55.5%; 69.9% boys): ADHD (44.5%; 68.5% boys); TD (17.7%; 77.8% boys) and ASD (6.1%; 76% boys). Among children with ND 70 (31.0%) had only one ND with no comorbid disorder, 49 (21.7%) had more than one ND (homotypic comorbidity) and 131 (58%) had a non-ND psychiatric disorder (heterotypic comorbidity). Anxiety disorders were the most frequently occurring heterotypic comorbidity in all three ND. Comorbid depressive disorder was associated with older age, and comorbid anxiety disorder with female gender.
Conclusion: In children referred to CAMHS, ND constitute the most frequently occurring group of disorders, with high rates of both homotypic and heterotypic comorbidity. This needs to be taken into consideration in health service planning and treatment delivery.
Background
Neurodevelopmental disorders (ND) are increasingly being recognized as a leading cause of morbidity in children, causing great suffering for patients and their families and large costs for society [Citation1]. Comorbid disorders may contribute to cognitive impairment [Citation2], reduced quality of life [Citation3,Citation4], and poor long-term prognosis [Citation5,Citation6], and they have implications for choice of treatment [Citation7,Citation8]. Accurate prevalence rates of ND and comorbid conditions in child and adolescent mental health services (CAMHS) are essential for treatment planning and organization of health care [Citation9]. However, valid and reliable prevalence estimates from Nordic CAMHS populations are scarce, and the published findings vary.
In the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V), the psychiatric disorders grouped under the term ND include attention-deficit hyperactivity disorder (ADHD), tic disorders (TD) and autism spectrum disorders (ASD) [Citation10]. These disorders share neuropathology, symptoms, and genetics to such an extent that they may be viewed as a spectrum rather than distinct disorders [Citation11,Citation12]. In this article, we will use the designation ND to indicate ADHD, TD and ASD.
Prevalence estimates of ND from Nordic CAMHS populations vary considerably and the use of different assessment methods may account for a great part of this variation. Prevalence estimates of ADHD range from 20.8 [Citation13] to 44.5% [Citation14], of TD from 1.8 [Citation13] to 17.7% [Citation14], and of ASD from 2.3 [Citation13] to 10.3% [Citation15]. Despite different diagnostic procedures across studies, ADHD clearly seems to be the most frequent ND encountered in CAMHS.
As to comorbidity in ADHD, overall psychiatric comorbidity reported from national patient registers ranges from 29.5 [Citation16] to 52% [Citation17], while a comorbidity rate of 30% was reported in a Swedish study in which hospital records were retrospectively investigated [Citation13]. In comparison, rates of comorbid psychiatric disorders in non-Nordic ADHD populations assessed by means of standardized diagnostic interviews range from 55.6 [Citation18] to as high as 96.3% [Citation19], the latter in a study from a tertiary university clinic. In the multinational ADHD Observational Research in Europe (ADORE) study, an overall comorbidity rate of 68.1% (including learning disorders) was reported [Citation20], quite similar to a comorbidity rate of 66% reported from an Italian multicenter study [Citation21].
Concerning the specific comorbid conditions in ADHD, prevalence rates of TD ranged from 4.8 [Citation17] to 7.5% [Citation16] in the Nordic studies, compared to 8.6 [Citation18] to 14.8% [Citation19] in non- Nordic countries using standardized diagnostic interviews. Correspondingly, comorbid ASD in the Nordic studies was reported in 7.2 [Citation16] to 12.4% [Citation17] of cases compared to 15.4% in the ADORE population [Citation20]. The most frequent non-ND comorbid condition (heterotypic comorbidity) reported from both the Nordic and non-Nordic countries in clinical ADHD populations have been disruptive behavior disorders (DBD) [oppositional defiant disorder (ODD) and conduct disorder (CD)]; albeit with the Nordic countries reporting lower rates ranging from 4.2 [Citation16] to 28.1% [Citation22] compared to rates from 31.9 [Citation18] to 75.0% [Citation19] in the non-Nordic countries. Rates of comorbid anxiety disorder were also lower in the Nordic studies (1.3 [Citation17] to 13.6% [Citation22]) compared to those from other countries (from 19.8 [Citation18] to 49.1% [Citation19]).
For TD, we found no Nordic studies from ordinary CAMHS clinics, while two studies report from specialized TD clinics. In a Swedish study, ADHD was reported in 64%, ASD in 22%, DBD in 36% and obsessive compulsive disorder (OCD) in 38% of children with Tourette’s syndrome; anxiety and depressive disorders were not assessed [Citation23]. In a Danish study, comorbid ADHD and/or OCD was reported in 61.4% of children with Tourette’s syndrome [Citation24]. In studies from non-Nordic countries, comorbidity rates as high as 90% have been reported in clinical TD populations [Citation25]. Among children with TD recruited for a behavioral intervention study, 64% had one or several comorbid conditions: ADHD in 26%, any anxiety disorder in 37%, OCD in 19%, ODD in 6% and enuresis in 5% [Citation26].
Regarding comorbidity in ASD, 75% of children with ASD in a Finnish study had one or more comorbid disorders. Comorbidity was assessed with a standardized diagnostic interview [Citation27]. A similar rate of 72% was reported in a sample of Norwegian children with ASD attending a special school, also assessed by means of a standardized diagnostic interview [Citation28]. In both studies, the most frequent comorbid disorder was anxiety (in 45 and 41%, respectively), followed by ADHD (in 40 and 31%, respectively) and TD (in 23 and 11%, respectively). There is a notable difference in the prevalence of comorbid DBD in the Finnish and the Norwegian study: 22.5% in the former [Citation27] and 4% in the latter study [Citation28]. In comparison, 80.9% of Dutch children with PDD-NOS had one or more comorbid disorders: anxiety in 55.3%; ADHD in 44.7%; ODD in 37.2%; CD in 9.6% and comorbid TD was not assessed [Citation29].
There have been few studies and varying results concerning the influence of gender and age on comorbidity in children with ND. In a Danish ADHD population, male gender was associated with neuropsychiatric comorbidity, and female gender with anxiety and depression [Citation17], while similar levels of coexisting psychiatric impairment in boys and girls were reported in the multinational ADORE study [Citation30]. Gender did not influence comorbidity rates in ASD in a Norwegian study [Citation28], while male gender was associated with comorbid ADHD in a US study based on hospital records [Citation31]. Conflicting results have been reported regarding age influence on comorbidity in ASD from Nordic studies; while one study reported comorbid OCD to be associated with higher age [Citation28], another reported less comorbidity in older age [Citation27].
In summary, Nordic prevalence studies of ND and comorbid conditions in CAMHS are scarce, and the results vary. The influence of gender and age on comorbidity rates is understudied with conflicting findings. Even though the use of a standardized diagnostic interview is recommended in the diagnostic assessment [Citation32], few studies have included such an instrument. Concern has been raised as to the accuracy of prevalence estimates of psychiatric diagnoses in CAMHS as standardized diagnostic methods often are not applied [Citation14,Citation33]. Hence, more studies using standardized diagnostic methods are needed to obtain more valid and reliable prevalence estimates, including knowledge about the influence of gender and age on comorbidity.
The aim of the present study was to assess prevalence rates of ND and comorbid disorders, including the influence of gender and age, in consecutive CAMHS referrals by using a standardized diagnostic interview with their parents.
Materials and methods
This was a cross-sectional study among children referred to two Norwegian CAMHS outpatient clinics situated in the Akershus county/Oslo area. The catchment areas have a combined population of ∼75,000 children aged 0–18 years. The government-funded CAMHS clinics are the main service providers for child and adolescent psychiatric disorders in Norway, and outpatient clinics are the primary treatment settings [Citation34]. The CAMHS clinics in Norway offer treatment to ∼5% of the population 0–18 years of age [Citation34].
Participants
Participants were consecutive referrals admitted between September 2007 and February 2009, 7–13 years of age. A total of 552 children in this age group were admitted to the two clinics, and parents of 421 of these children (76.3%) were interviewed. For 407 of the children, written consent was obtained from both parents. The main reason for nonparticipation was failure to show up after one reminder was given, and in a few cases due to an emergency referral. We did not have consent to use information from nonparticipating children. Details on demographic and clinical data have been published previously [Citation14].
Procedure
The recruitment procedure was part of a study on anxiety disorders in children 7–13 years of age. Parents of consecutive referrals in the relevant age group admitted to the clinics were invited to a diagnostic interview. Details of the procedure have been published previously [Citation35]. The study was approved by the Regional Committee for Medical Research Ethics.
Instruments
Psychiatric disorders were assessed using the semi-structured diagnostic interview Schedule for Affective Disorders and Schizophrenia, present and lifetime version (Kiddie-SADS-PL), DSM-IV version [Citation36]. Good values on validity and acceptable values for inter-rater reliability [Citation36–38] have been reported. The validity of Kiddie-SADS-PL interview (when both parents and child were interviewed) versus longitudinal expert all data (LEAD) procedure were reported as excellent for anxiety disorders, depression, behavioral disorders, and TD; good for ADHD, and good-to-moderate for ASD [Citation39]. This study reports the prevalence of present diagnoses based on interviews with the parents. All interviews were undertaken by experienced clinicians.
The psychiatric disorders derived from the Kiddie-SADS-PL interview were grouped as follows:
Depressive disorders: major depressive disorders, dysthymia, and depressive disorder NOS
DBD: ODD, CD, and disruptive disorder NOS
TD: chronic tics, transient tics, Tourette’s syndrome, and tics INA
ASD: infantile autism, Asperger’s syndrome, and pervasive developmental disorder NOS
Anxiety Disorders: panic disorder, agoraphobia, separation anxiety disorder, social anxiety disorder, specific phobias, generalized anxiety disorder, and post-traumatic stress disorder
Statistical analyses
Data were analyzed using IBM SPSS Statistical Software version 24.0 and Stata version 14.0 (College Station, TX). The Chi-square (χ2) test was used to compare differences in distributions of categorical variables. Differences in comorbidity rates between the specific ND were analyzed with the two-sample z-test for proportions. Normality of distribution was tested on Q–Q-plot and by Kolmogorov–Smirnov test. The Student’s t-test was used to compare means of age when assumption of normality of distribution was satisfied; otherwise, the Mann–Whitney U-test was used. Significance level was set as p < .05 and adjusted with Bonferroni correction to p < .05/n (where n is the number of analyses).
Results
A total of 226 children met criteria for at least one ND, comprising 55.5% of the total sample. Boys comprised a significant majority of referred children (66.3%). There was no significant difference in gender distribution or mean age between the ND group and the non-ND psychiatric disorder group () or between any specific ND and the other ND (for example ADHD versus non-ADHD ND) (data not shown).
Table 1. ND, non-ND psychiatric disorders, and no psychiatric disorder in 407 consecutive referrals aged 7–13 years.
Among children with ND, 31.4% (N = 71) had no comorbid disorder, 21.2% (N = 48) had more than one ND (homotypic comorbidity) (), and 58.0% (N = 131) had one or more non-ND comorbid disorder (heterotypic comorbidity).
Figure 1. Homotypic comorbidity (neurodevelopmental disorder, ND) in 226 children aged 7–13 years with ND according to parent interview with Kiddie-SADS-PL (DSM IV version). ASD: autism spectrum disorders; TD: tic disorders.
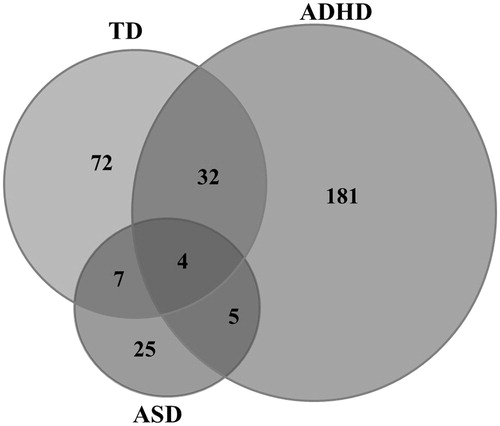
A significantly lower proportion of children with ADHD had a comorbid condition compared to children with TD (p < .001) or ASD (p = .008) (). This was driven by a significantly lower proportion of children with ADHD having a homotypic comorbid condition compared to children with TD or ASD (ADHD: 22.6%, TD: 59.7%, ASD: 64.0%; for both comparisons p < .001), while there was no significant difference in heterotypic comorbidity. Anxiety disorders comprised the most prevalent heterotypic comorbidity in all three groups, followed by DBD ().
Table 2. Homotypic comorbidity (neurodevelopmental disorder, ND) and heterotypic (non-ND) comorbidity in 226 children aged 7–13 years with ND according to parent interview with Kiddie-SADS-PL (DSM IV version).
Comorbid anxiety disorders were associated with female gender (). This association was driven by a significantly higher proportion of girls with ADHD having a comorbid anxiety disorder compared to boys with ADHD (ADHD girls: 43.9%, ADHD boys: 18.5%, p < .001). The higher median age related to comorbid depressive disorder was due to a significantly higher median age in children with TD and comorbid depressive disorder compared to children with TD and no comorbid depressive disorder [median (interquartile range) 12.42 (10.85–13.40) years versus 10.00 (8.33–11.54) years; p = .018].
Table 3. Gender distribution and mean age by comorbid status in 226 children aged 7–13 years with a ND.
Discussion
Children with ND constituted 55.5% of children referred to CAMHS, and thus comprised the largest group of patients. This prevalence estimate is comparable to those reported from a Nordic study in adolescents, also using a standardized diagnostic interview [Citation33]. The rates of the specific ND were in line with a Danish study using a standardized diagnostic interview [Citation40] and, with one exception (ASD rate in Kriz et al. [Citation15]), higher than previously reported from the studies using a less structured assessment method [Citation13,Citation15].
Overall, our prevalence estimates may be too low, as they are based on a parental interview at one time-point only. Applying the LEAD procedure may yield higher prevalence estimates of the neurodevelopmental disorders ADHD and TD [Citation39,Citation41]. Applying the gold standard assessment techniques for ASD diagnosis, the ADI-R and ADOS may give a more correct estimate of this group of disorders. Child and parents provide unique information to the diagnostic process, and children are generally considered better informants of their inner states [Citation42], hence including child interview could have resulted in higher estimates of internalizing comorbid disorders. Such a procedure would also enable a direct observation of the child, and provide useful clinical information on presence of tics, as well as social and communicative skills.
Nevertheless, the specific ND in our study had a comorbidity rate in a magnitude comparable to that previously reported in Nordic ADHD CAMHS populations [Citation17] and in non-Nordic clinical ND populations (ADHD, TD and ASD) [Citation20,Citation21,Citation43–45]. The rate of comorbidity in ASD in our study (92%) was higher than previously reported in Nordic studies (74 and 72%) [Citation27,Citation28]. This may be explained by differences in recruitment procedure (via hospital records and not assessment of consecutive referrals) [Citation27] and differences in study populations (CAMHS samples versus special school attendees) [Citation28]. In our sample, comorbidity was especially prevalent among children with TD and ASD and significantly higher compared to children with ADHD. These results contrast with those of a Dutch study that found no difference in comorbidity rate between children with ADHD and those with ASD [Citation46]. However, our study and theirs are difficult to compare, as the diagnoses of ADHD and ASD were not established by structured interview, and neither TD nor elimination disorders were included in comorbidity analyses. As for the higher comorbidity rate in ASD, there is a phenotypic overlap between psychiatric disorders and ASD [Citation28], which may explain the higher load of reported comorbid psychiatric disorders in children with ASD.
As to TD, the reason for the higher rate of comorbidity in children with TD compared to those with ADHD is unclear. We can, however, speculate that referral practices play a role. In TD, more so than in ADHD, comorbid conditions might be conceived as the most impairing [Citation25] and be the reason for referral to CAMHS. Hence, clinical TD samples may be more biased towards higher comorbidity load than clinical ADHD samples.
Anxiety disorders were the most frequent heterotypic comorbid condition among children with ND in our study. Our rates were higher than what was reported in the Nordic studies, which used a less structured diagnostic approach [Citation13,Citation17], and comparable to rates reported from studies using a structured diagnostic approach [Citation20,Citation21,Citation26,Citation28]. Hence, the previously reported lower rates of comorbid anxiety disorder in clinical ND populations from the Nordic countries compared to those from other countries could result from differences in assessment methods rather than true differences by geographical location. Previous reports have expressed concerns as to whether anxiety disorders are properly identified in CAMHS unless structured diagnostic procedures are used [Citation14,Citation32]. Comorbid anxiety disorders have a known impact on behavior, choice of treatment, and prognosis in ADHD and ASD [Citation8,Citation47]. In TD, research has mainly focused on comorbidity with ADHD and OCD, and to some extent ASD [Citation23,Citation25]. However, recognition of the importance of comorbid anxiety on quality of life, psychosocial functioning, and impact on symptom severity in TD is emerging [Citation4,Citation25,Citation26,Citation48]. For instance, a relationship between anxiety, propensity of aggressive fear response, and tic severity in children with TD has recently been discussed [Citation49]. Consequently, it is of importance to recognize that comorbid anxiety may aggravate aggressive behavior and tic severity in children with TD. As a conclusion, efforts should be made to identify comorbid anxiety disorders in all ND, and if present specific treatment should be offered.
The rates of DBD among children with ADHD, TD or ASD in our study are, with one exception [Citation27], comparable to previous reports from the Nordic countries, including those using structured diagnostic assessments [Citation17,Citation23,Citation28], but lower compared to those reported from non-Nordic countries [Citation20,Citation44]. Hence, the lower prevalence rates of comorbid DBD in our study may be due to a true difference in prevalence of comorbid DBD between the Nordic and non-Nordic countries. This interpretation is supported by findings from a study comparing Norwegian and British children’s rates of DBD [Citation50]. DBD is a condition considerably influenced by psychosocial factors [Citation51], and lower rates of DBD in Norwegian children have been associated with a more favorable socioeconomic profile and lifestyle in the Nordic countries [Citation50]. However, cultural differences regarding parent’s perception and reporting of their child’s behavioral problems must be considered: Nordic parents generally score lower on questionnaires measuring child mental health problems compared to parents from other countries [Citation50]. Also, differences in health care organizations may play a role. For example, in Norway, counseling of families with a child having DBD without other psychiatric conditions has mainly been the responsibility of the child welfare system and/or other primary care institutions, not CAMHS. Hence, the possibility of children with DBD with an undetected ND not being referred to a CAMHS clinic in Norway cannot be ruled out [Citation52]. This may lower the occurrence of children with ND and comorbid DBD in Norwegian CAMHS samples.
Elimination disorder—enuresis and/or encopresis—was the third most frequent heterotypic comorbidity in children with ADHD and in children with TD. Co-occurrence of elimination disorders in ADHD and TD has previously been reported [Citation26,Citation43,Citation48]. Elimination disorders cause significant distress and low self-esteem in children [Citation53] and thus deserve clinical attention, including assessment and evidence-based treatment [Citation54,Citation55].
Boys comprised a significant majority of all referred children, but had no higher propensity for having ND or a comorbid disorder than referred girls. We found a significantly higher proportion of girls with ADHD who had a comorbid anxiety disorder compared to boys with ADHD. An association between female gender and comorbid anxiety disorders in ADHD has previously been reported from Nordic [Citation17] and non-Nordic clinical populations [Citation19], but it was not reported in the multinational ADORE study, even though girls had significantly more parent-rated emotional symptoms [Citation30]. The association between older age and comorbid depression in TD in our study is in line with at least one previous study [Citation56]. Clearly, more research is needed regarding the relationship between comorbidity, gender and age in ND.
ND comprised the largest group of patients in our sample of referrals to CAMHS, and rates of both homotypic and heterotypic comorbidity were high. This needs to be considered when planning health services for children with ND. Rather than centralized and specialized ND units, as has been suggested [Citation57], the general CAMHS clinics need to develop sufficient competence, procedures, and skills to identify and treat all ND and their comorbid conditions [Citation21,Citation58]. Structured diagnostic procedures should be implemented as routine in CAMHS clinics, and including a standardized diagnostic instrument in the diagnostic procedure is advisable. Further, sufficient competence in specialized treatment, such as habit reversal therapy for TD and cognitive behavior therapy for comorbid anxiety, as well as psychosocial interventions based on a thorough understanding of the underlying ND, are needed.
Given the chronic course of these disorders and their high degree of continuity into early adulthood for many individuals [Citation59], interventions in a long-term perspective will be needed, much in the same way as for other chronic diseases. Children with ND have a high occurrence of cognitive dysfunction, motor coordination disorders, language, and other learning disorders [Citation12,Citation16,Citation60–63]. As a consequence, multidisciplinary health care coordinated with educational and social support is needed to secure optimal development [Citation59,Citation64]. Further research on models of care is needed, including on how ND are best managed across the lifespan and across transition periods [Citation59].
Limitations
A note of caution is needed when interpreting the results. We only interviewed the parents, not the child; our sample consisted of referred children; this may introduce both information and selection bias. We only included 76.3% of referred children, which may have caused selection bias and hence reduce the generalizability of our findings. Children and adolescents with comorbid disorders are more likely to be referred to CAMHS than children and adolescents with non-comorbid conditions. This may possibly inflate the rates of comorbidity in our material and limit the generalizability of our findings. Further, we did not interview parents of all referred children and do not have any information on the nonparticipating children. The strength of the study is the use of a standardized diagnostic interview, carried out by a limited number of experienced clinicians, in a large sample of consecutive referrals to CAMHS.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jeste SS. Neurodevelopmental behavioral and cognitive disorders. Continuum. 2015;21:690–714.
- Yerys BE, Wallace GW, Sokoloff JL, et al. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009;2:322–333.
- Sørensen L, Plessen KJ, Nicholas J, et al. Is behavioral regulation in children with ADHD aggravated by comorbid anxiety disorder? J Atten Disord. 2011;15:56–66.
- Eapen V, Cavanna AE, Robertson MM. Comorbidities, social impact, and quality of life in Tourette syndrome. Front Psychiatry. 2016;7:97.
- Newcorn J, Miller SR, Ivanova I, et al. Adolescent outcome of ADHD: impact of childhood conduct and anxiety disorders. CNS Spectr. 2004;9:668–678.
- Gillberg CI, Helles A, Billstedt E, et al. Boys with Asperger syndrome grow up: psychiatric and neurodevelopmental disorders 20 years after initial diagnosis. J Autism Dev Disord. 2016;46:74–82.
- El Malhany N, Gulisano M, Rizzo R, et al. Tourette syndrome and comorbid ADHD: causes and consequences. Eur J Pediatr. 2015;174:279–288.
- Manassis K. When attention-deficit/hyperactivity disorder co-occurs with anxiety disorders: effects on treatment. Expert Rev Neurotherapeut. 2007;7:981–988.
- Caron C, Rutter M. Comorbidity in child psychopathology: concepts, issues and research strategies. J Child Psychol Psychiatry. 1991;32:1063–1080.
- Diagnostic and statistical manual of mental disorders, 4th edition (DSM-IV). Washington (DC): American Psychiatric Association; 1994.
- Kern JK, Geier DA, King PG, et al. Shared brain connectivity issues, symptoms,and comorbidities in autism spectrum disorder, attention deficit/hyperactivity disorder, and Tourette syndrome. Brain Connect. 2015;5:321–335.
- Gillberg C. The ESSENCE in child psychiatry: early symptomatic syndromes eliciting neurodevelopmental clinical examinations. Res Dev Disabil. 2010;31:1543–1551.
- Kopp S, Gilberg C. Swedish child and adolescent psychiatric out-patients - a five-year cohort. Eur Child Adolesc Psychiatry. 2003;12:30–35.
- Hansen BH, Skirbekk AB, Oerbeck B, et al. Non-obsessive – compulsive anxiety disorders in child and adolescent mental health services – are they underdiagnosed, and how accurate is referral information? Nord J Psychiatry. 2016;70:133–139.
- Kriz S, Thomsen P. Doubling of the capacity of child psychiatric services in a region of southwestern Norway - how did it affect the composition of the clinical population? Nord J Psychiatry. 2009;63:322–330.
- Oerbeck B, Overgaard KR, Aspenes ST, et al. ADHD, comorbid disorders and psychosocial functioning: how representative is a child cohort study? Findings from a national patient registry. BMC Psychiatry. 2017;17:23.
- Jensen CM, Steinhausen H-C. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. ADHD Atten Def Hyp Disord. 2015;7:27–38.
- Huh Y, Choi I, Song M, et al. A comparison of comorbidity and psychological outcomes in children and adolescents with attention-deficit/hyperactivity disorder. Psychiatry Investig. 2011;8:95–101.
- Yüce M, Zoroglu SS, Ceylan MF, et al. Psychiatric comorbidity distribution and diversities in children and adolescents with attention deficit/hyperactivity disorder: a study from Turkey. Neuropsychiatr Dis Treat. 2013;9:1791–1799.
- Steinhausen H-C, Nøvik TS, Baldursson G, et al. Co-existing psychiatric problems in ADHD in the ADORE cohort. Eur Child Adolesc Psychiatry. 2006;15(Suppl. 1):I25–I29.
- Reale L, Bartoli B, Cartabia M, et al. Comorbidity prevalence and treatment outcome in children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2017;26:1443–1457.
- Joelsson P, Chudal R, Gyllenberg D, et al. Demographic characteristics and psychiatric comorbidity of children and adolescents diagnosed with ADHD in specialized healthcare. Child Psychiatry Hum Dev. 2016;47:574–582.
- Kadesjø B, Gilber C. Tourette’s disorder: epidemiology and comorbidity in primary school children. J Am Acad Child Adolesc Psychiatry. 2000;39:548–555.
- Eysturoy AE, Skov L, Mol Debes N. Genetic predisposition increases the tic severity, rate of comorbidities, and psychosocial and educational difficulties in children with Tourette syndrome. J Child Neurol. 2015;30:320–325.
- Robertson MM, Eapen V, Singer HS, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers. 2017;3:16097.
- Specht MW, Woods DW, Scahill L, et al. Clinical characteristics of children and adolescents with a primary tic disorder. J Dev Phys Disabil. 2011;23:15–31.
- Mattila M-L, Hurtig T, Haapsamo H, et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: a community- and clinic-based study. J Autism Dev Disord. 2010;40:1080–1093.
- Gjevik E, Eldevik S, Fjaeran-Granum T, et al. Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorders. J Autism Dev Disord. 2011;41:761–769.
- de Bruin EI, Ferdinand RF, Meester S, et al. High rates of psychiatric co-morbidity in PDD-NOS. J Autism Dev Disord. 2007;37:877–886.
- Nøvik TS, Hervas A, Ralston SJ, et al. Influence of gender on attention-deficit/hyperactivity disorder in Europe – ADORE. Eur Child Adolesc Psychiatry. 2006;(Suppl. 1)15:I/15–I/24.
- Supekar K, Iyer T, Menon V. The influence of sex and age on prevalence rates of comorbid conditions in autism. Autism Res. 2017;10:778–789.
- Esbjorn BH, Hoeyer M, Dyrborg J, et al. Prevalence and co-morbidity among anxiety disorders in a national cohort of psychiatrically referred children and adolescents. J Anxiety Disord. 2010;24:866–872.
- Olofsdotter S, Vadlin S, Sonnby K, et al. Anxiety disorders among adolescents referred to general psychiatry for multiple causes: clinical presentation, prevalence and comorbidity. Scand J Child Adolesc Psychiatr Psychol. 2016;4:55–64.
- Krogh F, Bukten MIK. Activity data for mental health services for children and youth 2013 (Aktivitetsdata for psykisk helsevern for barn og unge 2013). Oslo: Helsedirektoratet; 2014.
- Skirbekk B, Hansen BH, Oerbeck B, et al. The relationship between sluggish cognitive tempo, subtypes of attention-deficit/hyperactivity disorder, and anxiety disorders. J Abnorm Child Psychol. 2011;39:513–525.
- Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988.
- Lauth B, Magnusson P, Ferrari P, et al. An Icelandic version of the Kiddie-SADS-PL: translation, cross-cultural adaptation and inter-rater reliability. Nord J Psychiatry. 2008;62:379–385.
- Villabo MA, Oerbeck B, Skirbekk B, et al. Convergent and divergent validity of K-SADS-PL anxiety and attention deficit hyperactivity disorder diagnoses in a clinical sample of school-aged children. Nord J Psychiatry. 2016;70:358–364.
- Jarbin H, Andersson M, Råstam M, et al. Predictive validity of the K-SADS-PL 2009 version in school-aged and adolescent outpatients. Nord J Psychiatry. 2017;71:270–276.
- Sørensen MJ, Becker Nissen J, Mors O, et al. Age and gender differences in depressive symptomatology and comorbidity: an incident sample of psychiatrically admitted children. J Affect Disord. 2005;84:85–91.
- Spitzer RL. Psychiatric diagnosis: are clinicians still necessary? Compr Psychiatry. 1983;24:399–411.
- Brown-Jacobsen AM, Wallace DP, Whiteside SPH. Multimethod, multi-informant agreement, and positive predictive value in the identification of child anxiety disorders using the SCAS and ADIS-C. Assessment. 2011;18:382–392.
- Jans T, Weyers P, Schneider M, et al. The Kiddie-SADS allows a dimensional assessment of externalizing symptoms in ADHD children and adolescents. ADHD Atten Def Hyp Disord. 2009;1:215–222.
- Joshi G, Petty C, Wozniak J, et al. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: a large comparative study of a psychiatrically referred population. J Autism Dev Disord. 2010;40:1361–1370.
- Robertson MM. The Gilles De La Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97:166–175.
- van Steensel FJA, Bogels SM, de Bruin EI. Psychiatric comorbidity in children with autism spectrum disorders: a comparison with children with ADHD. J Child Fam Stud. 2013;22:368–376.
- Sukhodolsky DG, Bloch MH, Panza KE, et al. Cognitive-behavioral therapy for anxiety in children with high-functioning autism: a meta-analysis. Pediatrics. 2013;132:e1341–e1350.
- Hirschtritt ME, Lee PC, Pauls PL, et al. Lifetime prevalence, age of risk, and etiology of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72:325–333.
- Godar S, Bortolato M. What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome. Neurosci Biobehav Rev. 2017;76:123–133.
- Heiervang E, Goodman A, Goodman R. The Nordic advantage in child mental health: separating health differences from reporting style in a cross-cultural comparison of psychopathology. J Child Psychol Psychiatry. 2008;49:678–685.
- Bassarath L. Conduct disorder: a biopsychosocial review. Can J Psychiatry. 2001;46:609–616.
- Jozefiak T, Kayed N, Rimehaug T, et al. Prevalence and comorbidity of mental disorders among adolescents living in residential youth care. Eur Child Adolesc Psychiatry. 2016;25:33–47.
- von Gontard A. The impact of DSM-5 and guidelines for assessment and treatment of elimination disorders. Eur Child Adolesc Psychiatry. 2013;22(Suppl. 1):S61–S67.
- von Gontard A. Enuresis. In: Rey J, editor. IACAPAP e-textbook of child and adolescent mental health. Geneva: International Association for Child and Adolescent Psychiatry and Allied Professions; 2012.
- von Gontard A. Encopresis. In: Rey J, editor. IACAPAP e-textbook of child and adolescent mental health. Geneva: International Association for Child and Adolescent Psychiatry and Allied Professions; 2012.
- Wanderer S, Roessner V, Freeman R, et al. Relationship of obsessive-compulsive disorder to age-related comorbidity in children and adolescents with Tourette syndrome. J Dev Behav Pediatr. 2012;33:124–133.
- Surén P, Bakken IJ, Lie KK, et al. Differences across counties in the registered prevalence of autism, ADHD, epilepsy and cerebral palsy in Norway. Tidsskriftet . 2013;133:1929–1934.
- Gillberg C, Gillberg IC, Rasmussen R, et al. Co-existing disorders in ADHD – implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004;13(Suppl. 1):80–92.
- Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387:1240–1250.
- Korrel H, Mueller KL, Silk T, et al. Research review: language problems in children with attention-deficit hyperactivity disorder – a systematic meta-analytic review. J Child Psychol Psychiatr. 2017;58:640–654.
- Eddya CM, Rizzob R, Cavannac AE. Neuropsychological aspects of Tourette syndrome: a review. J Psychosom Res. 2009;67:503–513.
- Burd L, Freeman RD, Klug MG, et al. Tourette syndrome and learning disabilities. BMC Pediatr. 2005;5:34.
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;8:896–910.
- Bourgeron T. The genetics and neurobiology of ESSENCE: the third birgit olsson lecture. Nord J Psychiatry. 2016;70:1–9.
