Abstract
Purpose: To compare the rate of remission, rate of response, change in depressive symptoms, and adverse effects between repetitive transcranial magnetic stimulation (rTMS) and electroconvulsive therapy (ECT).
Materials and methods: In this retrospective case-control study, 35 patients treated for depression with rTMS (left dorsolateral prefrontal cortex, 90% observed motor threshold, 10 Hz, 2000 pulses/session, 15 sessions) at Örebro University Hospital, Sweden (cases), were compared with a matched group of 35 patients treated for depression with ECT (controls). Data on controls were obtained from the Swedish National Quality Register for ECT (Q-ECT). Severity of depression was evaluated using the Montgomery-Åsberg Depression rating scale (MADRS).
Results: Remission rate was 26% for cases and 43% for controls (p = .3). Response rate was 40% for cases and 51% for controls (p = .63). The median decrease in MADRS was 11 (IQR 3–19) vs. 17 (IQR 6–27; p = .10) for rTMS and ECT, respectively. There was no statistically significant difference in any measure of treatment effect between rTMS and ECT. More than half of the patients of the rTMS group experienced scalp discomfort and 11% of the ECT group had memory disturbances.
Conclusions: All measures of therapeutic efficacy were numerically inferior in the rTMS group compared to the ECT group. The differences were not statistically significant, probably because the sample size was small. More studies are required to find the optimal place for rTMS within the Swedish health care system. Such studies could be facilitated by inclusion of rTMS in the Q-ECT.
Background
Major depressive disorder (MDD) impairs social functioning, causes personal suffering, and has economic consequences for the individual and society due to long-term incapacitation and loss of production [Citation1]. The 12 month prevalence of MDD is about 5%. The symptoms can differ between patients and 30–60% of all cases are not improved significantly by antidepressant medication [Citation2,Citation3]. These cases are considered to have treatment-resistant depression (TRD) [Citation4].
An emerging treatment for TRD is repetitive transcranial magnetic stimulation (rTMS) [Citation5]. This is a noninvasive technique that utilizes pulsating shifting magnetic fields generated by extracorporeal electromagnetic coils to focally excite or inhibit cortical neurons through electromagnetic induction [Citation6]. Although the therapeutic mechanism of rTMS remains unclear, it may involve changes in neuroplasticity due to long-term potentiation, long-term depression, and dendritic growth owing to increases in neurotrophic factors [Citation7,Citation8]. There is evidence that rTMS affects depressive symptoms [Citation9–12], but it is unclear if the effects are large enough to be clinically relevant [Citation13,Citation14]. The therapeutic effects correlate with the number of pulses and number of sessions in some studies [Citation10–12,Citation15,Citation16] but not others [Citation17]. Patients often have more than one concommittant treatment [Citation18]. There is some evidence of a synergistic antidepressant effect of rTMS with antidepressant medication [Citation12]. In one study, younger age predicted the efficacy of rTMS for patients with unipolar depression but not bipolar depression [Citation19]. Other reported predictors of efficacy are short duration of depressive symptoms [Citation19,Citation20] and cognitive rather than somatic depressive symptoms [Citation21].
Electroconvulsive therapy (ECT) is an established treatment for MDD, particularly TRD [Citation22]. In ECT, generalized seizures are induced under general anesthesia by stimulation with electrical current [Citation22]. The efficacy of rTMS for depression is inferior to that of ECT [Citation23]. ECT seems especially preferable to rTMS for treatment of MDD with psychotic symptoms [Citation23,Citation24], while for non-delusional MDD rTMS may have similar effect [Citation23]. ECT, but not rTMS, is associated with transient memory loss [Citation25].
In 2017, the Swedish National Board of Health and Welfare issued national recommendations for the use of rTMS for the treatment of MDD [Citation26]. These were based on results of international studies and include rTMS as a treatment that ‘can be offered’ for moderate to severe MDD. There are no data on the treatment results of rTMS in Swedish psychiatry and there is a need for scientific evaluation of this novel approach and its role in the treatment of depression.
Aim: To establish the rate of remission, response, change in depressive symptoms, and adverse effects with rTMS and to compare it with ECT when used for the treatment of depression in a Swedish population.
Materials and methods
Study design
This was a retrospective case-control study of 35 patients who were treated with rTMS at the University Hospital Örebro, Sweden, between 2003 and 2007. Controls (the ECT group) were 35 patients treated with ECT in Sweden between 2012 and 2016 and were reported to the the Swedish national quality register for ECT (Q-ECT) [Citation27]. rTMS and ECT groups were matched for age, gender, diagnosis, psychotic symptoms, and depression severity, evaluated using the Clinical Global Impressions Severity [Citation28] scale. All cases and controls had TRD of at least stage 1 on the Thase and Rush scale [Citation4], i.e. failure to achieve response after a trial with antidepressant medication in adequate dose and duration.
Patient selection and data sources
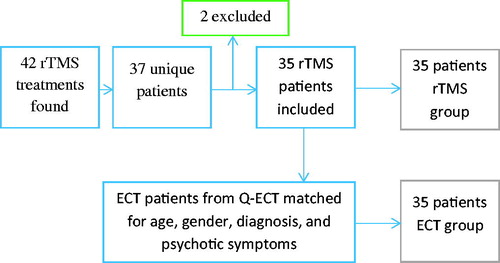
Data on cases were obtained from medical records. There were 42 medical records of rTMS treatment series within the identified time period, distributed over 37 patients. Five patients had more than one rTMS treatment series on record, and all but the first treatment series were discarded. Two patients were excluded because treatment was stopped prematurely due to overheating of the magnetic coil causing machine malfunction. Data from the remaining 35 patients were included in the analysis (). Data on controls were obtained from the (Q-ECT) [Citation27]. Thirty-five ECT-treated patients were matched for gender, age, diagnosis, depression severity (Clinical Global Impressions Severity score), and psychotic symptoms, in that order, to the rTMS population (). The ECT patients were registered from 19 different psychiatric clinics in Sweden. Information about prior personality disorder was collected from the Swedish national patient register, which includes diagnoses from more than 99% of hospital discharges. Information about prior antidepressant treatment was collected from the Swedish prescribed drug register, which has a coverage of 100% of medication dispensed through pharmacies.
Figure 1. Flow chart of patient selection. Forty-two rTMS treatments were found, of which thirty-five were unique first-time rTMS treatments. Two rTMS patients were excluded due to technical malfunction. Thirty-five rTMS patients was included in the study and were matched to thirty-five ECT patients from the Swedish National Quality Register for ECT to function as control group.
Repetitive transcranial magnetic stimulation (rTMS)
All rTMS was performed with the Magventure MagPro R30 stimulator and the Magventure MCF-75 circular coil, both manufactured by Magventure A/S Farum, Denmark. The coil was first positioned at 90°over the central sulcus on the patient’s scalp and then shifted to a 45 degree angle to find the left motor cortex. The coil was moved until stimulation induced a movement of the right index finger. The coil was then kept in this location and the stimulation intensity was gradually reduced until no visible movement of the index finger was observed. The stimulation intensity at this point was defined as the observed motor threshold (OMT). After OMT was established, the coil was moved 5 cm anterior so that it was over the left dorsolateral prefrontal cortex and the stimulation intensity was set to 90% of OMT. rTMS was then performed, consisting of 2000 pulses divided into 20 trains of 100 pulses at a frequency of 10 Hz. A 20 s inter-train pause allowed for cooling of the coil. A treatment series consisted of 15 sessions and was not adjusted according to treatment response.
Electroconvulsive therapy (ECT)
ECT is usually administered thrice weekly under general anesthesia. Patients were sedated with intravenous propofol or thiopental, succinylcholine was administered as a muscle relaxant, and atropine or another anticholinergic drug was administered to alleviate the risk of cardiac arrhythmias during stimulation when indicated. Of the 35 ECT patients included in this study, 75% had unilateral and 25% had bilateral electrode placement. The median number of sessions were six (inter quartile range 6–10). ECT was delivered with the following parameters: charge, 318 ± 132 mC; current, 845 ± 55 mA; duration, 69 ± 12 s; frequency, 53 ± 21 Hz; pulse width, 50 ± 6.8 ms; seizure time, 41 ± 25 s (all values are mean ± standard deviation). Detailed information about the ECT of each patient is available in Supplementary Table 1.
Table 1. Baseline characteristics.
Outcome measures
In the rTMS group, the severity of depression was evaluated using the Montgomery-Åsberg Depression Rating scale (MADRS) [Citation29] at baseline (pre-treatment), after seven sessions of rTMS, at the final session of rTMS, and at follow-up (45 days after the first session of rTMS). In the ECT group, the severity of depression was evaluated using MADRS or the self-rated version of the MADRS scale (MADRS-S) [Citation30], before ECT and within one week after the last session. Remission was defined as MADRS or MADRS-S score ≤10 after last stimulation. Response was defined as a 50% decrease in MADRS or MADRS-S score after last stimulation compared with baseline. For each rTMS session, the treating clinician used a questionnaire to document patient fatigue, headache, subjective flushing, discomfort, and epileptic seizures. Each item was rated as either present or absent. These data were used to evaluate adverse effects in the rTMS group. In the ECT-group, subjective memory dysfunction was evaluated using a clinician rated scale ranging from zero (no memory dysfunction) to six (total inability to remember). This was combined with clinical records of clinician assessed adverse effects available in the Q-ECT.
Statistical analysis
The primary outcome was remission. Secondary outcomes were response and change in MADRS or MADRS-S score (ΔMADRS) from baseline to last treatment. Outcomes were compared across rTMS and ECT groups using Fisher’s exact test, due to the presence of cells with values <5 within nominal variables and the Mann-Whitney U-test for interval and ordinal non-normally distributed variables. The significance level was p < .05. In two separate subgroup analyses, cases and controls were compared after stratification for age (<50 and ≥50 y) and the presence or absence of psychotic symptoms. The cutoff for the age-stratified analysis was chosen as 50 y in accordance with evidence of decreased efficacy of rTMS in patients ≥50 y [Citation18]. Patients for whom the post-treatment MADRS score was missing were included in the post-treatment analyses with the patient’s nearest historical assessment scale score (last observation carried forward). If there was no post-baseline assessment, drop-outs were defined as non-remissive.
Ethics
All cases consented to rTMS treatment and detailed clinical follow-up to facilitate evaluation of the treatment and were not required to provide additional consent for this chart review. Data from controls were obtained from the Q-ECT. Participation in this register is voluntary and at the time of entry to the register patients were informed of future scientific use of their data. The study was approved by the regional ethics committee in Uppsala (approval no. 2016/196).
Results
Included patients
Seventy patients were included in the study: 35 cases who received rTMS and 35 controls who received ECT. There was a larger proportion of in-patients among controls than cases, and a history of ECT treatment was more common in controls than cases (). There were no other statistically significant differences between groups among measured variables ().
Table 2. Classes of concommitant antidepressants used.
Remission and response rate
The rate of remission was 26% in cases and 43% in controls (p = .3). The rate of response was 40% in cases and 51% in controls (p = .63). Median ΔMADRS was 11 in cases and 17 in controls (p = .10; ).
Table 3. Treatment details and results.Table Footnotee
Three cases dropped out during treatment and did not complete all MADRS assessments: one relapsed into alcohol abuse after six sessions of rTMS, one opted out of treatment due to lack of effect after nine sessions of rTMS, and one was admitted to emergency psychiatric care after an attempted suicide. Four controls received fewer than six sessions of ECT. These four individuals were non-remissive and non-responding, reasons for terminating treatment was not reported.
Subgroup analyses
Remission, response and median ΔMADRS in rTMS and ECT groups after stratification according to age or the presence or absence of psychotic symptoms are presented in and . There were no statistically significant differences but the ECT group had numerically superior results in all subgroups.
Table 4. Treatment effect in patients stratified according to age.Table Footnotee
Table 5. Treatment effect in psychotic and non-psychotic patients.e
Adverse effects
Twenty-five cases (71%) reported at least one adverse effect in at least one rTMS session. Twenty cases (57%) reported scalp discomfort, 18 (51%) reported headache, 14 (40%) reported fatigue, and five (14%) reported flushing. No rTMS patient had an epileptic seizure. In the ECT population, 11% reported impairing or embarrassing memory dysfunction.
Discussion
The rates of remission and response were lower in the rTMS-group as compared to the ECT group (26 vs. 43% and 40 vs. 51%, respectively), but these differences were not statistically significant. Subgroup analyses revealed similar results across subgroups after stratification according to age (<50 and ≥50 years) or psychotic symptoms. Our results are in line with previous studies showing rTMS to be significantly inferior to ECT for treatment of depression [Citation23]. The likely explanation for insignificant differences in this study is the small sample size examined.
Regarding the technical aspect of the rTMS treatment relatively low stimulus intensity (90% MT) was chosen to limit the risk for epileptic seizures. The amount of pulses (2000 per session) was higher than seems to be optimal [Citation10], which seems to be 1200–1500 pulses per stimulation. This may have limited the therapeutic effect. The determination of OMT and the position of the coil used in the treatment of cases included in this study was imprecise compared with that achieved with neurographic methods [Citation31] and does not account for differences in head size, skull asymmetry, or individual neuroanatomy. Correct positioning of the coil is as important as stimulation intensity and the efficacy of rTMS may be enhanced by utilizing neuroimaging-guided positioning [Citation17]. With precise positioning of the coil, rTMS at an intensity of 90% motor threshold (MT) appears to have therapeutic effect [Citation17,Citation24]. However, with imprecise positioning of the coil, even stimulation as high as 120% MT may be insufficient and there does not appear to be any increased therapeutic effect with stimulation intensities between 93 and 120% MT [Citation17]. However, the method of determining OMT used for patients in the current study may result in an overestimation of the required stimulation intensity by as much as 28% of maximum stimulator output [Citation31]. As such, it is uncertain if there was sufficient stimulation of the dorsolateral prefrontal cortex in all patients.
Comparing rTMS results across studies is complicated because of heterogenic rTMS protocols [Citation32]. There are also different coils being used [Citation33]. The pulse frequency and location used in this study also have heterogenic evidence of efficacy, with publications of both statistically significant therapeutic effect [Citation34], and of no significant therapeutic effect compared to sham-rTMS [Citation35,Citation36]. Nevertheless, the rate of remission in the patients in this study is comparable to that reported in international studies comparing rTMS to sham [Citation9,Citation24,Citation34,Citation36,Citation37].
The response rate with ECT (51%) was not optimal in the current study, compared to 80% in a broader sample from the Q-ECT [Citation38]. Technical aspects of ECT that could be improved for the ECT patients included in this analysis are longer pulse width, more frequent use of bilateral electrode placement, less use of benzodiazepines, and for some patients, longer treatment series [Citation39]. For example, if a patient does not respond to unilateral stimulation after six or more sessions, they could be switched to bilateral stimulation to potentiate efficacy. This was not done for any of the controls in the current study.
Limitations
The small sample size makes the comparisons uncertain, especially among subgroups. A potential confounder in the present analysis is the larger proportion of in-patients among ECT patients (77%) than rTMS patients (8%). In-patients are likely to have more severe symptoms than out-patients. Likely symptoms demanding in-patient care are high risk of suicide and catatonia. Although rTMS and ECT patients were matched for severity of depression as indicated by Clinical Global Impressions Severity scale (CGI-S) score, there may have been aspects of symptom severity that did differ between groups but were not captured by this measure and therefore not taken into account in the analysis. In the ECT group, severity of depression was evaluated by a mixture of clinician-rated MADRS (8%) and patient self-rated MADRS-S (92%), whereas in the rTMS group the severity of depression was evaluated by clinician-rated MADRS only and a single clinician evaluated all cases. The MADRS-S score does not correlate exactly with MADRS score [Citation40], introducing variability between groups. In addition, mild side effects and memory disturbances were not evaluated and recorded equally among cases and controls. In many cases mild side effects may not have been reported to the Q-ECT, hindering a comparison between mild side effects in rTMS and ECT groups. There is always a certain risk for bias in a retrospective case-control study like this, but the results may nevertheless be of scientific and clinical relevance. A randomized controlled study with a similar research question in focus is therefore called upon.
Clinical implications
This study does not provide sufficient evidence of the efficacy of rTMS to warrant any recommendation. Although rTMS seems inferior to ECT in rates of response and remission also in other studies [Citation23,Citation41], the treatment results are interesting. Swedish patients not suited for or willing to partake in ECT for TRD may be helped by rTMS. Advantages of rTMS include no need for anesthesia or post-anesthesia monitoring, which can be limited by the hospital’s availability of anesthesiologist care. More and larger studies comparing ECT and rTMS are needed. Further studies are also required to improve the treatment techniques. Such studies could be facilitated by the inclusion of rTMS in the Q-ECT in 2018.
Conclusions
rTMS may have lower response and remission rates than ECT, although the sample size of this study was too small to make firm conclusions. The side effect profile of rTMS differs from that of ECT, with high rates of mild side effects such as scalp discomfort and headache in the rTMS group, but memory disturbances were only reported in the ECT group. More studies are required to find the optimal place for rTMS within the Swedish health care system.
Supplementary_Table_1.docx
Download MS Word (19.5 KB)Acknowledgments
The authors thank Ole Brus, statistician Region Örebro county for help with the matching procedure.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- de Sousa RT, Zanetti MV, Brunoni AR. Challenging treatment-resistant major depressive disorder: a roadmap for improved therapeutics. Curr Neuropharmacol. 2015;13:616–635.
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917.
- Park SC, Kim JM, Jun TY, et al. How many different symptom combinations fulfil the diagnostic criteria for major depressive disorder? Results from the CRESCEND study. Nord J Psychiatry. 2017;71:217–222.
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659.
- Aleman A, Slotema CW, Sommer IE. rTMS deserves a fair chance as a novel treatment for depression. Acta Psychiatr Scand. 2014;130:324–325.
- George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. 2011;168:356–364.
- Chervyakov AV, Chernyavsky AY, Sinitsyn DO, et al. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci. 2015;9:303.
- Bakker N, Shahab S, Giacobbe P, et al. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 2015;8:208–215.
- Berlim MT, Van den Eynde F, Daskalakis ZJ. High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry. 2013;74:e122–e129.
- Teng S, Guo Z, Peng H, et al. High-frequency repetitive transcranial magnetic stimulation over the left DLPFC for major depression: session-dependent efficacy: a meta-analysis. Eur Psychiatry. 2017;41:75–84.
- Concerto C, Lanza G, Cantone M, et al. Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: A six-month clinical follow-up study. Int J Psychiatry Clin Pract. 2015;19:252–258.
- Kedzior KK, Reitz SK, Azorina V, et al. The antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) in the absence of maintenance treatment in major depression: a systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials. Depress Anxiety. 2015;32:193–203.
- Rodriguez-Martin JL, Barbanoj MJ, Schlaepfer TE, et al. Transcranial magnetic stimulation for treating depression. Cochrane Database Syst Rev. 2002;(2):CD003493.
- Health Quality Ontario. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Ont Health Technol Assess Ser. 2016;16(5):1–66.
- Richieri R, Jouvenoz D, Verger A, et al. Changes in dorsolateral prefrontal connectivity after rTMS in treatment-resistant depression: a brain perfusion SPECT study. Eur J Nucl Med Mol Imaging. 2017;44:1051–1055.
- Theleritis C, Sakkas P, Paparrigopoulos T, et al. Two versus one high-frequency repetitive transcranial magnetic stimulation session per day for treatment-resistant depression: a randomized sham-controlled trial. Response to Andrade and colleagues. J ECT. 2017;33:143.
- Johnson KA, Baig M, Ramsey D, et al. Prefrontal rTMS for treating depression: location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimul. 2013;6:108–117.
- Okkels N, Mogensen RB, Crean LC, et al. Treatment profiles in a Danish psychiatric university hospital department. Nord J Psychiatry. 2017;71:289–295.
- Brakemeier EL, Luborzewski A, Danker-Hopfe H, et al. Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS). J Psychiatr Res. 2007;41:395–403.
- Wu GR, Baeken C. Longer depressive episode duration negatively influences HF-rTMS treatment response: a cerebellar metabolic deficiency? Brain Imaging Behav. 2017;11:8–16.
- Rostami R, Kazemi R, Nitsche MA, et al. Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clin Neurophysiol. 2017;128:1961–1970.
- Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939–1945.
- Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychophar-macol Biol Psychiatry. 2014;51:181–189.
- Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;15:s415–s424.
- Schulze-Rauschenbach SC, Harms U, Schlaepfer TE, et al. Distinctive neurocognitive effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in major depression. Br J Psychiatry. 2005;186:410–416.
- Socialstyrelsen. Nationella riktlinjer för vård vid depression och ångestsyndrom-Stöd för styrning och ledning [Welfare. National guidelines for depression and anxiety disorder support-support for management and m anagement]. Stockholm: Socialstyrelsen; 2017. Swedish.
- Nordanskog P, Hulten M, Landen M, et al. Electroconvulsive therapy in Sweden 2013: data from the National Quality Register for ECT. J ECT. 2015;31:263–267.
- Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: National Institute of Health, Psychopharmacology research branch; 1976.
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389.
- Svanborg P, Asberg M. A comparison between the Beck Depression Inventory (BDI) and the self-rating version of the Montgomery Asberg Depression Rating Scale (MADRS). J Affect Disord. 2001;64:203–216.
- Westin GG, Bassi BD, Lisanby SH, et al. Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: safety implications. Clin Neurophysiol. 2014;125:142–147.
- Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74:143–152.
- Nordenskjold A, Martensson B, Pettersson A, et al. Effects of Hesel-coil deep transcranial magnetic stimulation for depression - a systematic review. Nord J Psychiatry. 2016;70:492–497.
- Avery DH, Holtzheimer PE, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59:187–194.
- Loo C, Mitchell P, Sachdev P, et al. Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry. 1999;156:946–948.
- Herwig U, Fallgatter AJ, Hoppner J, et al. Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. Br J Psychiatry. 2007;191:441–448.
- Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46:257–264.
- Nordenskjöld A, von Knorring L, Engström I. Predictors of the short-term responder rate of Electroconvulsive therapy in depressive disorders–a population based study. BMC Psychiatry. 2012;12:17–115.
- Brus O, Cao Y, Gustafsson E, et al. Self-assessed remission rates after electroconvulsive therapy of depressive disorders. Eur Psychiatry. 2017;45:154–160.
- Fantino B, Moore N. The self-reported Montgomery-Asberg Depression Rating Scale is a useful evaluative tool in Major Depressive Disorder. BMC Psychiatry. 2009;9:26.
- Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;2014:1.
