Abstract
Objectives: The purpose of this study was to assess the reliability and validity of Swedish translations of the Beliefs about Medicines Questionnaire-Specific (BMQ-Specific) and Brief Illness Perception Questionnaire (B-IPQ) for use in adolescents with ADHD.
Methods: Forward and backward translations of the BMQ-Specific and B-IPQ scales to Swedish were conducted and reviewed by adolescents with ADHD and professionals. The validity and reliability of both questionnaires were investigated in a cross-sectional study of 101 adolescents (13–17 years) on a long-term prescription of ADHD medication recruited from two child and adolescent psychiatric outpatient clinics in Sweden.
Results: Regarding the BMQ-Specific, principal component analysis (PCA) loadings confirmed the previously defined components of Specific-Necessity and Specific-Concern. The PCA for B-IPQ revealed two components, the first one, B-IPQ Consequences, captured questions regarding perceptions of the implication of having ADHD (items 1, 2, 5, 6 and 8) and the second one, B-IPQ-Control, the perceptions of the capability to manage the ADHD disorder (items 3, 4 and 7). The Cronbach alpha coefficients for BMQ-Specific-Necessity scale was α = 0.80, for BMQ-Specific-Concern scale α = 0.75, B-IPQ Consequences α = 0.74 and for B-IPQ-Control α = 0.44.
Conclusions: The present results prove the Swedish translation of BMQ-Specific and B-IPQ to be valid and reliable for utilization in adolescents with ADHD. The PCA confirmed the original components for BMQ-Specific and the recent findings of two main B-IPQ components describing emotional and cognitive implications versus the capability for self-care maintenance of ADHD.
Introduction
ADHD in children and adolescents is a common disorder with a reported prevalence around 5.9–7.1% [Citation1]. ADHD during adolescence has lifelong consequences for the individual [Citation2–4], impacts on the caregivers’ lives [Citation5] and yields high economic national cost [Citation6].
Some of the consequences of ADHD may be avoided by pharmacological treatment [Citation7–9] and adherence is therefore of importance in order to achieve the treatment effects [Citation10–13]. Emilsson et al. [Citation14] documented high adherence behavior in 46.5% of adolescents on long-term ADHD medication in line with reports of adherence failure in about 50% of children and adolescents [Citation15]. Beliefs about medication are probably the most crucial drivers of non-adherence [Citation16,Citation17] in longstanding disorders, and therefore need to be elucidated during treatment according to the World Health Organization [Citation10]. According to Petrie et al. [Citation18] postulation, it is also important to understand perceptions of the disorders to enable prediction of behavioral reactions [Citation18] such as adherence. In accordance, an association was revealed in adolescents between adherence and perceptions of ADHD, by using the Brief Illness Perception Questionnaire (B-IPQ), as well as beliefs in the necessity, concerns and experienced side effects of ADHD medication by using the Beliefs about Medicines Questionnaire-Specific (BMQ-Specific) [Citation14].
The BMQ-Specific [Citation16,Citation17] is based on Horne’s Necessity-Concerns Framework (NCF) [Citation19,Citation20] which elucidates the relative impact of beliefs about benefits versus risks of medication. The NCF is an expansion of Leventhal’s Common-Sense Model (CSM) [Citation19] which in turn the B-IPQ is based on [Citation21].
The BMQ-Specific [Citation16] has previously been translated to Swedish [Citation22] but a new translation and validation in a Swedish population was still necessary because of further development of the inventory whereas some sentences had been rephrased to optimize the ability to capture the correct feature of interest regarding persons belief about medicine.
The precondition for the secure use of questionnaires in a clinical context is that they actually measure the proposed phenomena. In order to enable the right conclusion to be drawn, validation is of importance [Citation23]. The latest version of BMQ-Specific has been validated for different conditions and languages [Citation24–28], although this does not apply for the Swedish version nor for adolescents with ADHD.
Similarly, B-IPQ has been validated for different conditions and languages [Citation29–34], although only lately translated to Swedish for use in adolescents with ADHD [Citation14]. The B-IPQ [Citation21] covers five domains: identity, timeline, cause, control and consequences [Citation35–39]. The original version of B-IPQ had no components so more overall information would be helpful in clinical contexts and research. After the original version of B-IPQ was published, later research on somatic disorders has revealed two or three components of B-IPQ by using confirmatory factor analysis or principal component analysis (PCA) [Citation33,Citation34,Citation40]. However, these somatic disorders arise during the life course and some of them are also life-threatening. The components of the perceptions of ADHD may therefore load items differently since it is usually a lifelong mental disorder and not a direct threat to survival.
The aim of this study was to assess the reliability and validity of Swedish translations of BMQ-Specific and B-IPQ questionnaires for use in adolescents with ADHD.
Materials and methods
Study design
A cross-sectional investigation was conducted in a group of adolescents on long-term ADHD medication and undergoing regular controls at child and adolescent psychiatric clinics (CAP).
Translation
The original author of the BMQ-Specific found that a renewal of the Swedish translation was necessary due to a revision of the scale [Citation16] after the first Swedish translation had already been performed [Citation22]. The development mainly consisted of minor changes where a few sentences had been rephrased; for instance, the question about the beliefs about effect of medicine has changed in Swedish from ‘utan mina mediciner skulle jag vara mycket sjukare’ to ‘utan mina mediciner skulle jag klara mig mycket sämre’. The process of translation and revision of the instrument mostly followed the recommendation given by Wild et al. [Citation41]. The new translation was based on the previous one [Citation22]. The forward translation to Swedish was done by a native Swedish speaker with a knowledge of the context and survey method as well as being a specialist in medicine and nursing (first author ME). First, the forward translation was thoroughly scrutinized the Swedish speakers research group. Second, a backward translation to English was performed by a native speaker who works as a professional English translator and has knowledge in the context and the survey method. The final English translation was accepted by the original author, Professor Horne. First, face validity was examined by specialists in child, adolescent and adult psychiatry. In addition, the Swedish translation of the BMQ-Specific items was second face validity tested [Citation42] in order to ascertain the comprehensibility of the items. Hence, the understandability, interpretation and culture relevance [Citation41] were tested in a group of adolescents with ADHD. Subsequently, the results were discussed with specialists in child- and adolescents psychiatry and adult psychiatry and some minor changes were done, i.e. the word health was changed to well-being. Thereafter, the new version was proofread by the Swedish part of the research group before it was distributed to the respondents.
B-IPQ is open for translation (www.uib.no/ipq/html/submitting.html). The same translation procedure as described above was repeated for B-IPQ [Citation21]. Since the face validity was high and the adolescents easily understood all questions, no changes were needed.
Procedure
Adolescents (13–17 years) on a long-term prescription of ADHD medication lasting for at least 6 months were recruited from two CAP in Sweden from March 2014 to June 2015. Exclusion criteria were autism spectrum disorder, intellectual development disorder (IQ < 70), neurological disorders and language barriers (e.g. inability to answer questionnaires in Swedish). Information about the study was sent with a letter setting up an appointment for ordinary monitoring of prescribed ADHD medication. Written informed consent was obtained both from the adolescent and parent/guardian at the time of the visit. In the presence of the study nurse, a sociodemographic questionnaire, BMQ-Specific [Citation16,Citation43] and B-IPQ [Citation44] were handed out and filled in. In order to get independent results and for confidentiality, it was explained that the answers would never reach the doctor or staff responsible for treatment.
The study was approved by the Regional Ethical Review Board in Linköping (Dnr 2013/402-31).
Participants
In total, 148 of the 160 patients invited to participate gave written informed consent and were included. The ADHD diagnosis was determined in accordance with DSM-IV criteria by an experienced CAP specialist after a thorough neuropsychological investigation encompassing careful clinical examination, questionnaires and, in most cases (93 of 101 or 92%), a computer-based assessment of the ADHD core symptoms, the QbTest (Qbtech, Quantitative behavior technology, https://www.qbtech.com/, accessed 31 March 2016). All participants had combined type ADHD [Citation14].
Instruments
Beliefs about Medicines Questionnaire-Specific
Beliefs about Medicines Questionnaire-Specific assess respondents’ beliefs about the prescribed medication and was first validated in the English original version [Citation16]. The BMQ-Specific comprises two subscales with five items each. The 10 items are rated on a five-point scale ranging from 1=‘strongly disagree’ to 5=‘strongly agree’ (Summing range 5–25). The Specific-Necessity scale evaluates respondents’ beliefs about the necessity of the prescribed medication for controlling their ADHD and maintaining health (e.g. ‘My health, at present, depends on my ADHD medication’). The Specific-Concern scale covers concerns regarding adverse consequences of taking the prescribed medication (e.g. ‘I sometimes worry about the long-term effect of my ADHD medication’). A higher score on the Specific-Necessity scale indicates stronger beliefs about the necessity of treatment, and a higher score on the Specific-Concern scale indicates stronger concerns [Citation16,Citation43].
Brief Illness Perception Questionnaire
The B-IPQ covers respondents’ perceptions regarding the actual disorder. The first eight items are rated between 0 and 10. Five items capture the cognitive representation of ADHD (personal and treatment control, timeline (chronic versus acute), identity and consequences of ADHD). Two items evaluate the emotional representation (concerns about ADHD and emotional impact of ADHD) and one item assess the comprehensibility of ADHD. The scoring is reversed in the items of personal and treatment control, and comprehensibility of ADHD. A higher score reflects a more negative perception of ADHD [Citation21]. Item 9 is an open question and the results were not analyzed in this study.
Data analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) 21.0 (SPSS Inc., Chicago, IL). Descriptive statistics encompassing frequencies, means, and standard deviations were calculated.
Principal component analysis groups together the items of the scales allowing consolidation of the results into only a few components [Citation45] and was therefore used to examine the construct validity of both scales. For BMQ-Specific, the oblique rotation (direct oblimin) was chosen for selection of items since it was used in the development of BMQ-Specific [Citation16] and also in prior validation of the BMQ-Specific [Citation25]. For the B-IPQ scale, the orthogonal rotation (Varimax) was chosen since it was used for validation of B-IPQ [Citation33]. The scale items were found suitable for participating in a PCA as the Kaiser–Meyer–Olkin (KMO) was >0.5 and Bartlett’s test of sphericity showed significant p values, thereby indicating correlations between the included items. The components of the scales generated by PCA were accepted if the Eigenvalues were >1 (Kaiser’s criterion). The hypotheses used for the convergent-related validation were tested by Pearson’s correlation coefficient [Citation46]. The questionnaires’ internal consistency reliability was evaluated using Cronbach’s alpha coefficient [Citation47].
Results
Demographic data
A total of 101 (68.2%) individuals with a mean age of 15.6 years filled in the BMQ-Specific and B-IPQ of which 66 (66.3%) were boys and 35 (34.7%) girls. Eighty-one of the adolescents (80.2%) were taking long-acting methylphenidate (MPH) formulations, nine (8.9%) atomoxetine (ATX) and 11 (10.9%) ATX in combination with MPH. The mean time on medication was 50.7 months. Attrition analysis compared the 47 non-respondents with the 101 participants. No significant differences regarding gender, age at the start of medication and time on medication were detected.
Principal component analysis BMQ-specific
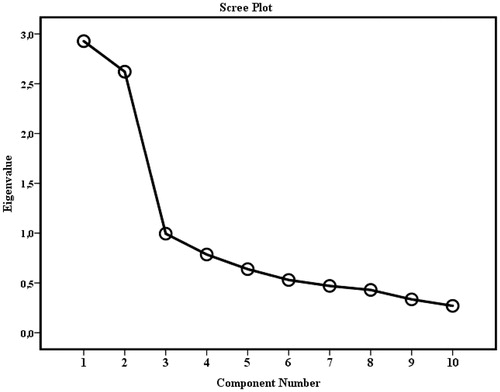
The adequacy of the items for use in PCA was confirmed as the result of the KMO was a middling (0.748) and Bartlett’s test showed significant (p< .001) and sufficiently large correlations between the items for including them in the PCA. Exploratory PCA with oblique rotation (direct oblimin) was conducted on the 10 items of the Swedish translated BMQ-Specific scale and generated two components with Eigenvalues fulfilling Kaiser’s criterion >1 and which together explained 55.48% of the variance. The scree plot is shown in . The first component had an Eigenvalue of 2.93 and explained 29.28% of the variance. It consisted of the previously established necessity items 1–5, which after rotation showed convergent loadings (0.522–0.838) and represents the Specific-Necessity component. The second component had an Eigenvalue of 2.62 and explained 26.21% of the variance. It contained the established concern items 1–5 which showed convergent loadings (0.606–0.777) and represents the Specific-Concern component.
Principal component analysis B-IPQ
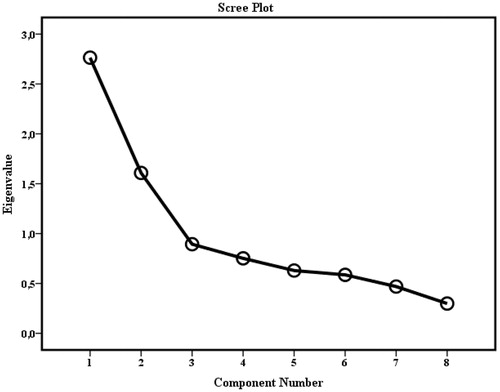
The adequacy of the items for use in PCA was confirmed as the result of the KMO was a middling (0.669) and Bartlett’s test showed significant (p< .001) and sufficiently large correlations between the items for inclusion of the items in PCA. Exploratory PCA with orthogonal rotation was conducted on the eight items of the Swedish translated B-IPQ scale (Varimax) which generated two components with Eigenvalues fulfilling Kaiser’s criterion >1 and which together explained 54.65% of the variance. The scree plot is shown in . The first component had an Eigenvalue of 2.76 and explained 34.6% of the variance. It consisted of the following items of consequences of ADHD, timeline, identity, concerns about ADHD and emotional impact of ADHD, which after rotation showed convergent loadings (0.603–0.752), and represents the consequences component. The second component had an Eigenvalue of 1.61 and explained 20.1% of the variance. It contained the following items of personal and treatment control, and comprehensibility of ADHD, which showed convergent loadings (0.549–0.699), and represents the Control component.
Convergent-related validity
The tested hypotheses for validation of BMQ-Specific components were confirmed as the Necessity component was positively correlated (r = 0.235, p = .019) but the Concern component negatively correlated (r = –0.310, p = .002) to the B-IPQ item ‘How much do you think your treatment can help your ADHD?’ [Citation21].
The tested hypotheses for validation of B-IPQ were confirmed as the B-IPQ ‘Consequences’ (r = 0.233, p = .019) and ‘Control’ (r = 0.364, p<.001) components were positively correlated to the BMQ-Specific Concern scale.
Internal consistency reliability
The Cronbach alpha coefficient for the BMQ-Specific-Necessity scale was α = 0.803 and for the Specific-Concern scale α = 0.753.
The Cronbach alpha coefficient for the B-IPQ Consequences component was α = 0.743 and for the Control component α = 0.438.
Discussion
The aim was to study the reliability and validity of the Swedish translations of BMQ-Specific and B-IPQ in adolescents with ADHD, which proved to be satisfactory for the BMQ-Specific but middling for the B-IPQ.
The construct validation [Citation48] by PCA revealed two components of BMQ-Specific, the Specific-Necessity component and the Specific-Concern component, thus reproducing the pattern of the original English version [Citation16] also found in other validation studies [Citation24,Citation26–28]. This illustrates that the original BMQ-Specific scale is robust and that the original validation was solid. Summarized, the BMQ-Specific seems suitable for use in different cultures and disorders.
Further evaluation of the convergent validity of BMQ-Specific supported the concepts of the two components as they correlated to a statement of a theoretically similar concept derived from the B-IPQ item: ‘How much do you think your treatment can help your ADHD?’ [Citation21]. Notably, this is a different statement compared to the ones used in the original validation which were derived from an earlier version of the Illness Perception Questionnaire (IPQ) [Citation49]. The treatment control item used in the present validation was added to the IPQ later during the development of IPQ-R [Citation50], the forerunner to B-IPQ [Citation21]. As the content of B-IPQ’s treatment control item is closer to the concept of BMQ-Specific exploring beliefs about treatment, we came to the conclusion that it was more suitable to use in the convergent validation.
The Cronbach alpha coefficient results indicated satisfactory internal consistency reliability for both of the BMQ-Specific components and in addition the face validity was good. In total, we conclude that the Swedish translation of BMQ-Specific retained the psychometric qualities of the original version and gives a valid and reliable picture of beliefs about medication in adolescents with ADHD.
The construct validation by PCA exhibited two components of B-IPQ, although no components were presented with the original version [Citation44]. Nevertheless, two previous investigators have described the same two components [Citation33,Citation34] and named them the Consequences and Control components [Citation33]. However, neither of these studies, on cancer and heart failure patients [Citation33,Citation34], included the B-IPQ’s Timeline item in the components, which however emerged as a distinct factor in a study on older adults with multiple illness [Citation40]. Among the adolescents with ADHD, the individual KMO value was good (>0.5) supporting that the timeline item belongs to the Control component. This discrepancy between the studies regarding the timeline could be due to the different age spans of the populations and different courses of the disorders. ADHD is usually a lifelong and non-fatal disorder while the nature of the somatic disorders may provoke perceptions of non-survival [Citation33,Citation34,Citation40], which can be closely linked to the concept of the timeline item.
The convergent validity test was low for the concepts of two B-IPQ components because the values of both the B-IPQ components correlated with the ‘BMQ-Specific Concern’ scale, supposed to measure a theoretically similar concept, i.e. concerns about medication versus concerns about disease in the B-IPQ. These findings are a replication of those reported by Timmermans et al. [Citation33], implementing the same statement for validation of the ‘Consequence component’ but not the ‘Control component’ as it was not included in their analysis [Citation33]. However, the convergent findings as regards the B-IPQ Consequence component, despite the dissimilar populations and treatments, suggest that this component has an informative attribute which is independent of the actual condition.
The Cronbach alpha coefficient results revealed satisfactory reliability regarding the internal consistency of the Consequences component. When interpreting the Cronbach alpha coefficient results, the number of items included in the component tested must be taken into account [Citation51]. The Control component only consisted of three items, which probably compromised the Cronbach alpha coefficient value [Citation46,Citation51,Citation52]. However, the Cronbach alpha coefficient only indicates whether items ‘hand together’ but not whether the questionnaire measures what it is supposed to do [Citation47]. In addition, the face validity of B-IPQ was good.
In total, the validation of the Swedish translation of B-IPQ based on PCA components gives a picture that is somewhat weak regarding validity and reliability of adolescents’ perceptions of having ADHD.
However, according to the PCA, the Control component comprised the items of concern and emotions besides the comprehensibility item. On the other hand, the Consequence component comprised three cognitive items and one purely emotional item along with the concerns item representing both cognitive and emotional elements. This distribution of items in the presented components in adolescents with ADHD indicates that B-IPQ mainly represents two concepts of perceptions representing emotional and cognitive implications of ADHD on one hand and the perceived capability for self-care maintenance of ADHD on the other hand.
Although the results on the components loadings are in agreement with previous validations study results based on PCA [Citation33] they are in part inconsistent with Leventhal’s CSM [Citation35–37,Citation39] in which two parallel processes of illness perceptions are postulated in terms of cognitive and emotional representations.
Since, the PCA analyses results did not support the existence of solid components which in turn also were inconsistent with Leventhal’s CSM [Citation35–37,Citation39], it seems more suitable to follow the original use of B-IPQ procedures which also allows comparisons of results with prior B-IPQ studies.
A strength of this study was the possibility of identifying all suitable patients undergoing treatment at the participating clinics and the well-characterized population diagnosed after thorough neuropsychological investigation. The KMO and Bartlett’s test of sphericity indicated that the data fulfilled the assumptions for PCA. In addition, the identified components of both the BMQ-specific and the B-IPQ have been previously described by other researchers from different countries [Citation16,Citation24,Citation26–28,Citation33,Citation34], highlighting the Swedish translation’s equivalency.
Limitations
The weak correlations of both BMQ-specific and B-IPQ components to the statements chosen for convergent validation to some degree limit the interpretation of the components’ concept in the Swedish translation. It is likely that the choice of validation statements was not optimal. Unfortunately, this data material does not contain the statements used by previous researchers to perform convergent validation.
The instruments were not test-retested for confirming their stability over time [Citation48]. Such reliability testing should be the subject of future studies. The translation process may have generated some inaccuracy since only one forward translation was conducted. Furthermore, conceptual equivalence between this and other translations as well as the original versions has not been investigated so far to ensure inter-translation validity, i.e. the so-called harmonization step. This may render obstacles in comparing or pooling future results of this translation with international data [Citation41]. For instance, the item regarding that medication is a mystery may lead to conceptual differences as the best available translation is the Swedish word ‘mystiskt’, which also vaguely implies mystification in the Scandinavian languages [Citation53].
However, by using an English native speaker who works as a professional English translator and has knowledge in the context and the survey method in the backward translation step, the risk for divergent meaning was minimized to some degree.
In addition, the translation to Swedish of the mutual text in the first and second version of BMQ-Specific are in principal congruent although performed by different translations. This supports the correctness of the present translation and suggests it may be considered as acceptable.
There were fewer girls than boys in the ADHD population. This however reflects the natural occurring proportions in the general population, so the findings should be representative on a population basis [Citation54].
Conclusions
The Swedish translation of BMQ-specific and B-IPQ proved to be valid and reliable for use in future research as well as in clinical work to assess the perception of ADHD and beliefs about ADHD medication in adolescents. Our findings confirm the previously described Consequence and Control components of B-IPQ and suggest that B-IPQ assess two main concepts of perceptions: emotional and cognitive implications of ADHD versus the capability for self-care maintenance of ADHD.
Notes on contributors
ME, PAG, and IM designed and conducted the study. ME collected the data. ME analyzed the data. ME, IB, PAG, RH and IM drafted the manuscript.
ME, IB, PAG, RH and IM contributed to the redrafting and editing of the manuscript.
Acknowledgements
The authors would like to thank the young people participating and their parents, and also the staffs of the participating CAP units for their time and involvement in the data collection. The Medical Research Council of Southeast Sweden (FORSS-466211) for financial support. The first author received founding to write from Child and Youth Studies at University West. The funding sources had no further role in study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Disclosure statement
All authors declare that they have no conflict of interest.
References
- Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–499.
- Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(02):192–201.
- Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192–202.
- Molina BS, Pelham WE, Gnagy EM, et al. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcohol Clin Exp Res. 2007;31(4):643–654.
- Fridman M, Banaschewski T, Sikirica V, et al. Factors associated with caregiver burden among pharmacotherapy-treated children/adolescents with ADHD in the Caregiver Perspective on Pediatric ADHD survey in Europe. Neuropsychiatr Dis Treat. 2017;13:373–386.
- Le HH, Hodgkins P, Postma MJ, et al. Economic impact of childhood/adolescent ADHD in a European setting: the Netherlands as a reference case. Eur Child Adolesc Psychiatry. 2014;23(7):587–598.
- Biederman J, Monuteaux MC, Spencer T, et al. Do stimulants protect against psychiatric disorders in youth with ADHD? A 10-year follow-up study. Pediatrics. 2009;124(1):71–78.
- Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatr. 2014;55(8):878–885.
- Lichtenstein P, Larsson H. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2013;368(8):776.
- Sabaté E, editor. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003.
- Marcus SC, Durkin M. Stimulant adherence and academic performance in urban youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(5):480–489.
- Hong J, Novick D, Treuer T, et al. Predictors and consequences of adherence to the treatment of pediatric patients with attention-deficit/hyperactivity disorder in Central Europe and East Asia. Patient Prefer Adher. 2013;7:987–995.
- Gau SS, Chen SJ, Chou WJ, et al. National survey of adherence, efficacy, and side effects of methylphenidate in children with attention-deficit/hyperactivity disorder in Taiwan. J Clin Psychiatry. 2008;69(1):131–140.
- Emilsson M, Gustafsson PA, Ohnstrom G, et al. Beliefs regarding medication and side effects influence treatment adherence in adolescents with attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2016;26(5):559–571.
- Ferrin M, Taylor E. Child and caregiver issues in the treatment of attention deficit-hyperactivity disorder: education, adherence and treatment choice. Future Neurol. 2011;6(3):399–413.
- Horne R, Weinman J, Hankins M. The Beliefs about Medicines Questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24.
- Horne R, Chapman SC, Parham R, et al. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633.
- Petrie KJ, Broadbent E, Kydd R. Illness perceptions in mental health: issues and potential applications. J Mental Health. 2008;17(6):559–564.
- Horne R. Treatment perceptions and self-regulation. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. New York: Routledge; 2003. p. 138–153.
- Chapman SC, Barnes N, Barnes M, et al. Changing adherence-related beliefs about ICS maintenance treatment for asthma: feasibility study of an intervention delivered by asthma nurse specialists. BMJ Open. 2015;5(6):e007354.
- Broadbent E, Petrie KJ, Main J, et al. The Brief Illness Perception Questionnaire. J Psychosom Res. 2006;60(6):631–637.
- Jörgensen T, Andersson K, Bondesson Å, et al. The translation of the beliefs about medicines questionnaire to Swedish. Göteborg: Pharmaceutical Outcomes Research, Department of Social Medicine Göteborg University; 2003.
- Berntson E, Bernhard-Oettel C, Hellgren J, et al. Enkätmetodik. Stockholm: Natur & Kultur; 2016.
- Gatt I, West LM, Calleja N, et al. Psychometric properties of the Belief about Medicines Questionnaire (BMQ) in the Maltese language. Pharm Pract (Granada). 2017;15(1):886.
- Matoulkova P, Krulichova IS, Macek K, et al. Chronically III Czech patients' beliefs about medicines: the psychometric properties and factor structure of the BMQ-CZ. Drug Inf J. 2013;47(3):341.
- Alsous M, Alhalaiqa F, Abu Farha R, et al. Reliability and validity of Arabic translation of Medication Adherence Report Scale (MARS) and Beliefs about Medication Questionnaire (BMQ)-specific for use in children and their parents. PLoS One. 2017;12(2):e0171863.
- Fall E, Gauchet A, Izaute M, et al. Validation of the French version of the Beliefs about Medicines Questionnaire (BMQ) among diabetes and HIV patients. Rev Eur Psychol Appl/Eur Rev Appl Psychol. 2014;64(6):335–343.
- Perpiñá Tordera M, Moragón EM, Fuster AB, et al. Spanish asthma patients’ beliefs about health and medicines: validation of 2 questionnaires. Arch Bronconeumol (English Edition). 2009;45(5):218–223.
- Zhang N, Fielding R, Soong I, et al. Psychometric assessment of the Chinese version of the Brief Illness Perception Questionnaire in breast cancer survivors. PLoS One. 2017;12(3):1–10.
- de Raaij EJ, Schröder C, Maissan FJ, et al. Cross-cultural adaptation and measurement properties of the Brief Illness Perception Questionnaire-Dutch Language Version. Man Ther. 2012;17(4):330–335.
- Kossakowska MM, Stefaniak TJ. Psychometric properties for the Polish version of the Brief Illness Perception Questionnaire (Brief IPQ). Health Psychol Rep. 2017;5(1):67–83.
- Bazzazian S, Besharat MA. WCPCG-2010: reliability and validity of a Farsi version of the Brief Illness Perception Questionnaire. Proc Soc Behav Sci. 2010;5:962–965.
- Timmermans I, Versteeg H, Meine M, et al. Illness perceptions in patients with heart failure and an implantable cardioverter defibrillator: dimensional structure, validity, and correlates of the Brief Illness Perception Questionnaire in Dutch, French and German patients. J Psychosom Res. 2017;97:1–8.
- Karatas T, Ozen S, Kutluturkan S. Factor structure and psychometric properties of the Brief Illness Perception Questionnaire in Turkish Cancer Patients. Asia Pac J Oncol Nurs. 2017;4(1):77–83.
- Diefenbach MA, Leventhal H. The common-sense model of illness representation: theoretical and practical considerations. J Soc Distress Homeless. 1996;5(1):11–38.
- Leventhal H, Diefenbach M, Leventhal E. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cogn Ther Res. 1992;16(2):143–163.
- Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. New York: Routledge; 2003. p. 42–65.
- Leventhal H, Leventhal E, Contrada R. Self-regulation, health, and behavior: a perceptual-cognitive approach. Psychol Health. 1998;13(4):717–733.
- Leventhal H, Benyamini Y, Brownlee S, et al. Illness representations: theoretical foundations. In: Petrie KJ, Weinman J, editors. Perceptions of health & illness. Amsterdam: Harwood Academic; 1997. p. 19–45.
- Schuz B, Wolff JK, Warner LM, et al. Multiple illness perceptions in older adults: effects on physical functioning and medication adherence. Psychol Health. 2014;29(4):442–457.
- Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94–104.
- Vitolins MZ, Rand CS, Rapp SR, et al. Measuring adherence to behavioral and medical interventions. Control Clin Trials. 2000;21(5):S188–S194.
- Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567.
- Broadbent E. The Brief Illness Perception Questionnaire Scoring Instructions; [cited 2017 Apr 11]. Available from: http://www.uib.no/ipq/index.html2006
- Norman GR, Streiner DL. Biostatistics: the bare essentials. Hamilton: Decker; 2000.
- Field A. Discovering statistics using IBM SPSS statistics. 4th ed.; SAGA; London. 2012.
- Connelly LM. Research roundtable. Cronbach's alpha. MEDSURG Nurs. 2011;20(1):45–44.
- Behling O, Law KS. Translating questionnaires and other research instruments: problems and solutions. Thousand Oaks (CA): Sage; 2000.
- Weinman J, Petrie KJ, Moss-Morris R, et al. The Illness Perception Questionnaire: a new method for assessing the cognitive representation of illness. Psychol Health. 1996;11(3):431–445.
- Moss-Morris R, Weinman J, Petrie KJ, et al. The revised illness perception questionnaire (IPQ-R). Psychol Health. 2002;17(1):1–16.
- Cortina JM. What is coefficient alpha? An examination of theory and applications. J Appl Psychol. 1993;78(1):98–104.
- Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53–55.
- Granas AG, Norgaard LS, Sporrong SK. Lost in translation? Comparing three Scandinavian translations of the Beliefs about Medicines Questionnaire. Patient Educ Counsel. 2014;96(2):216–221.
- BUSA BehandlingsUppföljning av Säkerställd ADHD Årsrapport 2015. BUSA BehandlingsUppföljning av Säkerställd ADHD; Socialstyrelsen; Stockholm. 2016.