Abstract
Objectives
The aims of this study were to investigate whether baseline leisure-time physical activity (LTPA) is associated with future recovery from depression among patients with a depression diagnosis and whether baseline LTPA is associated with total physical activity after five years of follow-up.
Methods
A total of 258 patients aged ≥35 years with clinically confirmed depression at baseline participated. The study was conducted between 2008 and 2016 in municipalities within the Central Finland Hospital District. Depressive symptoms (DS) were determined with the Beck Depression Inventory (BDI) with a cutoff score ≥10, and depression diagnoses were confirmed by the Mini-International Neuropsychiatric Interview (MINI). Blood pressure and anthropometric parameters were measured and blood samples for glucose and lipid determinations were drawn at baseline. LTPA, physical activity, and other social and clinical factors were captured by standard self-administered questionnaires at baseline and the five-year follow-up point.
Results
Of the 258 patients, 76 (29%) had DS at follow-up. Adjusted odds ratio (OR) for future DS was 1.43 (confidence interval [CI] 0.69–2.95) for participants with moderate LTPA and 0.92 (CI 0.42–2.00) for participants with high LTPA, compared with low LTPA at baseline. Higher baseline LTPA levels were associated with higher total physical activity in the future (β=0.14 [95% CI: 0.02–0.26] for linearity = 0.024).
Conclusion
Baseline LTPA did not affect the five-year prognosis of depression among depressed patients in a Finnish adult population. Because the baseline LTPA level predicted the future total physical activity, it could be included as a part of the overall health management and treatment of depression in clinical practices.
Introduction
The benefits of physical activity for the treatment and prevention of depression are well known. Engaging in physical exercise or having a physically active lifestyle can reduce depressive symptoms (DS) and also reduces the risk of developing depression [Citation1–3]. In their Cochrane review, Cooney et al. [Citation4] concluded that physical exercise may have at least a moderate-sized effect for reducing DS and that physical exercise may be as effective as psychological therapies or medications. Similarly, Mammen and Faulkner [Citation5] stated in their review that any physical activity level, including low levels, such as walking, may prevent future depression. In turn, Ringen et al. [Citation6] found in their recent study that among inpatients with severe mental illness, low levels of physical activity were significantly associated with more severe depression.
Recent studies have also shown that independently of sociodemographic characteristics, leisure-time physical activity (LTPA) can benefit everyone with DS regardless of age, gender, education, family, social status, or living environment [Citation7]. In the body of literature, LTPA is defined as physical activity that an individual engages in during free time [Citation8] that is not related to regular work, housework, or transportation activities [Citation9]. In addition, lifetime physical activity has been shown to be linked to depression in such a manner that childhood physical activity may reduce the risk of depression in adulthood [Citation10]. Furthermore, promoting lifetime LTPA may reduce DS in older age [Citation11]. In the literature, physical activity is defined as any bodily movement produced by skeletal muscles that result in energy expenditure. Studies have shown that in daily life, physical activity can be categorized as occupational, sports, conditioning, household, or other activities [Citation12], and physical activity is used as an umbrella term that encompasses all daily physical activities in which LTPA is one part of total daily physical activity.
Because recent studies have suggested that physical activity and LTPA alone or as adjuncts to other treatments (medication and psychotherapy) [Citation2] are promising and plausible methods of treating and preventing depression and DS, it is relevant to explore whether self-assessed LTPA levels can predict future depression among those who have already been diagnosed as depression positive. Thus, the aims of this study, as a part of the Finnish Depression and Metabolic Syndrome in Adults (FDMSA) study, were to investigate whether baseline LTPA is associated with future recovery from depression among patients with a depressive diagnosis and whether baseline LTPA is associated with total physical activity after five years of follow-up.
Materials and methods
The study data from this prospective cohort study were drawn from the FDMSA baseline study (2008–2011) and its follow-up study (2012–2016), which were conducted within the municipalities in Central Finland Hospital District with a catchment area of 274,000 inhabitants. The Ethics Committee of the Central Finland Hospital District approved the study protocol prior to the commencement of the study. The study enrollment was based on written and oral patient information. All participants signed a written informed consent form before any study procedures commenced. The study population was enrolled from the group of new and old mild to moderately depressed patients who were experiencing a new depressive episode, were 35 years or older, and who scored ≥10 on the BDI. Participants were self-referred or referred by general practitioners to depression nurse case managers. Participants’ psychiatric diagnoses were confirmed by a diagnostic structured interview, the Mini-International Neuropsychiatric Interview (MINI), administered by trained depression nurse case managers.
At the baseline, a total of 706 referred patients scored 10 or more on the BDI. From these, 447 patients met the criteria for clinical depression (major depressive episode, major depressive episode with melancholic features, dysthymia) after the diagnostic interview. Of these, 258 patients took part through the follow-up stage, and those participants whose BDI scores were 10 or over at follow-up were classified as having DS. Depression (and Type 2 diabetes) are more prevalent in the Finnish population after 35 years of age; therefore, in this study, the authors decided to focus on the ≥35 year age group [Citation13]. The age limit of 35 years was also chosen to obtain a stable study population, which facilitates having a more prolonged follow-up [Citation14]. By including self-referred patients along with general practitioner referred patients, we obtained a representative sample comprising both mild and more severely depressive participants [Citation13,Citation14].
At baseline, all participants filled out a standard self-administered questionnaire containing questions about their socioeconomic background, health, and health behaviors, previously diagnosed somatic disorders, and LTPA. Participants also took part in a physical examination and collection of blood samples. Moreover, participant DS was assessed, and clinical depression was confirmed. At the five-year follow-up, participants filled out the same standard self-administered questionnaire. Participants’ DS, clinical depression, and total physical activity were also assessed at the five-year follow-up.
Participant LTPA was assessed by asking ‘How often do you participate in physical activity for at least half an hour so that you are out of breath and sweating?’ Answers were classified into three levels: low (twice per month or less), moderate (about once or twice per week), or high (three times per week or more). In previous studies, self-assessed LTPA has shown a high correlation with physical fitness as measured by maximal oxygen uptake [Citation15].
At follow-up participants’ total physical activity was assessed using the short-form International Physical Activity Questionnaire (IPAQ). The IPAQ was developed as an instrument for cross-national monitoring of physical activity [Citation16], and it has been proved to be a valid and reliable method of collecting physical activity data in cross-sectional studies [Citation17]. The IPAQ short form consists of seven questions about physical activity (in work, leisure time, commuting, exercising, or for sport) during the previous seven days. Participants were asked to assess the number of times they had engaged in at least 10 min of vigorous activity (hard physical effort that makes one’s breathing labored), moderate activity (moderate physical effort that makes breathing a little difficult), or walking, as days per week, hours per day, and minutes per day. Further, daily sitting time as hours and minutes per day were also assessed. Answers were then classified as IPAQ grades (low, moderate, high) via the IPAQ scoring protocol [Citation18]. At the five-year follow-up, answers for physical activity were converted and expressed as metabolic equivalent (MET) [Citation19] hours per week (METh).
The severity of DS was captured using the 21-item Beck Depression Inventory (BDI) [Citation20] completed by participants. The cutoff point was set at ≥10 [Citation13]. The psychiatric diagnosis was confirmed with the diagnostic Mini-International Neuropsychiatric Interview (MINI), version 5.0.0 [Citation21], delivered by a trained study nurse. In Finland, depression nurse case managers are an important part of the basic health care team, and they primarily organize the treatment of depressed patients together with the general practitioners [Citation13]. The patients were treated according to the current Finnish treatment guidelines for depression, meaning with antidepressive medications and/or psychotherapy [Citation13].
At baseline, the participant’s weight, height, and blood pressure were measured. Weight and height were measured with the participant wearing light clothing and were accurate to the nearest 0.5 cm and 0.1 kg, respectively. Blood pressure was measured twice after 15 min rest time with a mercury sphygmomanometer and the patient in a sitting position. The physical examination was conducted by trained study nurses [Citation22]. The body mass index (BMI) was defined as the person’s weight (kg) divided by the square of the height (m). The World Health Organization (WHO) has defined overweight as a BMI ≥25 and obesity as a BMI ≥30 [Citation23].
The blood samples were drawn between 8:00 a.m. and 11:00 a.m. after 12 h of fasting, for glucose and lipid determination. Serum total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and plasma glucose were analyzed using Modular Analytics SWA (Hitachi High-Technologies Corporation, Tokyo, Japan) [Citation13,Citation13,Citation24].
Statistical analysis
Data are presented as means with standard deviation (SD) or as counts with percentages. Statistical comparisons between DS groups were made using a t-test for continuous variables and Pearson’s chi‐square for categorical variables. The adjusted hypothesis of linearity (orthogonal polynomial) was evaluated using generalized linear models (analysis of covariance and logistic models), with the appropriate distribution and link function. Models included age, gender, years of education, diabetes, and BDI as covariates. The bootstrap method was used when the theoretical distribution of the test statistics was unknown or in the case of violation of the assumptions (e.g. non-normality). Rank-based (Spearman) partial correlations were calculated between baseline and follow-up LTPA, adjusted for age, sex, years of education and diabetes. Confidence intervals for the correlation was obtained by bias-corrected bootstrapping (5000 replications). The normality of variables was evaluated graphically and with the Shapiro–Wilk W-test. The Stata 16.0 statistical package (StataCorp LP, College Station, TX, USA) was used for the analysis.
Results
A total of 258 patients with confirmed clinical depression at baseline and who took part in the five-year follow-up were included in the analysis. Of these, DS (BDI ≥10) was confirmed in 76 patients (29%). The majority of participants were female (n = 195, 76%). shows the baseline sociodemographic and clinical characteristics of the participants according to the follow-up DS status. Those participants with DS at the follow-up had fewer years of education (p = 0.035), more DS (p = 0.011), and higher prevalence of diabetes (p = 0.014) at baseline than those participants without DS.
Table 1. Participants’ baseline social and clinical characteristics according to follow-up categorization as with or without depressive symptoms (DS).
In a comparison of the main baseline characteristics (age, gender, BDI level) of those who participated and those who did not participate in the follow-up, there was no statistically significant difference between the groups.
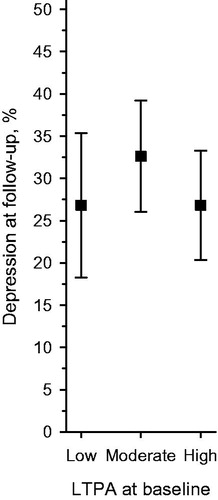
Statistical analysis revealed that the baseline LTPA level (adjusted for age, sex, years of education, diabetes, and BDI) was not associated with the DS status at the five-year follow-up (). Age, sex, years of education, diabetes and BDI adjusted odds ratio (OR) for future depression was 1.71 (CI 0.61–4.79) for participants with moderate LTPA and 1.00 (CI 0.35–2.85) for participants with high LTPA, as compared with low LTPA at baseline. Crude OR were 1.2 (CI 0.61–2.38) and 0.73 (CI 0.35–1.52) for participants with moderate or high LTPA and low LTPA, respectively. The result did not change even though the BDI adjusting was removed.
Figure 1. Proportion (%) of participants with DS (BDI score ≥10) at follow-up according to baseline LTPA level. Adjusted for baseline age, gender, years of education, diabetes, and BDI. BDI: beck depression inventory; DS: depressive symptoms; LTPA: leisure-time physical activity.
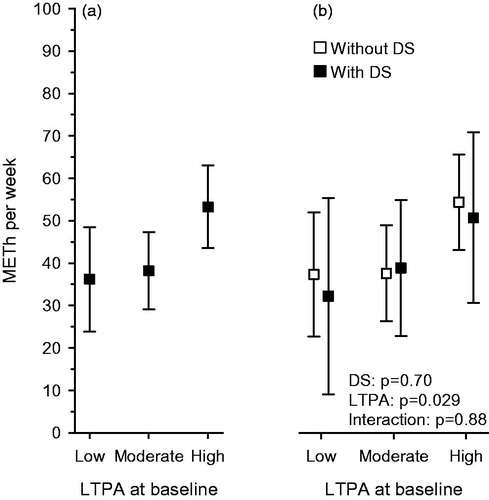
Instead, higher baseline LTPA levels were associated with higher physical activity in the future. Those participants with higher LTPA levels had more METh per week at follow-up than those with lower baseline LTPA levels [β = 0.15 (95% CI: 0.03–0.27) for linearity =0.021] (), and this association did not differentiate between follow-up DS status (). Correlation (adjusted for age, sex, years of education, and diabetes) between baseline and follow-up LTPA split by follow-up DS status was for participants with DS 0.52 (95% CI: 0.34–0.71) and 0.32 (95% CI: 0.18–0.46) for participants without DS, respectively.
Figure 2. (a) Participant (all) physical activity expressed as METh per week at follow-up according to baseline LTPA level. Adjusted for baseline age, gender, years of education, diabetes, and BDI. (b) Participant (separate with DS and without DS) physical activity expressed as METh per week at follow-up according to baseline LTPA level. Adjusted for baseline age, gender, years of education, and diabetes. BDI: beck depression inventory; DS: depressive symptoms; LTPA: leisure-time physical activity; METh: metabolic equivalent hours per week.
At the follow-up, among the participants without DS, 34% had low, 30% had moderate, and 36% had high physical activity levels (expressed as IPAQ grade). Among those participants with DS, 37% had low, 32% had moderate, and 32% had a high physical activity classification.
Discussion
This study showed that baseline LTPA levels did not predict an improvement in DS among depressive patients at a five-year follow-up. However, baseline LTPA did predict future total physical activity. Moreover, baseline depressive diagnosed participants who still had DS at the five-year follow-up had fewer years of education and poorer health status (more diabetes and DS) at baseline.
Our study findings are in line to a certain extent with some earlier studies. While physical activity has been found to be an effective method of reducing depression, as well as preventing and protecting against depression [Citation1–3], in our study sample, earlier physical activity levels were not related to a subsequent recovery from depression. On the other hand [Citation25], for example, found in their recent study after a two-year follow-up that physical activity predicts future remission of depressive disorder only in younger adults but not in older depressive patients. Our findings are in line with the findings from [Citation25] on older depressive patients. Also, although physical activity can be a protective factor and effective treatment method for depression, it might not be favorable for everyone. Some individuals may benefit less from physical activity and exercise in the same way that some benefit less from other treatments of depression, such as medication, due to different biological, clinical, psychological, and/or social moderators [Citation26]. Again, autonomous motivation and social support from the patient’s friends and family, as well as from health professionals, may play the key role in exercise adherence [Citation26]. From earlier studies, we also know that the course of depression fluctuates and the severity of DS can vary over time [Citation27–29].
Our finding that baseline LTPA was associated with the future total physical activity level is consistent with findings that physical activity stays relatively stable and the level of physical activity rarely changes over time [Citation25]. The physical activity level of depressed patients might also reflect a trait (feature of one’s personality) instead of the state of depression [Citation25]. Also, according to a study by [Citation20], as compared with adulthood physical activity, an earlier physical activity history does not contribute to the progression of DS to a greater degree. Further, there is evidence of a longitudinal and bi-directional relationship between a low physical activity and the severity of depression and anxiety symptoms and odds for disorders chronicity [Citation31]. In this light, our findings are important because being physically active can create better odds of successful protection from depression, and thus, health professionals should encourage their patients to engage in physical activity by endorsing it in everyday life and as an add-on to other treatments for depression. Once a physically active lifestyle has been gained and stabilized, the probability of it being a permanent situation is more plausible [Citation25].
Earlier studies have shown that depression is often associated with other illnesses [Citation32,Citation33], a poorer living environment, and lower socioeconomic status [Citation34,Citation35]. This kind of association was found in our study as well with years of education, diabetes, and DS. Our finding that depressed patients had more diabetes is in line with the recent knowledge that diabetes is a risk factor for depression and that depression is more prevalent in people with diabetes [Citation36]. In addition, the fact that depression may remain underdiagnosed when comorbid with diabetes underlines the importance of properly identifying and treating both illnesses [Citation36].
In Finland, LTPA has increased in recent decades among the general population, but total physical activity has remained fairly stable [Citation37]. According to the recent Finnish national FinHealth 2017 survey, one-third of the Finnish adult population were physically inactive, and approximately only half of the population achieved the national recommended physical activity level [Citation38]. Given that in our study sample, only one-third of the participants achieved the same level, it seems that depressive patient physical activity is lower than in the overall Finnish population. This could be one reason for the diminishing effects of physical activity on future DS among our study sample.
Overall, in light of earlier studies, it seems that factors other than physical activity alone influence the remission of DS. Patient traits, socioeconomic background, and other somatic illnesses such as diabetes might be, together or individually, more important elements of individual well-being and health than is a physically active lifestyle. Nonetheless, promoting physical activity is especially important because it certainly has positive impacts on overall health and well-being and seldom has any adverse effects.
Because many recent studies have focused mainly on the immediate or short-term effects of physical activity on depression, the main strength of this study is its five-year longitudinal design, nationally representative capture, and depression confirmation. In this study, we also had a nationally representative study population with a catchment area of 274,000 inhabitants. Another study strength is that we used a structured diagnostic interview (MINI) to confirm depression. The main limitations lie in the possibility of LTPA and total physical activity overestimation by participants and the study results’ generalizability. As the study population included only patients 35 years or older, the generalizability to younger persons is questionable. Also, follow-up was at only one time point, which can be considered a weakness because many depression patients cycle between periods. Again, one study weakness is the lack of data on intra- and inter-observer variability.
Conclusion
Baseline LTPA did not affect the five-year prognosis of depression among depressed patients in a Finnish adult population. However, as baseline LTPA predicted future physical activity, those in clinical practice should consider including it as a part of overall health management and the treatment of depression.
Acknowledgments
The authors would like to thank the depression nurse case managers who took part in the practical implementation of the FDMSA: Mari Alanko, Harri Back, Timo Hannula, Anu Holopainen, Ritva Häkkinen, Katja Johansson, Eija Kinnunen, Kaija Luoma, Hannele Niemi, Hillevi Peura, Inga Pöntiö, Kirsi Rouvinen, Tiina Silvennoinen, and Marianne Vihtamäki, as well as the FDMSA study nurses: Anne Kirmanen, Seija Torkkeli, Reetta Oksanen, and Olli Niemi. We would also like to thank Pia Jauhiainen, scientific secretary of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hallgren M, Nguyen T-T-D, Lundin A, et al. Prospective associations between physical activity and clinician diagnosed major depressive disorder in adults: a 13-year cohort study. Prev Med. 2019;118:38–43.
- Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67–86.
- Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175(7):631–648.
- Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
- Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prevent Med. 2013;45(5):649–657.
- Ringen PA, Faerden A, Antonsen B, et al. Cardiometabolic risk factors, physical activity and psychiatric status in patients in long-term psychiatric inpatient departments. Nord J Psychiatry. 2018;72(4):296–302.
- Marques A, Peralta M, Gouveia ÉR, et al. Leisure-time physical activity is negatively associated with depression symptoms independently of the socioeconomic status. Eur J Sport Sci. 2020;20(9):1268–1269.
- Steinbach D, Graf C. Leisure time physical activity and sedentariness. In: Encyclopedia of Public Health; 2008. p. 849–851.
- eseguer CM, Galán I, Herruzo R, et al. Leisure-time physical activity in a Southern European Mediterranean country: adherence to recommendations and determining factors. Revista Española de Cardiología. 2009;62(10):1125–1133.
- Jacka FN, Pasco JA, Williams LJ, et al. Lower levels of physical activity in childhood associated with adult depression. Journal of Science and Medicine in Sport. 2011;14(3):222–226.
- Korniloff K, Vanhala M, Kautiainen H, et al. Lifetime leisure-time physical activity and the risk of depressive symptoms at the ages of 65–74 years: The FIN-D2D survey. Preventive Medicine. 2012;54(5):313–315.
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131.
- Koponen H, Kautiainen H, Leppänen E, et al. Association between suicidal behaviour and impaired glucose metabolism in depressive disorders. BMC Psychiat. 2015;15(1).
- Koponen H, Kautiainen H, Leppänen E, et al. Cardiometabolic risk factors in patients referred to depression nurse case managers. Nord J Psychiatry. 2015b;69(4):262–267.
- Aires N, Selmer R, Thelle D. The validity of self-reported leisure time physical activity, and its relationship to serum cholesterol, blood pressure and body mass index. A population based study of 332,182 men and women aged 40–42 years. Eur J Epidemiol. 2003;18(6):479–485.
- Booth M. Assessment of physical activity: an international perspective. Res Quarter Exer Sport. 2000;71(sup2):114–120.
- Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395.
- Grupo IPAQ. 2016. IPAQ scoring protocol – International Physical Activity Questionnaire. Retrieved March 16, 2019, from: https://sites.google.com/site/theipaq/scoring-protocol.
- Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–565.
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571.
- Sheehan D, Lecrubier Y, Harnett-Sheehan K, et al. The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry. 1998;59(Suppl. 20):22–23.
- Raatikainen I, Vanhala M, Mäntyselkä P, et al. Does level of leisure time physical activity, in a sample of patients with depression, predict health care utilization over a subsequent 5-year period? Findings from a Finnish cohort study. Ment Health Phys Activ. 2018;15(June):40–44.
- World Health Organization. 2018. Obesity and overweight. Retrieved March 16, 2019, from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Auvinen P, Mäntyselkä P, Koponen H, et al. Prevalence of restless legs symptoms according to depressive symptoms and depression type: a cross-sectional study. Nord J Psychiatry. 2018;72(1):51–56.
- Wassink-Vossen S, Collard RM, Penninx BW, et al. The reciprocal relationship between physical activity and depression: does age matter? Eur Psychiatry. 2018;51:9–15.
- Schuch FB, Stubbs B. The role of exercise in preventing and treating depression. Curr Sports Med Rep. 2019;18(8):299–304.
- Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55(8):694–700.
- Kennedy N, Abbott R, Paykel ES. Longitudinal syndromal and sub-syndromal symptoms after severe depression: 10-year follow-up study. Br J Psychiatry. 2004;184(APR):330–336.
- Vergunst FK, Fekadu A, Wooderson SC, et al. Longitudinal course of symptom severity and fluctuation in patients with treatment-resistant unipolar and bipolar depression. Psychiatry Res. 2013;207(3):143–149.
- Kaseva K, Rosenström T, Hintsa T, et al. Trajectories of physical activity predict the onset of depressive symptoms but not their progression: a prospective cohort study. J Sports Med. 2016;2016:1–9.
- Hiles SA, Lamers F, Milaneschi Y, et al. Sit, step, sweat: Longitudinal associations between physical activity patterns, anxiety and depression. Psychol Med. 2017;47(8):1466–1477.
- Penninx BWJH. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74(Pt B):277–286.
- Penninx BWJH, Milaneschi Y, Lamers F, et al. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11(1):129.
- Hoebel J, Maske UE, Zeeb H, et al. Social inequalities and depressive symptoms in adults: the role of objective and subjective socioeconomic status. PLOS One. 2017;12(1):e0169764.
- Silva M, Loureiro A, Cardoso G. Social determinants of mental health: a review of the evidence. Eur J Psychiatry. 2016;30:259–292.
- Bădescu SV, Tătaru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120–125.
- Borodulin K, Harald K, Jousilahti P, et al. Time trends in physical activity from 1982 to 2012 in Finland. Scand J Med Sci Sports. 2016;26(1):93–100.
- Koskinen S, Lundqvist A, Ristiluoma N. 2018. Terveys, toimintakyky ja hyvinvointi Suomessa - FinTerveys 2017 -tutkimus. Terveyden ja hyvinvoinnin laitos (THL), Raportti 4/2018. In Terveyden ja hyvinvoinnin laitos. Retrieved from http://www.julkari.fi/handle/10024/136223%0Ahttp://www.julkari.fi/bitstream/handle/10024/90832/Rap068_2012_netti.pdf?sequence=1.
