Abstract
Objective
The use of antipsychotics in children and adolescents has increased rapidly. Little is known about psychotropic polypharmacy in children and adolescent initiating an antipsychotic drug. Thus, we investigated the frequency and predictors of polypharmacy during the first year of antipsychotic use in Finnish children and adolescents.
Methods
Between 2008 and 2016, 14 848 individuals aged 1–17 years initiating risperidone, quetiapine, aripiprazole, or olanzapine treatment were identified from Finnish Prescription Registry. Data on psychotropic drug prescriptions prior to and during antipsychotic treatment were collected. Associations between predictors and polypharmacy were analyzed with regression models.
Results
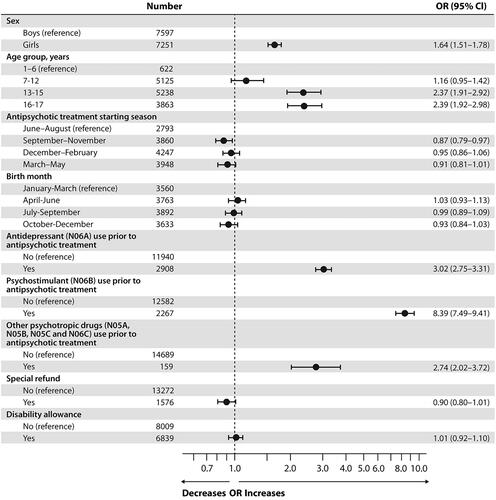
During the study period polypharmacy occurred in 44.9% of the new antipsychotic users, being more frequent in girls (55.5%) than in boys (44.5%, p < 0.001). The two most frequent concomitant psychotropic drug classes were antidepressants (66.2%) and psychostimulants/atomoxetine (30.8%). Adolescents aged 13–15 and 16–17 years, and girls showed an increased risk of polypharmacy during antipsychotic treatment (OR 2.37 [95% CI 1.91–2.92], OR 2.39 [95% CI 1.92–2.98], and OR 1.64 [95% CI 1.51–1.78], respectively). The use of psychostimulants/atomoxetine or antidepressants prior to initiation of antipsychotic treatment was strongly associated with polypharmacy during antipsychotic treatment (OR 8.39 [95% CI 7.49–9.41], OR 3.02 [95% CI 2.75–3.31]).
Conclusions
Polypharmacy was common in children and adolescents initiating antipsychotic treatment. Prior use of psychostimulants/atomoxetine and antidepressants increased the risk of polypharmacy. The use of antipsychotics was mainly off-label, thus, the risks of concomitant use of antipsychotics with other psychotropic drugs should be carefully weighed.
Introduction
The use of antipsychotic drugs has increased in children and adolescents in many European countries during the last fifteen years, whereas the prevalence has slightly decreased in the USA, nevertheless remaining higher than in Europe [Citation1–3]. The most common diagnoses in children and adolescents who receive second-generation antipsychotics (SGAs), such as risperidone, quetiapine, and aripiprazole, are attention-deficit/hyperactivity disorder, autism spectrum disorder, disruptive behavioral disorder, depression, and bipolar disorder [Citation4–8]. In these conditions, SGAs are used to treat target symptoms such as inattention/hyperactivity, disruptive behaviors, and anxiety [Citation2,Citation5,Citation7,Citation9]. In addition, SGAs are prescribed for short-term treatment of insomnia, aggression, and other symptoms not fulfilling the criteria of a mental disorder [Citation5,Citation6,Citation10,Citation11]. In children and adolescents, the prescribing of SGAs for indications other than diagnosed psychiatric conditions has contributed to the increasing number of antipsychotic users in many countries [Citation12,Citation13].
Although SGAs have a more favorable adverse effect profile than first-generation antipsychotics [Citation12], SGAs are associated with short- and long-term adverse effects such as weigh gain, changes in endocrine functions, daytime sedation, and increased risk for morbidity in later life [Citation14–17]. Furthermore, children and adolescents are at higher risk of adverse effects of antipsychotics than adults [Citation18]. The risk of adverse effects increases with longer treatment duration and with concomitant use of other psychotropic drugs (i.e. polypharmacy) [Citation19,Citation20,Citation21]. Several studies have investigated the frequency of psychotropic polypharmacy in children and adolescents, whereas only few studies have examined psychotropic polypharmacy in antipsychotic users [Citation7,Citation22–24]. The frequency of psychotropic polypharmacy has ranged from 7% to 85% depending on age group, data source, and especially the definition of polypharmacy applied [Citation7,Citation22–24]. However, none of the previous studies have evaluated frequency of psychotropic polypharmacy in children and adolescents initiating antipsychotic drug use for the first time.
In antipsychotic users, polypharmacy can be categorized to be within an antipsychotic class (within- class) or between different psychotropic drug classes (multiclass) [Citation25]. The most common drugs combined with antipsychotic treatment have been psychostimulants/atomoxetine, antidepressants, mood stabilizers, and other antipsychotics [Citation7,Citation23,Citation25,Citation26]. In adolescents with bipolar disorder, the evidence suggests that adding an adjuvant agent to the regimen may be useful when monotherapy does not provide sufficient efficacy [Citation27], whereas the efficacy of polypharmacy in other psychiatric disorders remains unknown. Polypharmacy is worrisome since simultaneous use of multiple psychotropic drugs increases the risk for drug-drug interactions, morbidity, and adverse effects such as type II diabetes and hyperlipidemia [Citation21,Citation28–32]. To the best of our knowledge, no published data exist on the predictors for psychotropic polypharmacy in children and adolescents who initiate an antipsychotic drug for the first time. Identifying the antipsychotic users who are especially at risk for psychotropic polypharmacy could result in a more intensive utilization of non-pharmacological treatments.
In this nationwide study, we aim to determine the frequency for psychotropic polypharmacy in Finnish children and adolescents aged 1–17 years initiating antipsychotic treatment between 2008 and 2016, to characterize which psychotropic drug classes are most combined with antipsychotic treatment in new antipsychotic users, and to identify the predictors for polypharmacy such as age, sex, starting season of antipsychotic treatment, and prior use of psychotropic drugs.
Patients and methods
This study included all Finnish children and adolescents aged 1–17 years, who had received antipsychotic drugs between 1 January 2008 and 31 December 2017 (n = 70 012) reimbursed by National Health Insurance. The drug purchases were collected from the Finnish Prescription Registry, which is maintained by the Social Insurance Institution of Finland (SII). The register includes information on individuals (e.g. age and sex) and prescription (e.g. Anatomical Therapeutic Chemical (ATC) numbers, purchase date, package size, and number of prescribed tablets).
First, we identified new antipsychotic users from whom an antipsychotic drug (risperidone, quetiapine, olanzapine, aripiprazole) had been purchased but who had not filled a prescription for antipsychotics in the previous 730 days [Citation3]. These four drugs accounted for 96.1% of all reimbursed SGA prescriptions to Finnish children and adolescents [Citation3]. The initiation of antipsychotic treatment was defined as the date of the initial purchase (i.e. index date), and the individual’s age at treatment initiation was calculated as the difference between birth date and index date [Citation3,Citation38]. The duration of antipsychotic treatment for new users was defined as the time between the initial medicine purchase date (i.e. index date) and the date to which their last purchase would theoretically last [Citation3]. The methods used to identify new users of antipsychotics and to calculate antipsychotic treatment duration have been described in detail before and used in previous prescription register studies [Citation3,Citation33–35,Citation38].
Next, we excluded antipsychotic users who had received only one purchase of the antipsychotic drug during the treatment duration to exclude temporary and short-term usage of antipsychotics. Additionally, we excluded individuals who became 18 years of age in 2017 since their maximum follow-up would have been too short (i.e. less than 365 days). Thus, the number of individuals included in analyses was 14 849. In these individuals, polypharmacy was defined as having at least one additional psychotropic drug at the same time as the antipsychotic drug treatment (i.e. at least two concomitant psychotropic drugs during follow-up) [Citation25,Citation36,Citation37,Citation11]. In cases where antipsychotic treatment duration was more than 365 days, polypharmacy during the first year of treatment was determined. Additionally, the individuals had to have at least two purchases of the concomitant psychotropic drug during the follow-up to fulfill the criteria of polypharmacy (Supplementary Figure 1). These inclusion criteria were set to exclude the short-term and temporary usage of the concomitant psychotropic drug. These drugs (i.e. polypharmacy drugs) were pooled into the main groups by ATC codes: N03A (antiepileptics), N05A (antipsychotics), N05B (anxiolytics), N05C (hypnotics and sedatives), N06A (antidepressants), N06B (psychostimulants, agents used for attention-deficit/hyperactivity disorder (ADHD), and nootropics), and N06C (psycholeptics and psychoanaleptics in combination) (WHO Collaborating Centre for Drug Statistics Methodology). Of note, N05A group polypharmacy included antipsychotic drugs other than risperidone, quetiapine, olanzapine, or aripiprazole since the calculation of duration of antipsychotic treatment allowed individuals to change within the four most used antipsychotic substances without discontinuation of treatment [Citation38].
We next determined what psychotropic drugs were purchased two years prior to initiation of antipsychotic treatment (i.e. prior to the index date). The data were searched for purchases of psychotropic drugs that had occurred at least twice during the two years before antipsychotic treatment initiation (Supplementary Figure 1). Additionally, we determined which of these drugs were purchased also during the antipsychotic treatment, indicating that the polypharmacy drug was initiated already before the antipsychotic treatment.
We included data on reimbursements of the drugs in the analyses. The psychotropic drugs were categorized into two different rates of reimbursement: the Basic Refund Category (reimbursement rate of 40%) or the Special Refund Category (reimbursement rate of 100%). Children and adolescents who have intellectual disability with aggressive behavior, psychotic disorder (including schizophrenia), or depressive disorder with psychosis or bipolar disorder/mania are eligible for Special Refund. Thus, the Special Refund Category illustrates a more severe psychiatric morbidity and on-label use of antipsychotics. In addition, Finnish children and adolescents with a severe somatic or psychiatric disorder (i.e. mental and behavioral disorders) that causes disability and those who need regular care, attention, and rehabilitation are entitled to a disability allowance. Information on the disability allowance is recorded in the Register of Disability Benefits of the Social Insurance Institution and was used in the analyses. The allowance is intended to provide monetary support to families of children with disabilities.
Since the increased burden of schoolwork including increased social demands vary between seasons, we evaluated how the season of antipsychotic treatment initiation predicts polypharmacy. Seasons were categorized into summer (June–August), autumn (September–November), winter (December–February), or spring (March–May).
This was a register-based study, and thus, no Ethics Committee approval was required. However, as a register holder, the Social Insurance Institution approved the study protocol.
Statistical analyses
Data were prepared with R for Windows (version 3.5.2), whereas statistical analyses were conducted with SPSS statistical software for Windows (version, 25.0 Chicago, IL, USA). Between-group comparisons were performed with χ2 test. Logistic regression model was used to evaluate factors associated with polypharmacy in new antipsychotic users. The variables were first analyzed in a unadjusted model and then pooled to a single adjusted regression model. Variables included in the model were age, sex, birth month, antipsychotic starting season, prior antidepressant (N06A) or psychostimulant (N06B) or other psychotropic drug use (N05A, N05B, N05C, N06C), special refund category, and granted disability allowance. The results of the regression model are presented with odds ratios (ORs) with 95% confidence intervals (CIs). In order to test the variation of time periods in a sensitivity analysis, a shorter follow-up period of 180 days for polypharmacy was applied, and evaluated how this affected the risk factors of polypharmacy. The level of statistical significance was set at p < 0.05.
Results
Between 2008 and 2016, a total of 14 849 children and adolescents aged 1–17 years initiated antipsychotic drug use (). Of these, 6660 (44.9%) had concomitant prescriptions of psychotropic drugs, and of them, 3540 (53.2%) had purchased the concomitant drug at least twice already before initiation of the antipsychotic treatment. The frequency of polypharmacy in individuals initiating antipsychotic treatment in 2016 was higher (51.0%) than in those starting the treatment in 2008 (41.0%) (p < 0.001).
Table 1. Characteristics of new antipsychotic users and those with polypharmacy between 2008 and 2016.
In children aged 7 to 12 years, boys had polypharmacy more often than girls (59.5% vs. 32.6%, p = 0.005), whereas in adolescents (age groups 13–15 and 16–17 years) girls had significantly more polypharmacy than boys (54.9% vs. 42.0%, p < 0.001 and 54.8% vs. 44.0%, p < 0.001, respectively) (). Polypharmacy was most frequent in the age group of 13–15 years (n = 2645, 39.7%), followed by individuals aged 16–17 years (n = 1981, 29.7%). The concomitant use was more frequent in girls (n = 3698) than in boys (n = 2962) (55.5% vs. 44.5%, p < 0.001).
Polypharmacy during antipsychotic treatment
Of the new antipsychotic users with polypharmacy, 95.9% had purchased one concomitant psychotropic drug, whereas 4.1% had two or more concomitant psychotropic drug prescriptions during the antipsychotic treatment (). The most frequent concomitant psychotropic drug classes were antidepressants (66.2%) and psychostimulants/atomoxetine (30.8%). Only 0.8% of the new antipsychotic users with polypharmacy had purchased another antipsychotic drug during the treatment. The frequency of antipsychotic polypharmacy was higher in those with purchases reimbursed in the special refund category (2.2%) than in those without it (0.2%) (p < 0.001). Psychostimulants/atomoxetine were the most used concomitant drugs with antipsychotics in younger age groups (1–6 and 7–12 years), whereas antidepressants accounted for most of polypharmacy in older age groups (13–15 and 16–17 years) (). In all age groups, boys had more frequently psychostimulants/atomoxetine combined with the antipsychotic treatment than girls (p < 0.001), whereas girls aged 7 to 17 years had more commonly a concomitant antidepressant than same-aged boys (p < 0.001).
Table 2. Frequency of concomitant psychotropic drugs during the first year of antipsychotic treatment in different age and sex groups.
Predictors of polypharmacy during antipsychotic treatment
Results of the unadjusted and adjusted regression models for predictors of polypharmacy are shown in and , respectively. In the adjusted model, adolescents aged 13 to 15 and 16 to 17 years and girls had the strongest association with polypharmacy (OR 2.37 [95% CI 1.91–2.92], OR 2.39 [95% CI 1.92–2.98], and OR 1.64 [95% CI 1.51–1.78], respectively) (). Notably, the prior use of psychostimulants/atomoxetine had strongest association with polypharmacy (OR 8.39 [95% CI 7.49–9.41]), followed with the prior use of antidepressants OR 3.02 [95% CI 2.75–3.31]). Using other antipsychotics, anxiolytics, or hypnotics (N05A, N05B, N05C, and N06C) prior to initiation of antipsychotic treatment also increased the risk of polypharmacy during antipsychotic treatment (OR 2.74 [95% CI 2.02–3.72]). Starting antipsychotic treatment in autumn slightly decreased the risk of polypharmacy during antipsychotic treatment (OR 0.87 [95% CI 0.79–0.97]), whereas birth month had no significant effect on polypharmacy. In the unadjusted model, the special refund category and disability allowance, which serve as surrogate markers of disease severity, decreased OR for polypharmacy (OR 0.82 [95% CI 0.74–0.91] and OR 0.79 [95% CI 0.74–0.84], respectively), but this effect vanished in the adjusted model ().
Figure 1. Univariate logistic regression model of predictors for psychotropic polypharmacy in Finnish children and adolescents (n = 14,849) who initiated antipsychotic treatment between 2008 and 2016. OR: odds ratio; CI: confidence interval. All variables analyzed separately (univariate) in a logistic regression model.
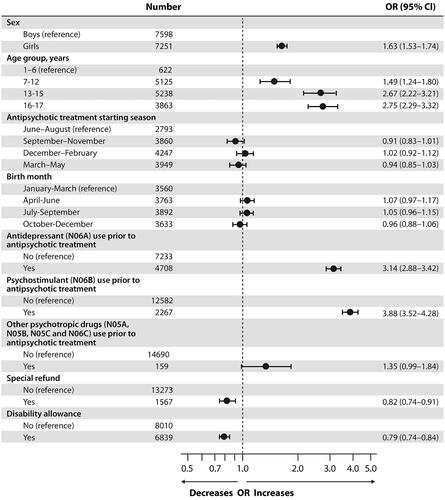
Figure 2. Adjusted logistic regression model of predictors for psychotropic polypharmacy in Finnish children and adolescents (n = 14 849) who initiated antipsychotic treatment between 2008 and 2016. OR: odds ratio; CI: confidence interval. All variables included in the same logistic regression model (adjusted).
In sensitivity analyses, a follow-up period of 180 days (Supplementary Table 1) was applied instead of 365 days. The results were very similar to those observed with a follow-up period of 365 days. In adjusted regression model, the use of psychostimulants/atomoxetine and use of antidepressants before initiation of an antipsychotic increased the risk of polypharmacy during the antipsychotic treatment (OR 8.38 [95% CI 7.46–9.41]) and OR 3.05 [95% CI 2.78–3.36], respectively), whereas the risk of polypharmacy was lower in antipsychotic users receiving a special refund for their medication (OR 0.88 [95% CI 0.78–0.99]). Additionally, we inspected the interaction between sex and age group, and the results aligned with those of the adjusted regression model (data not shown).
Psychotropic drug prescriptions two years prior to antipsychotic treatment
During the two years before the initiation of antipsychotic treatment 5157 (34.7%) of the antipsychotic users had one or more psychotropic drugs purchased at least twice (). Of these, 96.0% had one psychotropic drug and only 4.0% two or more psychotropic drugs purchased before initiation of antipsychotic treatment. The two most common substances were antidepressants (N06A, 54.2%) and psychostimulants, agents used for ADHD, and nootropics (N06B, 42.3%).
Discussion
In this nation-wide register study, we found that nearly half of new antipsychotic users had psychotropic polypharmacy during the first year of their antipsychotic treatment. In new users aged over 13 years, the girls exceeded the boys in frequency of polypharmacy, and the total frequency of polypharmacy was more common in girls than in boys. Antidepressants and psychostimulants/atomoxetine were mostly combined with antipsychotic drug treatment. Older age, female sex, and prior use of psychotropic drugs, especially psychostimulants/atomoxetine and antidepressants, were associated with increased risk of polypharmacy during antipsychotic treatment. These findings are worrisome given that psychotropic polypharmacy is often associated with short and long-term adverse effects.
Forty-four percent of the new antipsychotic users had polypharmacy during the first year of their antipsychotic treatment. In two nationwide US studies, Olfson et al. and Kreider et al. reported an even higher frequency of polypharmacy (85%) with antipsychotic users [Citation7,Citation23]. However, these cross-sectional studies included all antipsychotic users, whereas our study focused on polypharmacy in new antipsychotic users. In the current study, polypharmacy, especially concomitant use of antidepressants, was more frequent in adolescent girls than in boys, whereas boys aged 1–12 years had more commonly concomitant psychotropic medication, especially psychostimulants, than same-aged girls. A similar trend has been noted before [Citation7,Citation39]. These findings suggest that gender- and age-specific differences exist in polypharmacy, which may reflect the predominant disorders and diseases in these groups. In fact, ADHD is the most common neurobehavioral disorder in early childhood and more frequent in boys that in girls [Citation39,Citation40]. Additionally, younger age at diagnosis of ADHD has been reported to be associated with psychotropic polypharmacy [Citation41]. Almost two-thirds of these patients have psychiatric comorbidities, such as conduct disorders, autism spectrum disorder, or aggressive behavior, which are commonly treated with a combination of psychostimulants/atomoxetine and antipsychotics [Citation41]. On the other hand, the prevalence of anxiety and depressive disorders increases in adolescence, especially in girls [Citation7,Citation39,Citation40,Citation42,Citation43]. In contrast to the adult population, the major symptoms of depression in adolescents include weight change and insomnia, the latter of which may be treated by combining short-term antipsychotic drug treatment with antidepressants [Citation10,Citation44]. Similarly, in anxiety disorders, antipsychotics have been used to treat insomnia and high levels of anxiety [Citation6,Citation10,Citation11]. In Finland, the use of antipsychotics is authorized for children and adolescents only in the following indications: i) six weeks’ use for aggressive behavior in intellectually disabled children older than 5 years, ii) schizophrenia in individuals older than 15 years, and iii) bipolar I disorder for less than 12 weeks in individuals older than 13 years (Finnish Medicines Agency, European Medicines Agency). Consequently, antipsychotics in children and adolescents with other psychotropic drugs appear to be mainly off-label use and the use is based on clinical indication.
To the best of our knowledge, this is the first nationwide study to evaluate predictors for polypharmacy in new antipsychotic users. We found that older age, female sex, prior use of psychostimulants/atomoxetine and prior use of antidepressants increased the risk of psychotropic polypharmacy in the first year after antipsychotic treatment. Interestingly, the use of psychostimulants before the initiation of antipsychotic treatment was associated an increased risk for psychotropic polypharmacy during antipsychotic treatment. Supporting our finding, Medhekar et al. showed that ADHD was the most common diagnosis in children and adolescents with psychotropic polypharmacy [Citation25]. Although our data did not include information on diagnosis, in Finland the current clinical practice is that the diagnosis of ADHD is carefully made before ADHD medication is initiated. Furthermore, Olfson et al. reported in a large US population that the antipsychotics were prescribed for aggressive behavior or impulsivity in boys with ADHD [Citation7]. These findings suggest that SGAs are commonly combined with the treatment of ADHD. This prescribing pattern is disturbing since adding SGA to the combination of stimulant and parent training in aggressive children with ADHD is known to result in varying responses compared with the treatment regimen without SGA [Citation45,Citation46]. Kreider et al. reported the alarming result that SGAs are also combined with stimulant treatment in ADHD children without comorbidities such as aggressive behavior [Citation23]. Moreover, it has been suggested that adding antipsychotic drugs to the stimulant treatment may be a prolonged treatment decision rather than a short-term rescue treatment for acute symptoms [Citation23]. This is noteworthy given that the use of SGAs in this vulnerable population is often associated with short- and long-term adverse effects such as weight gain, daytime sedation, and metabolic and endocrine disruptions [Citation14,Citation15,Citation47]. Thus, the appropriateness and efficacy of current medication should be carefully evaluated, and exhaustion of all nonpharmacological treatments should occur before another psychotropic drug is added to the regimen.
Before initiation of SGA treatment and during the treatment, antidepressants were the most prescribed psychotropic drugs. In addition, the use of antidepressants prior to SGA treatment increased the risk of psychotropic polypharmacy during SGA treatment. In a Swedish register study, 62.1% of antidepressant users aged 0 to 24 years had simultaneous prescriptions of other psychotropic drugs, especially anxiolytics, hypnotics, and sedatives [Citation48]. These findings suggest that the efficacy of the antidepressant treatment might be insufficient to control the disabling symptoms, such as insomnia and anxiety, in children and adolescents, and other psychotropic drugs are added to control these symptoms [Citation10,Citation49]. Antidepressant treatment is known to increase anxiety and suicidal thoughts at the beginning of treatment, and thus, anxiolytics, sedatives, and antipsychotics are then combined with the antidepressant treatment. In children and adolescents, the use of antidepressants is associated with the risk of type 2 diabetes, and the risk increases even more if antipsychotic drugs are combined with the treatment [Citation21,Citation29]. Consequently, it should be remembered that the overall combination of psychotropic drugs, not only antipsychotics, contributes to the risk of adverse effects.
Only 0.8% of our individuals used at least two antipsychotics concomitantly, which is significantly lower than the figure reported by others [Citation22,Citation11]. In our study, the lower rate of antipsychotic polypharmacy may stem from the facts that the individuals were new antipsychotic users who had not filled prescriptions of antipsychotic drugs two years prior to treatment initiation and that within-class polypharmacy analysis included antipsychotic drugs other than the four most common ones used in treatment duration calculation (i.e. risperidone, quetiapine, olanzapine, or aripiprazole). Saldana et al. reported that the frequency of antipsychotic polypharmacy correlated positively with longer hospitalization, violence/aggression, and psychosis and that psychotic and bipolar disorders are overrepresented in individuals with antipsychotic polypharmacy [Citation11]. Similarly, we found that children and adolescents with purchases reimbursed with a special refund category, a surrogate marker of severe mental disorders such as bipolar disorders and psychosis, presented with a higher frequency of antipsychotic polypharmacy than those without the category. The concomitant use of different antipsychotics is associated with the risk of adverse effects [Citation29,Citation31] and may provide only marginal benefits to the treatment [Citation50].
The main strength of the study is the nationwide study design in which the new antipsychotic users and psychotropic polypharmacy were carefully determined.
We performed sensitivity analyses inspecting the influence of a shorter follow-up period and the interaction between sex and age group to the results. However, the data did not include the diagnoses of patients and drug usage was based on the assumption that the purchased drugs were in fact used. We excluded individuals who received only one prescription of an antipsychotic or polypharmacy drug, thus minimizing the bias from temporary and short-term usage of psychotropic drugs.
In conclusion, polypharmacy in new antipsychotic users during the first year of use was common, especially in girls. The frequency of polypharmacy increased between 2008 and 2016, which is a worrisome trend. The age of 13 years was the turning point when girls surpassed boys in prevalence of polypharmacy. After the initiation of antipsychotic drug use, antidepressants or psychostimulants/atomoxetine were most combined with the treatment. Older age, female sex, and prior use of psychostimulants/atomoxetine and antidepressants were associated with an increased risk of polypharmacy during antipsychotic treatment. These findings serve as a reminder to clinicians to carefully evaluate the efficacy of psychotropic polypharmacy in children and adolescents.
Author contributions
Eeva Aronen and Leena Saastamoinen were responsible for the concept and design of the study. Eveliina Varimo and Hanna Rättö drafted the manuscript. All authors contributed to data acquisition, analysis, and interpretation. All authors revised the manuscript critically and approved the final version.
Disclosure statement
The authors have no conflicts of interest.
Additional information
Funding
References
- Kalverdijk LJ, Bachmann CJ, Aagaard L, et al. A multi-national comparison of antipsychotic drug use in children and adolescents, 2005-2012. Child Adolesc Psychiatry Ment Health. 2017;11:55–51.
- Schroder C, Dorks M, Kollhorst B, et al. Outpatient antipsychotic drug use in children and adolescents in Germany between 2004 and 2011. Eur Child Adolesc Psychiatry. 2017;26(4):413–420.
- Varimo E, Saastamoinen LK, Ratto H, et al. New users of antipsychotics among children and adolescents in 2008-2017: a nationwide register study. Front Psychiatry. 2020;11:316.
- Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a Population-Based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449–455.
- Dinnissen M, Dietrich A, van der Molen JH, et al. Prescribing antipsychotics in child and adolescent psychiatry: guideline adherence. Eur Child Adolesc Psychiatry. 2020;29(12):1717–1727.
- Nesvåg R, Hartz I, Bramness JG, et al. Mental disorder diagnoses among children and adolescents who use antipsychotic drugs. Eur Neuropsychopharmacol. 2016;26(9):1412–1418.
- Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. 2015;72(9):867–874.
- Vanbronkhorst SB, Roberts DE, Edwards EM, et al. Diagnosis and use of psychotherapy among children and adolescents prescribed antipsychotics. J Psychiatr Pract. 2018;24(5):323–330.
- Rettew DC, Greenblatt J, Kamon J, et al. Antipsychotic medication prescribing in children enrolled in medicaid. Pediatrics. 2015;135(4):658–665.
- Chow ES, Zangeneh-Kazemi A, Akintan O, et al. Prescribing practices of quetiapine for insomnia at a tertiary care inpatient child and adolescent psychiatry unit: a continuous quality improvement project. J Can Acad Child Adolesc Psychiatry. 2017;26(2):98–103.
- Saldaña SN, Keeshin BR, Wehry AM, et al. Antipsychotic polypharmacy in children and adolescents at discharge from psychiatric hospitalization. Pharmacotherapy 2014;34(8):836–844.
- Patten SB, Waheed W, Bresee L. A review of pharmacoepidemiologic studies of antipsychotic use in children and adolescents. Can J Psychiatry. 2012;57(12):717–721.
- Verdoux H, Pambrun E, Cortaredona S, et al. Antipsychotic prescribing in youths: a french community-based study from 2006 to 2013. Eur Child Adolesc Psychiatry. 2015;24(10):1181–1191.
- Bobo WV, Cooper WO, Stein CM, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70(10):1067–1075.
- Cicala G, Barbieri MA, Santoro V, et al. Safety and tolerability of antipsychotic drugs in pediatric patients: Data from a 1-Year naturalistic study. Front Psychiatry. 2020;11:152.
- Krause M, Huhn M, Schneider-Thoma J, et al. Efficacy, acceptability and tolerability of antipsychotics in patients with schizophrenia and comorbid substance use. A systematic review and Meta-analysis. Eur Neuropsychopharmacol. 2019;29(1):32–45.
- Martínez-Ortega JM, Funes-Godoy S, Díaz-Atienza F, et al. Weight gain and increase of body mass index among children and adolescents treated with antipsychotics: a critical review. Eur Child Adolesc Psychiatry. 2013;22(8):457–479.
- Vitiello B, Correll C, van Zwieten-Boot B, et al. Antipsychotics in children and adolescents: increasing use, evidence for efficacy and safety concerns. Eur Neuropsychopharmacol. 2009;19(9):629–635.
- Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383–399.
- Olashore AA, Rukewe A. Polypharmacy among children and adolescents with psychiatric disorders in a mental referral hospital in Botswana. Pharmacotherapy. 2014;34(8):836–844.
- Rubin DM, Kreider AR, Matone M, et al. Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among medicaid-enrolled youths. JAMA Pediatr. 2015;169(4):e150285:
- Constantine RJ, Boaz T, Tandon R. Antipsychotic polypharmacy in the treatment of children and adolescents in the fee-for-service component of a large state medicaid program. Clin Ther. 2010;32(5):949–959.
- Kreider AR, Matone M, Bellonci C, et al. Growth in the concurrent use of antipsychotics with other psychotropic medications in medicaid-enrolled children. J Am Acad Child Adolesc Psychiatry. 2014;53(9):960–970.e2.
- Lohr WD, Creel L, Feygin Y, et al. Psychotropic polypharmacy among children and youth receiving medicaid, 2012-2015. JMCP. 2018;24(8):736–744.
- Medhekar R, Aparasu R, Bhatara V, et al. Risk factors of psychotropic polypharmacy in the treatment of children and adolescents with psychiatric disorders. Res Social Adm Pharm. 2019;15(4):395–403.
- Dosreis S, Yoon Y, Rubin DM, et al. Antipsychotic treatment among youth in foster care. Pediatrics. 2011;128(6):e1459-66–e1466.
- Kowatch RA, Fristad M, Birmaher B, Child Psychiatric Workgroup on Bipolar Disorder, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(3):213–235.
- Barbui C, Nosè M, Mazzi MA, et al. Persistence with polypharmacy and excessive dosing in patients with schizophrenia treated in four European countries. Int Clin Psychopharmacol. 2006;21(6):355–362.
- Burcu M, Zito JM, Safer DJ, Magder LS, et al. Concomitant use of atypical antipsychotics with other psychotropic medication classes and the risk of type 2 diabetes mellitus. J Am Acad Child Adolesc Psychiatry. 2017;56(8):642–651.
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126.
- McIntyre RS, McCann SM, Kennedy SH. Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Can J Psychiatry. 2001;46(3):273–281.
- Yalçin N, Özdemir N, Çak Esen HT, et al. Potential drug-drug interactions with psychotropic drugs in paediatric inpatients: a cross-sectional study. Int J Clin Pract. 2021;75(6):e14107.
- Helin-Salmivaara A, Lavikainen PT, Korhonen MJ, et al. Pattern of statin use among 10 cohorts of new users from 1995 to 2004: a register-based nationwide study. Am J Manag Care. 2010;16(2):116–122.
- Martikainen JE, Saastamoinen LK, Korhonen MJ, Enlund H, et al. Impact of restricted reimbursement on the use of statins in Finland: a register-based study. Med Care. 2010;48(9):761–766.
- Saastamoinen LK, Wallin M, Lavikainen P, et al. Treatment duration with selective serotonin reuptake inhibitors among children and adolescents in Finland: a nationwide register study. Eur J Clin Pharmacol. 2012;68(7):1109–1117.
- Saucedo RS, Liu X, Hincapie-Castillo JM, et al. Prevalence, time trends, and utilization patterns of psychotropic polypharmacy among pediatric medicaid beneficiaries, 1999-2010. Psychiatr Serv. 2018;69(8):919–926.
- Toteja N, Gallego JA, Saito E, et al. Prevalence and correlates of antipsychotic polypharmacy in children and adolescents receiving antipsychotic treatment. Int J Neuropsychopharm. 2014;17(07):1095–1105.
- Varimo E, Aronen ET, Mogk H, et al. Antipsychotic treatment duration in children and adolescents: a Register-Based nationwide study. J Child Adolesc Psychopharmacol. 2021;31(6):421–429.
- Oerbeck B, Overgaard KR, Hjellvik V, et al. The use of antidepressants, antipsychotics, and stimulants in youth residential care. J Child Adolesc Psychopharmacol. 2021;31(5):350–357.
- Cybulski L, Ashcroft DM, Carr MJ, et al. Temporal trends in annual incidence rates for psychiatric disorders and self-harm among children and adolescents in the UK, 2003-2018. BMC Psychiatry. 2021;21(1):229.
- Winterstein AG, Soria-Saucedo R, Gerhard T, et al. Differential risk of increasing psychotropic polypharmacy use in children diagnosed with ADHD as preschoolers. J Clin Psychiatry. 2017;78(7):e744–e781.
- Juul EML, Hjemdal O, Aune T. Prevalence of depressive symptoms among older children and young adolescents: a longitudinal population-based study. Scand J Child Adolesc Psychiatr Psychol. 2021;9:64–72.
- Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75–81.
- Rice F, Riglin L, Lomax T, et al. Adolescent and adult differences in major depression symptom profiles. J Affect Disord. 2019;243:175–181.
- Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47–60.e1.
- Barterian JA, Arnold LE, Brown NV, et al. Clinical implications from the treatment of severe childhood aggression (TOSCA) study: a Re-Analysis and integration of findings. J Am Acad Child Adolesc Psychiatry. 2017;56(12):1026–1033.
- Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45(7):771–791.
- Lagerberg T, Molero Y, D'Onofrio BM, et al. Antidepressant prescription patterns and CNS polypharmacy with antidepressants among children, adolescents, and young adults: a population-based study in Sweden. Eur Child Adolesc Psychiatry. 2019;28(8):1137–1145.
- Bushnell GA, Compton SN, Dusetzina SB, et al. Treating pediatric anxiety: Initial use of SSRIs and other antianxiety prescription medications. J Clin Psychiatry. 2018;79(1):16m11415.
- Gören JL, Parks JJ, Ghinassi FA, et al. When is antipsychotic polypharmacy supported by research evidence? Implications for QI. Jt Comm J Qual Patient Saf. 2008;34(10):571–582.
