Abstract
Purpose: Patients with schizophrenia or bipolar disorder are at increased risk of somatic illnesses and have more somatic complaints compared with the general population. Schizophrenia and bipolar disorder are highly heritable. Already during childhood, children at familial high risk of schizophrenia (FHR-SZ) or bipolar disorder (FHR-BD) are at increased risk of psychiatric disorders and cognitive and social impairments. Knowledge about physical conditions is sparse.Materials and methods: Through blood tests (n = 293), interviews, and questionnaires, we assessed inflammatory markers, somatic complaints, medication – and health care use in 11-year-old children at FHR-SZ, FHR-BD, and population-based controls (PBC).Results: Children at FHR-SZ had higher concentrations of leucocytes (mean 6.41, SD 0.73) compared with PBC (mean 5.78, SD 0.27, p = 0.005) and of neutrophilocytes (FHR-SZ: mean 3.11, SD 1.32, PBC: mean 2.70, SD 0.96, p = 0.024). Compared with PBC (26.6%), more children at FHR-SZ (40.5%, p = 0.007) reported somatic complaints. So did caregivers and teachers to children at FHR-BD. Somatic complaints, higher concentrations of leucocytes, and neutrophilocytes were associated with lower levels of physical activity. Children at FHR-BD with psychiatric disorders reported more somatic complaints compared with those without.Conclusion: Children at FHR-SZ had higher concentrations of leucocytes and neutrophilocytes than PBC. Children at FHR-SZ or FHR-BP displayed more somatic complaints than controls. Our study highlights rarely explored disadvantage of being born to parents with schizophrenia or bipolar disorder. To enhance understanding of how physical conditions in childhood may interplay with later transition to mental disorders in children at FHR-SZ and FHR-BD, further research is needed.
Introduction
Severe mental illnesses cause multiple disadvantages for the patient in terms of personal, social, and physical problems and disabilities [Citation1]. Mental illnesses have strong hereditary components [Citation2] and subtle signs of neurodevelopmental disturbances are present years before onset of manifest illness [Citation3,Citation4]. While it is well documented that children born to parents with schizophrenia or bipolar disorder show a higher incidence of psychopathology and display more impairments of a cognitive, social, and motor-functioning character compared with children born to parents with none of these disorders [Citation5–12], to our knowledge, the physical health in children at familial high risk of severe mental illness is yet relatively unexplored. However, compared to the general population, patients with psychiatric disorders are significantly more likely to experience not only mental but also somatic comorbid illnesses [Citation1]. A comprehensive population-based cohort study conducted in Denmark, encompassing the entirety of a 5.9-million-person population, discerned a pronounced correlation between mental illnesses and an extensive array of medical conditions, encompassing circulatory, neurological, endocrine, and pulmonary maladies or dysfunctions [Citation13]. Findings from another Danish population-based cohort study, focusing on heart disease among 4.6 million participants, revealed a modest yet statistically significant increase in healthcare interactions attributed to heart disease in individuals with severe mental illness when compared with those who had not undergone psychiatric hospitalization. However, over a five-year period, the mortality rate attributable to heart disease after the initial health care encounter for heart-related issues was markedly higher among patients with a mental disorder, standing at 8.26%, in contrast to the 2.86% observed among patients without mental disorders [Citation14]. Another extensive systematic review and meta-analysis focusing on the prevalence and incidence rates among patients with schizophrenia, bipolar disorder, or major depression unveiled a nearly 10% prevalence of cardiovascular disease comorbidity within this patient cohort. Furthermore, these individuals exhibited a heightened risk of both developing and succumbing to cardiovascular ailments compared with the general population [Citation15]. A systematic review and meta-analyses on type 2 diabetes in patients with severe mental illness including schizophrenia and bipolar disorder found that 1 out of 10 patients with those illnesses also suffer from type 2 diabetes, which is nearly double risk compared with the general population [Citation16]. Correspondently, in a study of 206 newly diagnosed bipolar patients, 50 unaffected relatives, and 109 healthy individuals, metabolic syndrome and insulin resistance was assessed. Bipolar patients showed significantly higher rates of metabolic syndrome and insulin resistance compared with healthy individuals, while unaffected relatives did not [Citation17]. Further, a comparative analysis of 679 in-patients from the University Hospital Birmingham, including patients with schizophrenia compared with an in-patient control group matched for age and gender, showed that the prevalence of type 2 diabetes mellitus was significantly higher in the schizophrenia group, standing at 11.3%, in contrast to the 6.3% in the control group. A follow-up study conducted seven years later unveiled that 24.0% of patients with schizophrenia had suffered from type 2 diabetes mellitus, a striking contrast to the 10.5% observed among deceased patients in the control group [Citation18].
Inflammatory markers have also been found to differ between patients with severe mental illness and healthy controls and as such, patients with schizophrenia [Citation19] or bipolar disorder have displayed higher levels of C-Reactive Protein compared with controls, and C-Reactive Protein levels were not influenced by lithium or antipsychotic medication use [Citation20]. Additionally, a systematic review and meta-analyses study found that compared with healthy control persons, patients with bipolar disorder showed moderately elevated C-Reactive Protein levels during depression and euthymia and significantly higher levels during mania, and the severity of symptoms did not correlate with C-reactive Protein levels. After resolving manic episodes, C-Reactive Protein levels decreased moderately, and after depressive episodes, they eased slightly. The findings indicate that C-Reactive Protein levels are consistently elevated in patients with bipolar disorder, with particularly high levels during manic episodes, indicating an increased inflammatory burden during mania [Citation21]. Further, a register-based study evaluated levels of C-Reactive Protein in first diagnosed patients with schizophrenia, bipolar disorder, and depression, and their association with psychiatric admission and mortality, and elevated C-Reactive Protein levels were found to be associated with increased all-cause mortality [Citation22]. Another large study (N = 17,808) showed evidence of elevated concentrations of the immunological marker neutrophilocytes in adults with mental problems or illnesses including schizophrenia or bipolar disorder when compared with controls [Citation23].
Another study including younger persons, revealed that adolescent girls with moderate or severe depression had a higher level of C-Reactive Protein than girls without or with few symptoms of depression [Citation24], and a growing body of research including children and young adults suggests that elevated inflammatory markers could be clinical indicators of severe mental illnesses, not influenced by any other health circumstances [Citation25–27].
A large study on life expectancy and cause-specific mortality in patients with bipolar disorder and their siblings revealed that compared with the general population, patients with bipolar disorder, but not their unaffected siblings, experience increased mortality and a decreased life expectancy of 7.7 years [Citation28]. Accordingly, it is documented that people with severe mental illness are at largely increased risk for comorbid somatic illnesses, which partly cause an expected lifespan reduced with 15–20 years compared to the general population [Citation29]. Nonetheless, we know little about whether physical health diversities – as well as psychological – present already in childhood, and investigating physical conditions in children at familial high risk of schizophrenia or bipolar disorder may provide valuable additional insights into the risk profile of these children.
In this study we aimed to compare physical health status in a cohort of 11-year-old children at familial high risk of schizophrenia (hereafter FHR-SZ), children at familial high risk of bipolar disorder (hereafter FHR-BD), and population-based controls (hereafter PBC) through subjective somatic complaints, health care use, medication, and blood-based biomarkers. The purpose of using biomarkers was to analyze immune system and infectious markers and diabetes markers. Information on the cohort’s background characteristics encompassed psychiatric disorder, BMI, VO2 max, onset of puberty, and physical activity. Based upon findings from previous studies [Citation30–32], additionally, our objective was to explore whether our findings correlated with the children’s level of physical activity or the presence of a psychiatric disorder.
Materials and methods
Study design and participants
The Danish High Risk and Resilience Study – VIA 11, conducted between 1 March 2017 and 30 June 2020, is the first follow-up study of the nationwide longitudinal study; The Danish High Risk and Resilience Study. The baseline study, The Danish High Risk and Resilience Study – VIA 7 was conducted in the period of 1 January 2013 and 31 January 2016. We established a cohort of 522 seven-year-old children with no, one, or two parents with schizophrenia spectrum psychosis (defined as ICD-10 codes F20, F22 and F25 or ICC-8 codes 295, 297, 298, 29 –39, −89 and 298,99) or bipolar disorder (defined as ICD-10 codes F30 and F31 or ICD-8 codes 296,19 and 296,39). Children with at least one parent diagnosed with schizophrenia spectrum psychosis in the Danish Register were matched according to age, sex, and municipality to population-based controls. Children born to parents diagnosed with bipolar disorder were an unmatched sample but comparable to the other two groups in terms of sex and age. Parents of the control children could not be registered with schizophrenia spectrum psychosis diagnoses or bipolar disorder.
The Danish Data Protection Agency and The Danish National Committee on Health Research Ethics approved the study protocol (Protocol number H16043682). All adult participants gave written informed consent and the child’s custody holder(s) gave written informed consent on behalf of the child. The study design of The Danish High Risk and Resilience Study is thoroughly described elsewhere [Citation33,Citation34]. All data were collected within the same timeframe. Due to our extensive assessment battery, our participants were assessed over a three-day period.
Blood-based biomarkers
Blood samples were collected from the child following the application of local anesthetic. Each sample was obtained only after the child provided informed consent and was comfortable with the procedure. In cases where the initial sample failed, a second attempt was made only with the child’s agreement. Our measurements included HbA1c, B-haemoglobin, highly sensitive C-reactive protein, leucocytes including differential count of monocytes, neutrophils, and basophils.
Reports of somatic complaints
We assessed self-reported somatic complaints using the Strength and Difficulties Questionnaire self-rated version intended for 11–17-year-old children (SDQ-CE), item three: ‘I get a lot of headaches, stomachaches or sickness’. Information from the child’s primary caregiver was obtained by the Strength and Difficulties Questionnaire for parents or teachers (SDQ-PE) [Citation35], item three: ‘Often complaints of headaches, stomach aches or sickness’ and the Child Behavior Checklist (CBCL), question number 56a to survey: ‘Physical problems, aches or pain without known medical cause (exclusive head and stomach aches)’. Information from the child’s teacher was obtained using the Teachers Report Form (TRF) [Citation36], question number 53a: ‘Physical problems, aches or pain without known medical cause (exclusive head and stomach aches)’. Data on somatic complaints were dichotomized into ‘yes’ or ‘no’.
The Danish High Risk and Resilience Study – VIA 11 consists of a large assessment-battery including the above-mentioned questionnaires. To avoid asking our participants the same kind of question more than once (e.g. about somatic complaints) data regarding somatic complaints employed in this study originate from those forms.
Medication and health care
Through an anamnestic interview with the primary caregiver, we gained information on the child’s use of medication and contacts with the health care system including both in- and outpatient contacts. For this study, we have drawn date on current use – and use within the last month of prescribed and over-the counter medication. Medication was categorized into desmopressin, melatonin, asthma & allergies medication, central stimulants, other prescribed medication (including insulin, laxative, eyedrops, worms, anti-fungal, acne/dandruff shampoo, acne, vitamin D, acid-reflux, psoriasis creme, nsaids, anti-ep, puberty delay, growth hormones, hydro cream), and painkiller (over-the-counter medication). Use of health care included data on use of health care within the last six month. Data were dichotomized into ‘yes’ or ‘no’.
Psychiatric disorder
We examined potential psychiatric disorder(s), Axis 1 disorder, using the Kiddie Schedule for Affective Disorders and Schizophrenia for school-aged children (6–18 years) [Citation37]. This is a semi-structured clinical interview assessing present and previous psychopathology in children and adolescents. The primary caregiver was interviewed and afterwards the child. For this study only current Axis 1 disorders were included. Methods and results are described in detail elsewhere [Citation38]. We used The Children’s Global Assessment Scale [Citation39] (CGAS) to assess the child’s global level of daily function.
Body Mass Index, VO2 max and onset of puberty
For Body Mass Index (BMI) measures we sampled height and weight three time and used the median value to calculate BMI. A standardized method was used to assess the child’s VO2 max (the maximum (max) rate (V) of oxygen consumption (O2) in mL/min/kg BW) during a progressive exhaustive bicycle test. In collaboration with an external scientific unit from The Department of Nutrition, Sports and Exercise, University of Copenhagen, our portable INNOCOR system, using a standardized progressive exhaustive bicycle test was validated against the Douglas bag system [Citation40,Citation41]. Puberty stage was assessed with self-reported Tanner Stage [Citation42].
Physical activity
Physical activity was assessed by accelerometry data collected using the SENS motion System. It consisted of a small-sized waterproof sensor (weight 7 g, size 45 × 4.5 × 23 mm) with a tri-axial accelerometer, sampling acceleration at 12 Hz, with a range of ±4 G. The sensor was attached to the skin approximately 10 cm from the lateral epicondyle of the knee on the lateral aspect of the thigh. The SENS motion system was able to differentiate between the amount of time with inactivity and activity, as well as the level of activity. Further details are described elsewhere [Citation43]. In this study we report the amount of high intensity physical activity.
Socioeconomic status of the primary caregiver
Socioeconomic information was obtained through anamnestic interviews with the primary caregivers, and for this study current employment engagement was used as the socioeconomic status marker of the primary caregiver. Information on employment engagement was dichotomized into ‘yes’ or ‘no’. Socioeconomic status is significantly correlated with physical (and psychological) health [Citation44] and in case of significant differences between groups, ANCOVA analyses were conducted using employment engagement as covariate to adjust for socioeconomic status of the primary caregiver.
Statistical analyses
IBM SPSS Version 25.0 was used for statistical analysis and the level of statistical significance was set at p < 0.05. Differences in continuous variables between the three groups were analyzed with one-way analysis of variance (ANOVA). When relevant, each one-way ANOVA test was followed by post-hoc Bonferroni-adjusted tests. Between group differences of questionnaire responses, use of medication, use of health care system, axis 1 disorder, physical activity during leisure time and working employment engagement were analyzed with Pearson’s Chi-square tests. In case of significant differences between the groups exploratively ANCOVA analyses were conducted using working employment engagement as covariate. Bivariate correlation analyses were conducted in analyses of association between high intensity physical activity and (1) leucocytes and (2) neutrophilocytes and (3) self-reported somatic complaints as well as between Axis 1 diagnoses and somatic complaints. Interaction effect of group status was analyzed with group status as interaction term, this was followed by cross sectional binary logistic regression analyses of associations between Axis 1 diagnoses and somatic complaints. Bivariate correlation analyses were used in cross sectional analyses of association between high intensity physical activity and (1) leucocytes and (2) neutrophilocytes.
Results
Background characteristics
This study consists of 461 children with the following group-distribution: FHR-SZ, n = 175, FHR-BD, n = 105 and controls n = 181. The mean age of all groups was 11.9. A significantly larger proportion of children at FHR-SZ or FHR-BD than PBC fulfilled criteria for at least one Axis 1 disorder including Affective disorders, Psychotic disorders, Anxiety disorders, Disruptive behavior disorders, ADHD, Pervasive developmental disorders, Post-traumatic stress disorder, Stress and adjustment disorders and Tic disorders. Mean score of daily level of current function (CGAS) was significantly lower in children at FHR-SZ or FHR-BD compared with PBC. The groups did not differ in terms of neither BMI, VO2 max or puberty onset. Hours of high intensity physical activity per day were significantly lower in children at FHR-SZ and in children at FHR-BD compared with PBC. A significantly smaller proportion of primary caregivers of children at FHR-SZ or FHR-BD were working employed compared with primary caregivers of PBC ().
Table 1. Characteristics of 11-year-old children at Familial High Risk of Schizophrenia (FHR-SZ) or Familial High Risk of Bipolar Disorder (FHR-BD) and Population-Based Controls (PBC) and their primary caregiver.
Blood-based biomarkers
Blood samples were collected from 293 children. Children at FHR-SZ (mean = 6.41, SD = 0.73) differed significantly from PBC (mean = 5.78, SD = 0.27, p = 0.005) regarding leucocytes, whereas children at FHR-BD (mean = 6.17, SD = 0.45) did not. Group differences remained after the adjustment for primary caregiver’s socioeconomic status (FHR-SZ vs. controls: p = 0.008). Regarding concentration of neutrophilocytes we also found a significant difference between children at FHR-SZ (mean = 3.11, SD = 1.32) and PBC (mean = 2.70, SD = 0.96, p = 0.024) but children at FHR-BD (mean = 3.00, SD = 1.11) did not differ significantly from any of the other groups. Significant differences between FHR-SZ and PBC remained after adjusting for primary caregiver’s working employment status (p = 0.018). No other differences in the blood tests were found ().
Table 2. Blood test results in 11-year-old children at familial high risk of schizophrenia (FHR-SZ) or familial high risk of bipolar disorder (FHR-BD) and population-based controls (PBC).
Self-reported somatic complaints
The prevalence of the children’s self-reported ‘Headaches, stomach aches or sickness’ (children’s self-rated SDQ) (hereafter ‘self-reported somatic complaints’) were significantly higher in children at FHR-SZ (40.5%), compared with PBC (26.6%, p = 0.007), and higher, but not significantly, in children at FHR-BD (38.0%) compared with PBC (p = 0.050) (). After adjusting for the primary caregiver’s working employment status differences between children at FHR-SZ and PBC remained significant (p = 0.035).
Table 3. Somatic complaints within the last month in 11-year-old children at Familial High Risk of Schizophrenia (FHR-SZ) or Familial High Risk of Bipolar Disorder (FHR-BD) and Population-Based Controls (PBC).
Primary caregiver’s report of somatic complaints of the child
Primary caregivers of children at FHR-SZ (37.1%) and FHR-BD (33.3%) reported significantly more complaints of ‘Headaches, stomach aches or sickness’ (SDQ-PE) compared with primary caregivers of controls (17.7%, p < 0.001 and p = 0.003 respectively) (). Between differences remained after adjusting for primary caregiver’s working employment status (FHR-SZ vs. PBC: p = 0.002, FHR-BP vs. controls: p = 0.004). Regarding ‘Physical problems exclusive head- and stomach aches’ (CBCL), we did not find significant differences between primary caregivers of children at FHR-SZ (13.9%) and PBC (10.9%, p = 0.073) whereas primary caregivers of children at FHR-BD (22.0%) reported significantly more physical problems than primary caregivers of PBC (p = 0.011) (). The latter difference did not remain significant after adjusting for primary caregiver’s working employment status.
Teacher’s report of somatic complaints of the child
Teachers reports of ‘Physical problems exclusive head and stomach aches’ (TRF) showed significantly more somatic complaints in children at FHR-SZ (16.0%) and FHR-BD (5.7%) compared with PBC (4.0%, p = 0.001 and p = 0.025 respectively) (). Only the difference between children at FHR-SZ and PBC remained significant after adjusting for primary caregiver’s working employment status (p = 0.017).
Use of medication and the health care system
We did not find significant differences in the children’s use of medication. Due to lack of power, statistical differences in use of melatonin and central stimulants could not be calculated, but use of melatonin was only reported by primary caregivers of children in the two high risk groups (FHR-SZ: 2.2%; FHR-BD: 4.8%; PBC: 0%) and results of central stimulants were: Children at FHR-SZ = 6.1%, children at FHR-BP = 2.9% and PBC = 1.1%. In use of two or more medications the groups did tend to differ, although not significantly (FHR-SZ:8.9%, FHR-BD:8.3%, and controls:3.5%; overall p = 0.069) (). No differences between the groups were found in terms of contacts with the health care system (neither in in- or outpatients).
Table 4. Prescribed and over-the-counter medication within the last month for 11-year-old children at Familial High Risk for Schizophrenia (FHR-SZ) or Familial High Risk of Bipolar Disorder (FHR-BD) and Population-Based Controls (PBC).
Correlation analysis of high intensity physical activity and self-reported somatic complaints
Bivariate correlation analysis of amounts of high intensity physical activity and self-reported somatic complaints revealed that lower amount of high intensity physical activity and higher incidence of self-reported somatic complaints were highly association (p < 0.001). We did not find different interaction across the three groups (p = 0.793).
Cross sectional correlation analyses of high intensity physical activity and (1) leucocytes, and (2) neutrophilocytes
Bivariate correlation analysis revealed that lower amounts of high intensity physical activity were associated with both higher concentrations of leucocytes (p = 0.032) and neutrophilocytes (p = 0.015). Interaction analyses did not show significant differences across the three groups (leucocytes: p = 0.105 and neutrophilocytes: p = 0.106). However, explorative group stratified bivariate correlation analyses showed that both higher concentrations of leucocytes (p = 0.036) and neutrophilocytes (p = 0.011) were associated with lower amount of high intensity physical activity in children at FHR-SZ, which was not the case in neither FHR-BD nor the control group.
Cross sectional correlation analyses of Axis 1 disorders and self-reported somatic complaints
Bivariate correlation analyses showed significant associations between more self-reported somatic complaints and at least one Axis 1 disorder (p < 0.001). Interaction analyses revealed significantly different associations across the three groups (overall: p = 0.031, FHR-SZ vs. PBC: p = 0.413, FHR-BD vs. PBC: p = 0.006, and FHR-SZ vs. FHR-BD: p = 0.050).
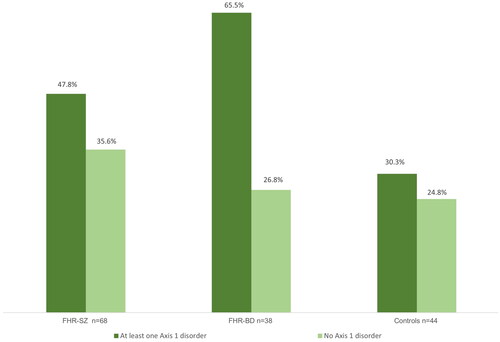
Group stratified binary logistic regression analyses of any Axis 1 disorder and self-reported somatic complaints revealed that children at FHR-SZ with at least one Axis 1 disorder (47.8%) did not differ significantly from children at FHR-SZ with no Axis 1 disorder (35.6%, p = 0.118), neither did controls with (30.3%) or those without Axis 1 disorders (24.8%, p = 0.519). In contrast, incidence of self-reported somatic complaints was significantly higher in children at FHR-BD with at least one Axis 1 disorder (65.5%) compared with children at FHR-BP with no disorder (26.8%, p = 0.001) ().
Figure 1. Self-reported headaches, stomach-aches or sickness (SDQ) in 11-year-old children at Familial High Risk of Schizophrenia (FHR-SZ) or Bipolar Disorder (FHR-BD) and controls with either at least one Axis 1 disorder or no Axis 1 disorder. FHR-SZ: At least one Axis 1 disorder vs. no disorder: p = 0.118, OR = 0.606, 95%CI = 0.323–1.136. FHR-BD: At least one Axis 1 disorder vs. no disorder: p = 0.001, OR = 0.192, 95%CI = 0.076–0.487. Controls: At least one Axis 1 disorder vs. no disorder: p = 0.519, OR = 0.759.
Discussion
This nationwide cohort study consisted of 11-year-old children at familial high risk of schizophrenia (n = 179) or familial high risk of bipolar disorder (n = 105) and a population-based control group (n = 181). A subsample of 293 children provided blood and measurements included HbA1c, B-haemoglobin, highly sensitive C-reactive protein, leucocytes including differential count of monocytes, neutrophils, and basophils. We found that children at high risk of schizophrenia had higher concentrations of the immunological markers, leucocytes and neutrophilocytes when compared with population-based controls. Children with a familial high risk of schizophrenia had significantly more somatic complaints when compared with population-based controls, both self-reported and based on data from primary caregivers and teachers. Children with a familial high risk of bipolar disorder had significantly more somatic complaints based on data from primary caregivers and teachers but not based on self-reports. We did not find any significant differences in use of medication or health service contacts, including both in- and outpatients between the three groups. We found that lower amounts of high intensity physical activity were associated with both self-reported somatic complaints and higher concentrations of leucocytes and neutrophilocytes. Cross sectional analyses showed that higher incidence of somatic complaints and presence of psychiatric disorders were only associated in the group of children with familial risk of bipolar disorder whereas no such relation appeared in neither of the other two groups.
In comparison with our control group, the standard deviation results of blood-based biomarkers were substantially wider among the children in the high-risk groups. Assuming, that not only manifest but also (genetic hereditary) future severe mental illness is associated with immunological markers [Citation27], it is reasonable that we observe the increased deviation specifically within this group. Since physical inactivity has a negative impact on the immune system and physical activity improves it [Citation45], higher concentrations of leucocytes and neutrophilocytes [Citation23] could reflect that patients with severe mental illness are more likely to present sedentary behavior [Citation46–48], but also poorer physical health in general [Citation49]. According to our study, by the age of 11 lower amounts of high intensity physical activity is already highly associated with higher concentrations of neutrophilocytes. We did not find significant interaction effects across the three groups, but explorative group stratified analyses suggested that the association were driven by the group of children at familial high risk of schizophrenia. Any conclusions based on this result would be premature, but one may speculate that the immune system of children at high risk of schizophrenia is more vulnerable to lower levels of physical activity compared with children without this increased risk. Potentially, our results are supported by the findings of a large study (n = 12,000) investigating the genetic factors influencing the levels of ten cytokines in newborn babies. These findings revealed that certain regulatory elements in the genome play a significant role in determining the levels of these inflammatory markers in the blood and support that inflammatory markers could serve as indicators of severe mental illness [Citation50]. In this way, the heightened impact of low activity levels on immunological markers may be indicative of a genetic risk variant.
Additionally, our study revealed that high occurrence of somatic complaints was associated with lower amounts of high intensity physical activity. Correlations between somatic complaints and amount of high intensity physical activity assessed by a highly accurate instrument, have to our knowledge not been investigated in similar cohorts. Within a wider study area, our results correspond well with previous studies [Citation51,Citation52], Aiming to reduce somatic complaints or illnesses, intervention including increased physical activities are common treatments of people with both somatic complaints/illnesses and psychiatric illnesses [Citation53]. Causality of lower amounts of physical activity and somatic complaints and lower amounts of physical activity and higher concentrations of the immunological marker of neutrophilocytes cannot be inferred from our study. However, our findings may indicate that one physical disadvantage is leading to another, and that there is an enlarged probability of an association between lower amount of physical activity and higher concentrations of neutrophilocytes in children at familial high risk of schizophrenia.
Only within the group of children at familial high risk of bipolar disorder did we find an association between occurrence of somatic complaints and psychiatric disorders. Frequency of somatic complaints of children in this group was remarkably high (65.5%). In addition, primary caregivers, and teachers of children at familial high risk of bipolar disorder reported significantly more somatic complaints in all questionnaires, whereas reports of primary caregivers of children at familial high risk of schizophrenia and population-based controls did not differ regarding physical problems exclusive headaches and stomach aches. Those findings indicate that psychiatric illness has a stronger physical impact in terms of somatic complaints in children at risk of bipolar disorder than in children at familial high risk of schizophrenia and controls. Contrary to this, there is no evidence of higher physical vulnerabilities in people with bipolar disorder compared with people with schizophrenia, in fact the opposite seems to be evident [Citation14]. Since the significantly higher occurrence of somatic complaints in children at familial high risk of schizophrenia was not associated with presence of psychiatric disorders and did exist regardless of their primary caregiver’s socioeconomic status, our study may allow us to conclude that a higher occurrence of somatic complaints in children at familial high risk of schizophrenia depends on the children’s risk status. Our findings correspond well with another study suggesting, that somatic complaints in preschool children at familial high risk of internalizing disorders predict anxiety, depression and somatic complaints at school age [Citation54].
Not only are children at familial high risk of schizophrenia or bipolar disorder disadvantaged in multiple psychological domains and more likely to suffer from psychiatric illness, but additionally they seem to suffer from aches and pains to a much larger degree compared with same-aged peers born without the risk of schizophrenia or bipolar disorder. Despite these findings, we found no group differences in use of the health care system. Only use of melatonin and central stimulants could reflect true group differences, but due to insufficient statical power none of these findings were qualified. Similarly, the use of two or more medications were more frequent in the two high risk groups although not to a significant degree. These discrepancies could not be explained within our study design, but based on a large register study showing higher incidence of somatic illness in children aged 0–6 born to parents with severe mental disorders [Citation55], it should be considered if the children’s somatic complaints might be an expression of underdiagnosed somatic diseases. Furthermore, the results might turn out differently if information was also gathered from medical records directly from the children’s general practitioner. Thus, our findings suggest that study designs with higher sensitivities towards aspects of the children’s physical conditions possibly could contribute with deeper insights into this area of a very vulnerable group of children. Currently, our cohort, now 15–16 years old adolescents are undergoing follow-up assessments of physical conditions.
Strengths and limitations
An important strength of the study is the large sample size of both participants with familial high risk of schizophrenia and bipolar disorder and controls. Furthermore, use of the Danish National Register ensures narrow age range and high representativity. Use of well validated questionnaires is a strength but using only selected items could be a limitation. However, each questionnaire was included in our assessment battery in its complete form, and to ensure not asking our participants the same kind of questions more than once, we chose to conduct our study as presented. Interview-based information on the children’s medication and use of health care system might be a limitation. Some primary caregivers were troubled remembering treatments of their child. Information obtained by register-based medical records would have been more accurate. Another limitation is our inability to gather blood samples from the entire group. This was due to some participants opting out of this aspect of the study, and because we had limited access to our biochemist collaborators, meaning that we were not able to conduct blood sampling during holidays and weekends. However, in this subsample, for all goups it applies that the mean score of CGAS (daily level of current functioning) was slightly and non-significantly higher compared with the mean scores of the complete cohort (FHR-SZ: 65.7 vs. 64.6, FHR-BD: 71.0 vs. 68.1 and PBC: 76.0 vs. 75.2). Sex distributions in the complete sample and the subsample did not differ significantly, neither did level of physical activity. Thus, we consider our sample of children providing blood comparable with the complete cohort, which is a strength It would have strengthened our study if we had investigated a wider range of inflammatory biomarkers, but our priority was to minimize any discomfort for the participants. Therefore, we exercised restraint in our number of assessments, resulting in some exclusions. Our FHR-SZ and PBC groups are larger than our FHR-BD group, and this weakens our estimates of differences for the groups which is a limitation.
Conclusion
We found higher concentrations of leucocytes and neutrophilocytes in children at familial high risk of schizophrenia when compared with population-based controls, and higher incidence of somatic complaints in both children at familial high risk of schizophrenia and bipolar disorder, although, for children at familial high risk of bipolar disorder, only primary caregivers and teachers reported more complaints. Our results did not reveal significantly more self-reported complaints. Higher concentrations of neutrophilocytes were associated with lower amounts of high intensity physical activity, supposedly especially in children at familial high risk of schizophrenia. Thus, our findings indicate physical vulnerabilities in children at familial risk of schizophrenia and bipolar disorder. Ongoing follow-up studies of this cohort may elucidate if these vulnerabilities are to be considered as independently contributing risk factors or subtle signs prior to the development of manifest mental illness. Additionally, since children born to parents with schizophrenia or bipolar disorder are not only at high risk of developing severe psychiatric disorders but also of somatic comorbidity, the possibility of somatic illness founded already in childhood seems relevant to consider. Our study suggests that an additional awareness of children with a familial risk of schizophrenia or bipolar disorder presenting high degrees of somatic complaints should be considered.
Acknowledgement
We would like to express our gratitude to all our participants, to H. B. Stadsgaard, Å. K. Prøsch, M. Melau, A. M. Bundsgaard, A. F. Bundgaard, M. Birk, and N. L. Steffensen for contributing to the data collection, and to C. B. Pedersen and M. G. Pedersen for retrieving the register extract.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Momen NC, Plana-Ripoll O, Agerbo E, et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. 2020;382(18):1721–1731. doi: 10.1056/NEJMoa1915784.
- Rasic D, Hajek T, Alda M, et al. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull. 2014;40(1):28–38. doi: 10.1093/schbul/sbt114.
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–1238. doi: 10.1038/mp.2012.23.
- Owen MJ, O’Donovan MC. Schizophrenia and the neurodevelopmental continuum: evidence from genomics. World Psychiatry. 2017;16(3):227–235. doi: 10.1002/wps.20440.
- Niemi LT, Suvisaari JM, Tuulio-Henriksson A, et al. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res. 2003;60(2-3):239–258. doi: 10.1016/s0920-9964(02)00234-7.
- Sanchez-Gistau V, Romero S, Moreno D, et al. Psychiatric disorders in child and adolescent offspring of patients with schizophrenia and bipolar disorder: a controlled study. Schizophr Res. 2015;168(1–2):197–203. doi: 10.1016/j.schres.2015.08.034.
- Bora E, Özerdem A. A meta-analysis of neurocognition in youth with familial high risk for bipolar disorder. Eur Psychiatry. 2017;44:17–23. doi: 10.1016/j.eurpsy.2017.02.483.
- Schreiber H, Stolz-Born G, Heinrich H, et al. Attention, cognition, and motor perseveration in adolescents at genetic risk for schizophrenia and control subjects. Psychiatry Res. 1992;44(2):125–140. doi: 10.1016/0165-1781(92)90047-7.
- Burton BK, Thorup AAE, Jepsen JR, et al. Impairments of motor function among children with a familial risk of schizophrenia or bipolar disorder at 7 years old in Denmark: an observational cohort study. Lancet Psychiatry. 2017;4(5):400–408. doi: 10.1016/S2215-0366(17)30103-7.
- Christiani CJ, Jepsen JRM, Thorup A, et al. Social cognition, language, and social behavior in 7-year-old children at familial high-risk of developing schizophrenia or bipolar disorder: the Danish high risk and resilience study VIA 7-a population-based cohort study. Schizophr Bull. 2019;45(6):1218–1230. doi: 10.1093/schbul/sbz001.
- Hemager N, Plessen KJ, Thorup A, et al. Assessment of neurocognitive functions in 7-year-old children at familial high risk for schizophrenia or bipolar disorder: the Danish high risk and resilience study VIA 7. JAMA Psychiatry. 2018;75(8):844–852. doi: 10.1001/jamapsychiatry.2018.1415.
- Ellersgaard D, Jessica Plessen K, Richardt Jepsen J, Soeborg Spang K, Hemager N, Klee Burton B, Jerlang Christiani C, Gregersen M, Søndergaard A, Uddin MJ, Poulsen G, Greve A, Gantriis D, Mors O, Nordentoft M, Elgaard Thorup AA. Psychopathology in 7-year-old children with familial high risk of developing schizophrenia spectrum psychosis or bipolar disorder - The Danish High Risk and Resilience Study - VIA 7, a population-based cohort study. World Psychiatry. 2018;17(2):210–219. doi: 10.1002/wps.20527.
- Plana-Ripoll O, Pedersen CB, Holtz Y, et al. Exploring comorbidity within mental disorders among a danish national population. JAMA Psychiatry. 2019;76(3):259–270. doi: 10.1001/jamapsychiatry.2018.3658.
- Laursen TM, Nordentoft M. Heart disease treatment and mortality in schizophrenia and bipolar disorder - changes in the Danish population between 1994 and 2006. J Psychiatr Res. 2011;45(1):29–35. doi: 10.1016/j.jpsychires.2010.04.027.
- Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. doi: 10.1002/wps.20420.
- Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15(2):166–174. doi: 10.1002/wps.20309.
- Coello K, Vinberg M, Knop FK, et al. Metabolic profile in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Int J Bipolar Disord. 2019;7(1):8. doi: 10.1186/s40345-019-0142-3.
- Schoepf D, Potluri R, Uppal H, et al. Type-2 diabetes mellitus in schizophrenia: increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry. 2012;27(1):33–42. doi: 10.1016/j.eurpsy.2011.02.009.
- Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is elevated in schizophrenia. Schizophr Res. 2013;143(1):198–202. doi: 10.1016/j.schres.2012.10.041.
- Dargél AA, Godin O, Kapczinski F, et al. C-reactive protein alterations in bipolar disorder: a meta-analysis. J Clin Psychiatry. 2015;76(2):142–150. doi: 10.4088/JCP.14r09007.
- Fernandes BS, Steiner J, Molendijk ML, et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(12):1147–1156. doi: 10.1016/S2215-0366(16)30370-4.
- Horsdal HT, Köhler-Forsberg O, Benros ME, et al. C-reactive protein and white blood cell levels in schizophrenia, bipolar disorders and depression-associations with mortality and psychiatric outcomes: a population-based study. Eur Psychiatry. 2017;44:164–172. doi: 10.1016/j.eurpsy.2017.04.012.
- Brinn A, Stone J. Neutrophil-lymphocyte ratio across psychiatric diagnoses: a cross-sectional study using electronic health records. BMJ Open. 2020;10(7):e036859. doi: 10.1136/bmjopen-2020-036859.
- Tabatabaeizadeh S-A, Abdizadeh MF, Meshkat Z, et al. There is an association between serum high-sensitivity C-reactive protein (hs-CRP) concentrations and depression score in adolescent girls. Psychoneuroendocrinology. 2018;88:102–104. doi: 10.1016/j.psyneuen.2017.11.014.
- Kofod J, Elfving B, Nielsen EH, et al. Depression and inflammation: correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur Neuropsychopharmacol. 2021;54:116–125. doi: 10.1016/j.euroneuro.2021.09.006.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940–948. doi: 10.1016/j.jad.2020.09.025.
- Perry BI, Zammit S, Jones PB, et al. Childhood inflammatory markers and risks for psychosis and depression at age 24: examination of temporality and specificity of association in a population-based prospective birth cohort. Schizophr Res. 2021;230:69–76. doi: 10.1016/j.schres.2021.02.008.
- Kessing LV, Ziersen SC, Andersen PK, et al. A nation-wide population-based longitudinal study on life expectancy and cause specific mortality in patients with bipolar disorder and their siblings. J Affect Disord. 2021;294:472–476. doi: 10.1016/j.jad.2021.07.065.
- Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. 2021;18(2):136–145. doi: 10.1038/s41569-020-00463-7.
- Fang S-H, Suzuki K, Lim CL, et al. Associations between sleep quality and inflammatory markers in patients with schizophrenia. Psychiatry Res. 2016;246:154–160. doi: 10.1016/j.psychres.2016.09.032.
- Egger HL, Costello EJ, Erkanli A, et al. Somatic complaints and psychopathology in children and adolescents: stomach aches, musculoskeletal pains, and headaches. J Am Acad Child Adolesc Psychiatry. 1999;38(7):852–860. doi: 10.1097/00004583-199907000-00015.
- Sackl-Pammer P, Özlü-Erkilic Z, Jahn R, et al. Somatic complaints in children and adolescents with social anxiety disorder. Neuropsychiatr. 2018;32(4):187–195. doi: 10.1007/s40211-018-0288-8.
- Thorup AAE, Jepsen JR, Ellersgaard DV, et al. The Danish High Risk and Resilience Study–VIA 7–a cohort study of 520 7-year-old children born of parents diagnosed with either schizophrenia, bipolar disorder or neither of these two mental disorders. BMC Psychiatry. 2015;15(1):233. doi: 10.1186/s12888-015-0616-5.
- Thorup AAE, Hemager N, Søndergaard A, et al. The Danish high risk and resilience study-VIA 11: study protocol for the first follow-up of the VIA 7 cohort -522 children born to parents with schizophrenia spectrum disorders or bipolar disorder and controls being re-examined for the first time at age 11. Front Psychiatry. 2018;9:661. doi: 10.3389/fpsyt.2018.00661.
- Obel C, Dalsgaard S, Stax H-P, et al. [Strengths and Difficulties Questionnaire (SDQ-Dan). A new instrument for psychopathologic screening of children aged 4–16 years]. Ugeskr Laeger. 2003;165(5):462–465.
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles: child behavior checklist for ages 6-18, teacher’s report form, youth self-report: an integrated system of multi-informant assessment. Burlington (VT): University of Vermont, Research Center for Children Youth & Families; 2001.
- Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021.
- Gregersen M, Søndergaard A, Brandt JM, et al. Mental disorders in preadolescent children at familial high-risk of schizophrenia or bipolar disorder - a four-year follow-up study: the Danish High Risk and Resilience Study, VIA 11: the Danish High Risk and Resilience Study, VIA 11. J Child Psychol Psychiatry. 2021;63(9):1046–1056. doi: 10.1111/jcpp.13548.
- Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010.
- Shephard RJ, Allen C, Benade AJ, et al. The maximum oxygen intake. An international reference standard of cardiorespiratory fitness. Bull World Health Organ. 1968;38(5):757–764.
- Jensen K, Jørgensen S, Johansen L. A metabolic cart for measurement of oxygen uptake during human exercise using inspiratory flow rate. Eur J Appl Physiol. 2002;87(3):202–206. doi: 10.1007/s00421-002-0616-2.
- Coleman L, Coleman J. The measurement of puberty: a review. J Adolesc. 2002;25(5):535–550. doi: 10.1006/jado.2002.0494.
- Søndergaard A, Wilms M, Gregersen M, et al. Physical activity and sleep in 11-year old children with a familial high risk of schizophrenia or bipolar disorder. The Danish High Risk and Resilience Study—VIA 11. Schizophrenia Bull Open. 2022;3(1):sgab055. doi: 10.1093/schizbullopen/sgab055.
- Wang J, Geng L. Effects of socioeconomic status on physical and psychological health: Lifestyle as a mediator. Int J Environ Res Public Health. 2019;16(2):281. doi: 10.3390/ijerph16020281.
- Wang J, Liu S, Li G, et al. Exercise regulates the immune system. Adv Exp Med Biol. 2020;1228:395–408. doi: 10.1007/978-981-15-1792-1_27.
- Strassnig MT, Harvey PD, Miller ML, et al. Real world sedentary behavior and activity levels in patients with schizophrenia and controls: an ecological momentary assessment study. Ment Health Phys Act. 2021;20:100364. doi: 10.1016/j.mhpa.2020.100364.
- Bort-Roig J, Briones-Buixassa L, Felez-Nobrega M, et al. Sedentary behaviour associations with health outcomes in people with severe mental illness: a systematic review. Eur J Public Health. 2020;30(1):150–157. doi: 10.1093/eurpub/ckz016.
- Vancampfort D, Firth J, Schuch FB, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry. 2017;16(3):308–315. doi: 10.1002/wps.20458.
- Reininghaus B, Riedrich K, Dalkner N, et al. Physical health in individuals with psychiatric disorders in Austria. J Affect Disord. 2019;257:38–44. doi: 10.1016/j.jad.2019.06.045.
- Wang Y, Nudel R, Benros ME, et al. Genome-wide association study identifies 16 genomic regions associated with circulating cytokines at birth. PLoS Genet. 2020;16(11):e1009163. doi: 10.1371/journal.pgen.1009163.
- Kleszczewska D, Mazur J, Bucksch J, et al. Active transport to school may reduce psychosomatic symptoms in school-aged children: data from nine countries. Int J Environ Res Public Health. 2020;17(23):8709. doi: 10.3390/ijerph17238709.
- Hrafnkelsdottir SM, Brychta RJ, Rognvaldsdottir V, et al. Less screen time and more physical activity is associated with more stable sleep patterns among Icelandic adolescents. Sleep Health. 2020;6(5):609–617. doi: 10.1016/j.sleh.2020.02.005.
- Knapen J, Vancampfort D, Moriën Y, et al. Exercise therapy improves both mental and physical health in patients with major depression. Disabil Rehabil. 2015;37(16):1490–1495. doi: 10.3109/09638288.2014.972579.
- Engel ML, Winiarski DA, Reidy BL, et al. Early-life somatic complaints: longitudinal associations with maternal and child psychopathology. J Dev Behav Pediatr. 2018;39(7):573–579. doi: 10.1097/DBP.0000000000000590.
- Davidsen KA, Munk-Laursen T, Foli-Andersen P, et al. Mental and pediatric disorders among children 0-6 years of parents with severe mental illness. Acta Psychiatr Scand. 2021;145(3):244–254. doi: 10.1111/acps.13358.
