Abstract
A time discrepancy of at least 200–300 million years exists between the generally accepted onset of metazoan evolution as currently evidenced from fossils compared with that from studies at the molecular level. That temporal disparity coincides with the existence and subsequent breakup of the Rodinia Supercontinent when the earliest evolutionary crucibles were isolated intracratonic basins rather than the globally connected shallow seaways of subsequent eras. However, the discovery of fossil evidence to complement the extrapolations from molecular data has been, and continues to be, hampered, by both the geographical limitations of these isolated intracratonic basins and the fact that any such discovery would conflict with the global geological mindset that macrofossils of such age do not exist. Yet recently identified within the intracratonic Amadeus Basin of central Australia is a suite of macroscopic metazoan fossils that dates to ca 850–840 Ma, exemplifying an early attempt at animal evolution within the restrictions of the Rodinia Supercontinent. Such early metazoan evolution within such isolated crucibles, however, was extremely vulnerable to variations in climatic conditions, which threatened total extinction because the isolation of these basins limited metazoan distribution by denying them the possibility of migrating to safer havens. The Amadeus Basin evidence suggests that early metazoan evolution perhaps comprised cycles of about 10 million years when evolution flourished followed by climate change induced extinction and a subsequent evolutionary void of between 50 and 100 million years. Such cycles possibly characterised metazoan evolution until the Rodinia Supercontinent broke-up, after which connections between smaller oceans, shallow seas and narrow waterways provided metazoan life passageway to sanctuary during times of environmental stress. As the Amadeus Basin was just one of a number of isolated Rodinian-aged intracratonic basins, it is likely that similar fossils of the earliest metazoans are preserved elsewhere awaiting discovery.
KEY POINTS
Metazoan evolution is widely believed to have followed the Gaskiers Glaciation at ca 580 Ma, yet studies at the molecular level suggest evolution began 200–300 million years earlier.
Fossils discovered in the Amadeus Basin of central Australia suggest that a diverse metazoan biota had evolved by 850 Ma.
Before the Rodinia Supercontinent fragmented during this temporal disparity, intracratonic basins acted as crucibles for early metazoan evolution.
Being isolated, intracratonic basins were vulnerable to periodic environmental change causing metazoans, with no means of escape, to periodically become extinct.
Amadeus Basin fossils suggest that pre-Ediacaran metazoan evolution possibly comprised cycles of ca 10 million years when evolution flourished before extinction and a following evolutionary void of between 50 and 100 million years.
Introduction
Life has been actively evolving upon planet Earth for at least 75% of its 4.6 billion year history, the oldest unequivical fossils being stromatolites (layered cyanobacteria) from Marble Bar in Western Australia dated at 3.45 billion years (Bontognali et al., Citation2012) as the biological affinity of stromatolite-like laminations from Greenland dated to ca 3.7 Ga remains debateable (Nutman et al., Citation2019). Metazoans (i.e. multicellular animals), however, are generally believed to have been around for just over half a billion years, a mere 13% of Earth’s history. In the early days of geological science, it was believed that the first appearance of metazoan life was a Cambrian event at the start of the Paleozoic Era (from the Greek palaiós: ‘old’, and zōḗ: ‘animate life’). So when organic macrofossils were found in sequences that seemingly pre-dated the Cambrian, the rocks were typically assigned an early Cambrian age (e.g. Gürich, Citation1933; Sprigg, Citation1947, Citation1949). This status quo was upset when fossils of a soft-bodied frondose metazoan were found in rocks that unquestionably pre-dated the Cambrian (Ford, Citation1958). Nevertheless organic-looking features from rocks any older than just prior to the start of the Cambrian continued to be viewed as ‘too old’ to be true fossils and were either described as pseudofossils (e.g. Hofmann, Citation1971, Jenkins et al., Citation1981) or ascribed to inorganic processes (e.g. Cloud et al., Citation1974, Plummer, Citation1980). Although, during the early 1980s, the concept of an ‘Ediacaran Period’ was being mooted to incorporate this era of pre-Cambrian metazoan evolution (Jenkins, Citation1981), it took the geological community a further quarter of a century before it was formally ratified to encompass the sequence between the top of the youngest near-global Neoproterozoic (Marinoan) glaciation, which ended at 635 Ma, and the beginning of the Cambrian, currently dated at 539 Ma (Knoll et al., Citation2006).
Subsequently, fossils of soft-bodied metazoans were discovered within the upper Ediacaran Period in several localities across the globe and classified into three faunal ‘assemblages’: the low energy, sub-tidal Avalon Assemblage; the moderate energy, shallow sub-tidal White Sea Assemblage; and the high-energy shallow water Nama Assemblage (e.g. Boag et al., Citation2016; Grazhdankin, Citation2004; Waggoner, Citation2003) that, combined, constituted the ‘Ediacara Biota’ (). Although these assemblages all post-date the Gaskiers Glaciation at 580 Ma and are essentially geographically and environmentally distinct, they display some overlap both temporally and in commonality of species, suggestive of a degree of global connectivity. Several theses have been (and continue to be) proposed to identify what triggered this evolutionary foray into metazoan life, such as the planet emerging from the Gaskiers glacial event (Pu et al., Citation2016; Wang, Shen, et al., Citation2023), or dramatic rises in oceanic and atmospheric oxygen (Evans et al., Citation2022; Kaiho et al., Citation2024; Och & Shields-Zhou, Citation2012; Shen et al., Citation2022, Wang, Peng, et al., Citation2023) and nutrient supply (Wood et al., Citation2019), or indeed the onset of ‘modern-type’ plate tectonics (Cordani et al., Citation2020).
Figure 1. Time ranges of the various Ediacaran assemblages of micro- and macro-biotas (from Xiao et al., Citation2014).
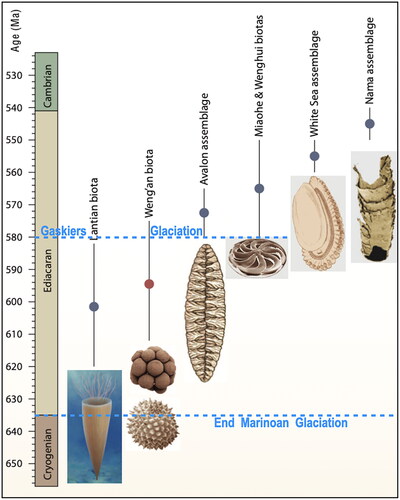
Meanwhile, Shu et al. (Citation2014, p. 892) argue that “the actual appearances of many non-skeletonised phyla may proceed their first fossil occurrences” and, indeed, the macrofossil Palaeopascichnus has been encountered in pre-Gaskiers deposits in Newfoundland (Liu & Tindal, Citation2020), and the Charnwood fossils of Ford (Citation1958) have been dated to 603 ± 2 Ma (McIlroy et al., Citation1998). This latter date coincides with biotic assemblages that have recently emerged out of China, namely the Lantian (Wan et al., Citation2016; Yang et al., Citation2022; Yuan et al., Citation2011) and Miaohe/Wenghui (Xiao et al., Citation2014), the former pushing the start date of metazoan evolution back possibly 40 million years towards the start of the Ediacaran Period (), again purported to have been triggered by post-Cryogenian ‘Snowball’ atmospheric/oceanic oxygenation (Yang et al., Citation2022) or phosphate fertilisation of the oceanic margins (Fru et al., Citation2023). Whether initially evolving at 620 or 580 Ma (i.e. early or mid in the Ediacaran Period), once metazoans had appeared they seemingly evolved as a continuum until the present day, albeit surviving episodes of possible biotic replacement (Laflamme et al., Citation2013) and a handful of post-Ediacaran mass extinction events (Tarlach, Citation2022) along the way.
Evolution and extinction
Evolution operates through genetic variation and inheritance, resulting in adaptation and natural selection, all of which requires time. Although seemingly contradictory, extinction is critical to the success of evolution (Raup, Citation1995), for without extinctions evolution would have no ‘space’ to evolve. Of all the species that have evolved throughout Earth’s history, more than 99% are extinct (Jablonski, Citation2004), and the average ‘life span’ of a species is a mere 10 million years (Newman, Citation1997). Contrary to human perception, planet Earth is a highly dynamic system that involves internal magmatic convection (possibly initiated 4.3 billion years ago by a gigantic Moon-forming impact; Yuan et al., Citation2024) shunting continental fragments about its surface on a series of tectonic plates. The drifting of these continental fragments causes local climates to vary, both above the waves (atmospheric climate) and below the waves (oceanic climate), resulting in environmental change. When life is exposed to these climatic/environmental variations, it either moves away, adapts (i.e. evolves), or goes extinct. When one species goes extinct, it frees up an environmental niche for new species to evolve into, thereby enabling evolution to progress.
Mass extinctions
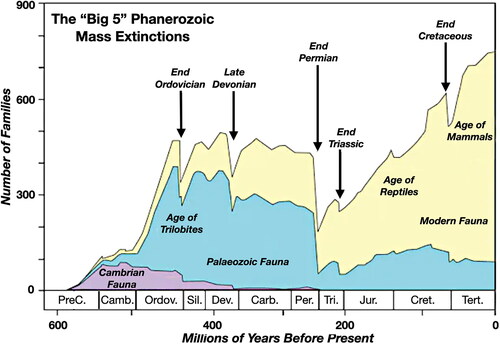
Occasionally these ordinary, ongoing climatic/environmental changes that induce extinctions are augmented by extraordinary events (Algeo & Shen, Citation2024). Some of these events are planetary, such as extensive and lengthy volcanic eruptions that globally alter the chemical make-up of the atmosphere and/or oceans, or glaciations that lock up much of the planet’s water into ice, while other events can be extra-planetary, such as asteroid impacts or possibly supernova-induced gamma ray bursts (Tretkoff, Citation2004). Over the last half a billion years, this planet has witnessed five major mass extinction events () when between 75 and 96% of all species became extinct (Tarlach, Citation2022). Evans et al. (Citation2022) argued for a sixth major mass extinction during the late Ediacaran Period (at ca 550 Ma) between the White Sea and Nama assemblages when at least 80% of species became extinct (see also Muscente et al., Citation2019).
Figure 2. Five major mass extinctions of the Phanerozoic Era (modified from Metcalfe & Isozaki, Citation2009). Published with permission from Elsevier.
Yet life has been able to survive these mass extinctions because, over the past 600 million years or so, evolution has produced a great diversity of species to occupy the myriad of environmental niches created by plate tectonics, including havens into which, at times of environmental stress, some species were able to migrate via the numerous seas and waterways between the various continental masses, thereby enabling life to maintain its evolutionary continuum.
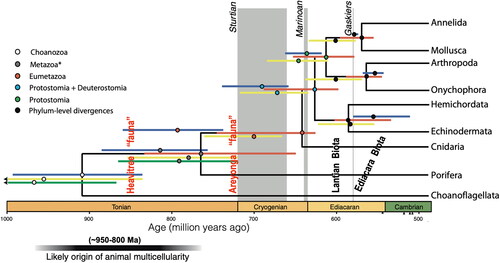
However, was that so when metazoan life was in its infancy? There is a disconnect between the timing of the origin of metazoan life as indicated from fossil evidence and that from research at the molecular level (Ho, Citation2008). On the one hand, the oldest fossils that constitute the various Ediacaran assemblages appear to indicate an origin early in the Ediacaran Period () no earlier than ca 620 Ma (Griffith et al., Citation2022; Waggoner, Citation2003; Xiao et al., Citation2014). On the other hand, research at the molecular level suggests that the metazoan molecular clock started ticking back in the Tonian Period () between ca 800 and 950 million years ago (Peterson et al., Citation2004, Citation2007, Sperling & Stockey, Citation2018). While the validity of the molecular clock continues to be debated (Budd & Mann, Citation2020), support lies in the initial emergence within eukaryotes of aerobic enzymes critical for metazoan evolution, which Bezerra et al. (Citation2021) concluded was a Mesoproterozoic, perhaps even a late Paleoproterozoic, event (see also Fedonkin, Citation2003). Support is also provided by the interpretation of vermiform microstructures as the fossilised tissue of keratose sponges from 890 Ma old reefs (Turner, Citation2021) and evidence of eukaryote predation, a common animal activity, from rocks of late Tonian age in the Grand Canyon (Porter, Citation2016). Although Cunningham et al. (Citation2016) believe the time discrepancy between fossil evidence and molecular modelling is commonly overstated, a discrepancy exists. So, was there, perhaps, a global event that explains this temporal disparity?
Figure 3. Estimated origin of metazoan evolution (950–800 Ma) from molecular studies (modified from Sperling & Stockey, Citation2018). Timing of pre-Ediacaran ‘faunas’ from the Amadeus Basin in red.
Supercontinents
Our dynamic planet is at the mercy of geological processes that have, over millennia, built, shaped and re-shaped its crustal surface. The crust’s component continental blocks are in constant motion, driven across the planet’s surface by plate tectonics. At times, these continental blocks are small and numerous, and scattered around the globe; at other times, they amalgamate into fewer, larger continental blocks, and at times into supercontinents (Nance et al., Citation2014).
Following the breakup and dispersal of the first true supercontinent of Nuna, the various continental fragments gradually reassembled, between ca 1300 and 900 Ma, into the vast Rodinia Supercontinent (Li et al., Citation2008). From ca 900 to 750 Ma, Rodinia existed as a solitary supercontinental mass within the single vast Mirovia Ocean that covered some 70% of the planet’s surface. At the time when molecular data suggest metazoan evolution was initiated, the principal stable and long-lasting protected watery worlds were isolated intracratonic basins scattered throughout Rodinia ( and ) and potentially the active passive margins bordering Rodinia (blue in ), but these are inferred and were subject to destruction during subsequent drift.
Figure 4. Simplified map of the Rodinia Supercontinent (modified from Li et al., Citation2008). Intracratonic basins in shades of yellow with the Amadeus Basin highlighted. Published with permission from Elsevier.
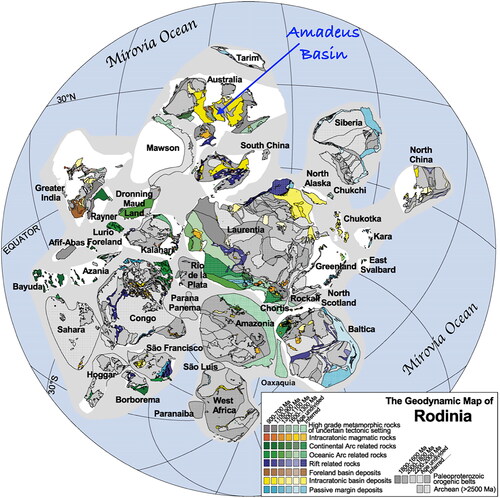
Figure 5. Progressive magmatic plume activity, rifting and break-up of the Rodinia Supercontinent (from Li et al., Citation2008). Published with permission from Elsevier.
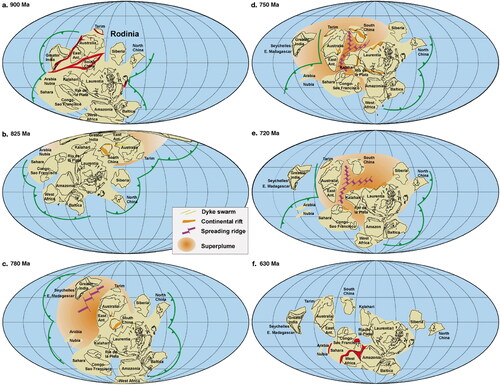
However, at ca 825, 780 and 750 Ma, convection within the mantle induced magmatic upwelling that caused the supercontinental crust to dome and stretch (), resulting in the formation of large-scale rifts (Li et al., Citation2008) within which new oceanic crust began to form (Caxito et al., Citation2021; McClellan & Gazel, Citation2014). At ca 720 Ma, Rodinia began to fragment into a series of smaller continental masses () that, over the next 100 million years or so, dispersed across the globe () on their individual oceanic crustal ‘conveyor belts’: modern-style plate tectonics had been born (Cordani et al., Citation2020). The dispersal of these continental fragments subdivided the Mirovia Ocean into smaller oceans, seas and narrow waterways.
Thus, when the metazoan evolutionary molecular clock began ticking in earnest, the Earth’s continental fragments were amalgamated into the Rodinia Supercontinent, whereas the subsequent fossil assemblages of the ‘Ediacara Biota’ evolved within a post-Rodinia world of dispersing continental blocks. Although each Ediacaran fossil assemblage is essentially geographically and environmentally distinct, their overlap both temporally and in some commonality of species (4% commonality between the Avalon and White Sea assemblages and 8% commonality between the White Sea and Nama assemblages; Muscente et al., Citation2018) indicates that, by the end of the Marinoan Glaciation at 635 Ma, a degree of global connectivity existed between the actively dispersing continental fragments, enabling some metazoans to migrate and disperse to find sanctuary from the extinctions that occurred within each of the various assemblages (Darroch et al., Citation2018, Citation2023; Evans et al., Citation2022) and thereby downgrading those extinctions from possibly ‘total’ to merely ‘severe’.
Evolution and extinction in a supercontinental world
Amadeus Basin, Central Australia
Prior to ca 750 Ma, the most likely protected watery worlds that could act as crucibles for metazoan evolution were intracratonic basins within the Rodinia Supercontinent, such as the Amadeus Basin of central Australia (Plummer, Citation2021; ). Therefore, when the metazoan molecular clock is proposed to have begun ticking, it was these intracratonic basins that provided the oxygenic environmental niches (Bezerra et al., Citation2021) required for evolution to progress to the macro scale. However, any metazoan life evolving within such an isolated and enclosed intracratonic basin was vulnerable to climatic and environmental change and thus, with no escape route to less severe climes, also to extinction.
Heavitree fauna
Such vulnerability is exemplified in the change in environmental conditions that swept across the Amadeus Basin at ca 840 Ma. Prior to that time, and central to the estimated timing of multicellular animal evolution as determined from molecular studies (), the basin was typified by generally shallow waters, with a significant delta situated in the northeastern corner through which clastic material entered the basin via nutrient-rich waters to be deposited both within the delta itself and westward along the adjacent northern shoreline (Plummer, Citation2015); clastics that now constitute the quartzose Heavitree Formation (). Within the uppermost member of that formation, macroscopic metazoans flourished as evidenced, within the delta itself, by vertical burrows and sessile carapace body fossils (, ) in addition to, along the adjacent shoreline, fossils of sessile frondose creatures, pelagic jellyfish (, ) and possible benthic crawlers (Plummer & Gorter, Citation2024a, Citation2024b).
Figure 6. Neoproterozoic stratigraphy of the Amadeus Basin, central Australia, with relevant isotopic ages. Fossils and trace fossils from the uppermost Heavitree Formation: (a, b) from Ormiston Gorge, respectively, Aspidella, with one attached to possible fallen stalk and medusoid, showing possible stranding drag marks; and (c, d) from Limbla Hills, respectively, walled vertical burrows with excavated sand and body fossils within cup-shaped carapaces (c, d from Lindsay, Citation1991). See for locations.
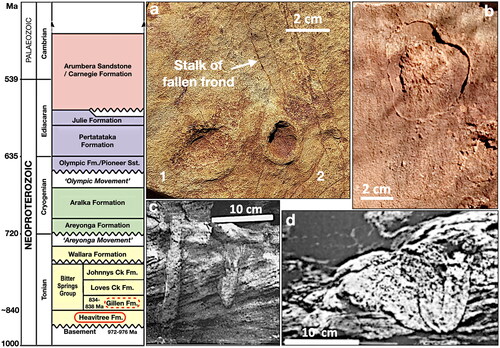
However, at ca 840 Ma, the region surrounding these shallow, oxygenated and nutrient-rich deltaic and shoreline environments began to experience a gradual drying of the climate. The rivers ceased their constant flow, becoming intermittent, and carbonates began to precipitate at times of low clastic input, forming limestone interbeds. Eventually the rivers dried up completely, and clastic input ceased altogether. With no fresh influx of oxygenated waters, the basin became increasingly salty, eventually transitioning into a vast evaporitic salt pan preserved as the Gillen Formation (see stratigraphic column ). For creatures trapped within the bounds of the basin and reliant upon oxygenated waters and a constant supply of nutrients (see Rozhnov, Citation2023), this transition spelt extinction: a ‘failed evolutionary trial’ of Plummer (Citation2022).
Areyonga fauna
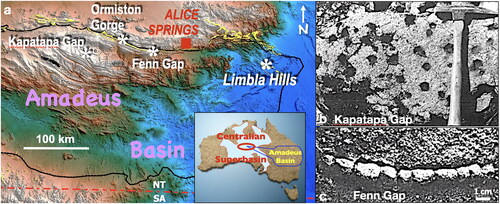
After a lengthy period of arid conditions and a considerable thickness of salt and gypsum had accumulated, the climate began to ameliorate as the supercontinent drifted into milder climate zones. Waters were once again flowing into the basin, and as the salinity began to drop, carbonates could once again precipitate. Algae relished these conditions, forming a variety of mounded and branching stromatolites. Then, as the climate cooled, and the waters continued to decrease in salinity, clastic sediments brought in by rivers began to accumulate. By ca 720 Ma, the basin comprised a conducive, oxygenated environment within which metazoans could once again evolve and thrive, as evidenced by the presence of numerous vertical, and occasional horizontal, burrows within sands along the northern shoreline of the basin (), although elsewhere metazoan evolution was seemingly well progressed by ca 770 Ma, as in Kazakhstan (Meert et al., Citation2011) and central India (Choudhuri, Citation2023). However, globally the climate continued to get increasingly colder until nearly the entire planet was plunged into the icy world of the Sturtian Glaciation. As waters became locked up in glaciers, rivers ceased to flush the basin with oxygenated and nutrient-rich waters, and the lifeforms that were flourishing in this new sanctuary were once again extinguished.
Figure 7. (a) Location of fossil localities along the northern margin of the Amadeus Basin, (b) plan view of vertical burrows from Kapatapa Gap (from Conybeare & Crook, Citation1968) and (c) horizontal burrow cast from Fenn Gap (from Wells et al., Citation1967) in sandstones related to the ca 720 Ma glaciogenic Areyonga Formation, Amadeus Basin, central Australia.
Globally, this period of glaciation lasted for about 60 million years (from ca 720 to 660 Ma) before the climate finally began to ameliorate, but the world that emerged from that glacial grip was very different, for Rodinia was breaking up, and its component fragments were dispersing. Seaways now existed between the various continental blocks as the vast Mirovia Ocean was segmented into smaller oceans, seas and waterways (), including a connection into the Amadeus Basin. Although globally metazoan life had evolved again by ca 620 Ma (see ), within the Amadeus Basin it was seemingly near the end of the Ediacaran Period before metazoan life had re-established itself.
Such an evolution/extinction scenario is likely to have occurred within any, perhaps all, similar intracratonic basins within the Rodinia Supercontinent wherein isolated pockets of fossilised early metazoan life could be awaiting discovery, as suggested by a seemingly chambered discoidal structure encountered within the 1.2–1.1 Ga Pandwa Fall Sandstone in the Vindhyan Basin of central India (Williams & Schmidt, Citation2003). Indeed, similar intracratonic basins within Rodinia’s predecessor, the Nuna Supercontinent, may harbour fossil evidence of even earlier metazoan life, as implied string-shaped traces in 2.1 Ga black silty shales from Gabon (El Albani et al., Citation2019), trail-like and discordant structures in sandstones within the 1.8 Ga Stirling Range Formation in southwestern Western Australia (Bengtson et al., Citation2007) and trail-like structures in the 1.6 Ga Chorhat Sandstone in the Vindhyan Basin of central India (Choudhuri, Citation2023; Choudhuri et al., Citation2023).
Conclusion: the ‘evolution’ of metazoan evolution
Evolution of the oldest metazoans is believed by many to have initiated at ca 580 Ma, stimulated by the Gaskiers glacial event, despite a body of evidence indicating that metazoans were extant considerably earlier, following the more severe Marinoan Glaciation that ended at 635 Ma. More recently, evidence has surfaced that metazoan evolution in fact extends back significantly further, to ca 850–840 Ma and perhaps even earlier. However, these earliest phases of metazoan evolution seemingly progressed, not as the post-Ediacaran continuum we are familiar with, but rather in a stop–start manner possibly involving a short (ca 10 million years) evolutionary flourish followed by extinction and a lengthy (50–100 million years) evolutionary void. It is proposed that such a stop–start process was a consequence of the earliest metazoan crucibles being primarily isolated and enclosed intracratonic basins within the Rodinia Supercontinent, rather than within globally connected shallow seas and waterways of the post-Rodinia world. Currently the Amadeus Basin of central Australia provides the most comprehensive suite of early metazoan phyla, but other isolated intracratonic basins of Rodinian (and indeed pre-Rodinian) age need to be searched for similar (and potentially older) forms of the earliest metazoan life.
Acknowledgements
The author wishes to thank Victor Gostin for half a century of encouragement to investigate various aspects of Australian Neoproterozoic geology and to reviewers Brian Jones and Phil Schmidt for their positive comments and encouragement to publish the synthesis of ideas presented herein.
Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
The author confirms that the data supporting the findings of this study are within the article.
References
- Algeo, T. J., & Shen, J. (2024). Theory and classification of mass extinction causation. National Science Review, 11(1), nwad237. https://doi.org/10.1093/nsr/nwad237
- Bengtson, S., Rasmussen, B., & Krapež, B. (2007). The Paleoproterozoic megascopic Stirling biota. Paleobiology, 33(3), 351–381. https://doi.org/10.1666/04040.1
- Bezerra, B. S., Belato, F. A., Mello, B., Brown, F., Coates, C. J., de Moraes Leme, J., Trindade, R. I. F., & Costa-Paiva, E. M. (2021). Evolution of a key enzyme of aerobic metabolism reveals Proterozoic functional subunit duplication events and an ancient origin of animals. Scientific Reports, 11(1), 15744. https://doi.org/10.1038/s41598-021-95094-4
- Boag, T. H., Darroch, A. F., & Laflamme, M. (2016). Ediacaran distributions in space and time: Testing assemblage concepts of earliest macroscopic body fossils. Paleobiology, 42(4), 574–594. https://doi.org/10.1017/pab.2016.20
- Bontognali, T. R. R., Sessions, A. L., Allwood, A. C., Fischer, W. W., Grotzinger, J. P., Summons, R. E., & Eiler, J. M. (2012). Sulfur isotopes of organic matter preserved in 3.45-billion-year-old stromatolites reveal microbial metabolism. Proceedings of the National Academy of Sciences, 109(38), 15146–15151. https://doi.org/10.1073/pnas.120749110
- Budd, G. E., & Mann, R. P. (2020). Survival and selection biases in early animal evolution and a source of systematic overestimation in molecular clocks. Interface Focus, 10(4), 20190110. https://doi.org/10.1098/rsfs.2019.0110
- Caxito, F. A., Basto, C. F., de Lira Santos, L. C. M., Dantas, E. L., de Medeiros, V. C., Dias, T. G., Barrote, V., Hagemann, S., Alkmim, A. R., & Lana, C. (2021). Neoproterozoic magmatic arc volcanism in the Borborema Province, NE Brazil: Possible flare-ups and lulls and implications for western Gondwana assembly. Gondwana Research, 92, 1–25. https://doi.org/10.1016/j.gr.2020.11.015
- Choudhuri, A. (2023). The advent of motile life in the Proterozoic: Scepticism and reality [Paper presentation]. In Presentation & Abstract T3/O-03, Vindhyan Supergroup: Recent Advances, Challenges & Opportunities (VISACOP) Conference, Geological Survey of India, Special Publication 128, 39.
- Choudhuri, A., El Albani, A., Mandal, S., & Sarkar, S. (2023). Biotic vs abiotic origin of unusual features from Mesoproterozoic of Vindhyan Supergroup, India. Annales de Paléontologie, 109(3), 102629. https://doi.org/10.1016/j.annpal.2023.102629
- Cloud, P., Wright, L. A., Williams, E. G., Diehl, P., & Walter, M. R. (1974). Giant stromatolites and associated vertical tubes from the Upper Proterozoic Noonday Dolomite, Death Valley region, eastern California. Geological Society of America Bulletin, 85(12), 1869–1882. https://doi.org/10.1130/0016-7606(1974)85%3C1869:GSAAVT%3E2.0.CO;2
- Conybeare, C. E. B., & Crook, K. A. W. (1968). Manual of sedimentary structures. Bureau of Mineral Resources, Geology and Geophysics Bulletin, 102.
- Cordani, U. G., Fairchild, T. R., Ganade, C. E., Babinski, M., & Leme, J. d. (2020). Dawn of metazoans: To what extent was this influenced by the onset of ‘modern-type plate tectonics’? Brazilian Journal of Geology, 50(2), e20190095. https://doi.org/10.1590/2317-4889202020190095
- Cunningham, J. A., Liu, A. G., Bengtson, S., & Donoghue, P. C. J. (2016). The origin of animals: Can molecular clocks and the fossil record be reconciled? BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 39(1), 1–12. https://doi.org/10.1002/bies.201600120
- Darroch, S. A. F., Smith, E. F., Laflamme, M., & Erwin, D. H. (2018). Ediacaran extinction and Cambrian explosion. Trends in Ecology & Evolution, 33(9), 653–663. https://doi.org/10.1016/j.tree.2018.06.003
- Darroch, S. A. F., Smith, E. F., Nelson, L. L., Craffey, M., Schiffbauer, J. D., & Laflamme, M. (2023). Causes and consequences of the end-Ediacaran extinction: An update. Cambridge Prisms: Extinction, 1(e15), 1–30. https://doi.org/10.1017/ext.2023.12
- El Albani, A., Mangano, M. G., Buatois, L. A., Bengtson, S., Riboulleau, A., Bekker, A., Konhauser, K., Lyons, T., Rollion-Bard, C., Bankole, O., Baghekema, S. G. L., Meunier, A., Trentesaux, A., Mazurier, A., Aubineau, J., Laforest, C., Fontaine, C., Recourt, P., Chi Fru, E., … Canfield, D. E. (2019). Organism motility in an oxygenated shallow-marine environment 2.1 billion years ago. Proceedings of the National Academy of Sciences, 116(9), 3431–3436. https://doi.org/10.1073/pnas.1815721116
- Evans, S. D., Tu, C., Rizzo, A., Surprenant, R. L., Boan, P. C., McCandless, H., Marshall, N., Xiao, S., & Droser, M. L. (2022). Environmental drivers of the first major animal extinction across the Ediacaran White Sea-Nama transition. Proceedings of the National Academy of Sciences, 119(46), e2207475119. https://doi.org/10.1073/pnas.2207475119
- Fedonkin, M. A. (2003). The origin of the Metazoa in the light of the Proterozoic fossil record. Paleontological Research, 7(1), 9–41. https://doi.org/10.2517/prpsj.7.9
- Ford, T. D. (1958). Pre-Cambrian fossils from Charnwood Forest. Proceedings of the Yorkshire Geological Society, 31(3), 211–217. https://doi.org/10.1016/S0016-7878(80)80014-9
- Fru, E. C., A., Bahri, J., Brosson, C., Bankole, O., Aubineau, J., El Albani, A., Nederbragt, A., Oldroyd, A., Skelton, A., Lowhagen, L., Webster, D., Fantong, W. Y., Mills, B. J. W., Alcott, L. J., Konhauser, K. O., & Lyons, T. W. (2023). Transient fertilization of a post-Sturtian Snowball ocean margin with dissolved phosphate by clay minerals. Nature Communications, 14(1), 8418. https://doi.org/10.1038/s41467-023-44240-9
- Grazhdankin, D. (2004). Patterns of distribution in the Ediacaran biotas: Facies versus biogeography and evolution. Paleobiology, 30(2), 203–221. https://doi.org/10.1666/0094-8373(2004)030<0203:PODITE>2.0.CO;2
- Griffith, H. J., Whittle, R. J., & Mitchell, E. G. (2022). Animal survival strategies in Neoproterozoic ice worlds. Global Change Biology, 29(1), 10–20. https://doi.org/10.1111/gcb.16393
- Gürich, G. v. (1933). Die Kuibis-Fossilien der Nama-Formation von Südwestafrika. Paläontologische Zeitschrift, 15(2-3), 137–154. https://doi.org/10.1007/BF03041648
- Ho, S. (2008). The molecular clock and estimating species divergence. Nature Education, 1(1), 168. https://www.nature.com/scitable/topicpage/the-molecular-clock-and-estimating-species-divergence-41971/
- Hofmann, H. J. (1971). Precambrian fossils, pseudofossils and problematica in Canada. Geological Survey of Canada Bulletin, 189.
- Jablonski, D. (2004). Extinction: Past and present. Nature, 427(6975), 589–589. https://doi.org/10.1038/427589a
- Jenkins, R. J. F. (1981). The concept of an ‘Ediacaran Period’ and its stratigraphic significance in Australia. Transactions of the Royal Society of South Australia, 105(4), 179–194.
- Jenkins, R. J. F., Plummer, P. S., & Moriarty, K. C. (1981). Late Precambrian pseudofossils from the Flinders Ranges, South Australia. Transactions of the Royal Society of South Australia, 105(2), 67–83.
- Kaiho, K., Shizuya, A., Kikuchi, M., Komiya, T., Chen, Z.-Q., Tong, J., Tian, L., Gorjan, P., Takahashi, S., Baud, A., Grasby, S. E., Saito, R., & Saltzman, M. R. (2024). Oxygen increase and the pacing of early animal evolution. Global and Planetary Change, 233, 104364. https://doi.org/10.1016/j.gloplacha.2024.104364
- Knoll, A. H., Walter, M. R., Narbonne, G., & Christie-Blick, N. (2006). The Ediacaran Period: A new addition to the geologic time scale. Lethaia, 39(1), 13–30. https://doi.org/10.1080/00241160500409223
- Laflamme, M., Darroch, S. A. F., Tweedt, S. M., Peterson, K. J., & Erwin, D. H. (2013). The end of the Ediacara biotas: Extinction, biotic replacement, or Cheshire Cat? Gondwana Research, 23(2), 558–573. https://doi.org/10.1016/j.gr.2012.11.004
- Li, Z. X., Bogdanova, S. V., Collins, A. S., Davidson, A., De Waele, B., Ernst, R. E., Fitzsimons, I. C. W., Fuck, R. A., Gladkochub, D. P., Jacobs, J., Karlstrom, K. E., Lu, S., Natapov, L. M., Pease, V., Pisarevsky, S. A., Thrane, K., & Vernikovsky, V. (2008). Assembly, configuration, and break-up history of Rodinia: A synthesis. Precambrian Research, 160(1–2), 179–210. https://doi.org/10.1016/j.precamres.2007.04.021
- Lindsay, J. F. (1991). New evidence for ancient metazoan life in the Late Proterozoic Heavitree Quartzite, Amadeus Basin, central Australia. In R. J. Korsch & J. M. Kennard (Eds.), Geological and geophysical studies in the Amadeus Basin, central Australia (pp. 91–95). Bureau of Mineral Resources, Geology and Geophysics Bulletin, 236.
- Liu, A. G., & Tindal, B. H. (2020). Ediacaran macrofossils prior to the ∼580 Ma Gaskiers glaciation in Newfoundland, Canada. Lethaia, 54(2), 260–270. https://doi.org/10.1111/let.12401
- McClellan, E., & Gazel, E. (2014). The Cryogenian intra-continental rifting of Rodinia: Evidence from the Laurentian margin in eastern North America. Lithos, 206–207, 321–337. https://doi.org/10.1016/j.lithos.2014.08.006
- McIlroy, D., Brasier, M. D., & Moseley, J. B. (1998). The Proterozoic–Cambrian transition within the ‘Charnian Supergroup’ of Central England and the antiquity of the Ediacaran fauna. Journal of the Geological Society, 155(2), 401–411. https://doi.org/10.1144/gsjgs.155.2.0401
- Meert, J. G., Gibsher, A. S., Levashova, N. M., Grice, W. C., Kamenov, G. D., & Ryabinin, A. B. (2011). Glaciation and ∼770 Ma Ediacara (?) fossils from the Lesser Karatau Microcontinent, Kazakhstan. Gondwana Research, 19(4), 867–880. https://doi.org/10.1016/j.gr.2010.11.008
- Metcalfe, I., & Isozaki, Y. (2009). Current perspectives on the Permian–Triassic boundary and end-Permian mass extinction: Preface. Journal of Asian Earth Sciences, 36(6), 407–412. https://doi.org/10.1016/j.jseaes.2009.07.009
- Muscente, A. D., Boag, T. H., Bykova, N., & Schiffbauer, J. D. (2018). Environmental disturbance, resource availability, and biologic turnover at the dawn of animal life. Earth-Science Reviews, 177, 248–264. https://doi.org/10.1016/j.earscirev.2017.11.019
- Muscente, A. D., Bykova, N., Boag, T. H., Buatois, L. A., Mángano, M. G., Eleish, A., Prabhu, A., Pan, F., Meyer, M. B., Schiffbauer, J. D., Fox, P., Hazen, R. M., & Knoll, A. H. (2019). Ediacaran biozones identified with network analysis provide evidence for pulsed extinctions of early complex life. Nature Communications, 10(1), 911. https://doi.org/10.1038/s41467-019-08837-3
- Nance, R. D., Murphy, J. B., & Santosh, M. (2014). The supercontinent cycle: A retrospective essay. Gondwana Research, 25(1), 4–29. https://doi.org/10.1016/j.gr.2012.12.026
- Newman, M. E. J. (1997). A model of mass extinction. Journal of Theoretical Biology, 189(3), 235–252. https://doi.org/10.1006/jtbi.1997.0508
- Nutman, A. P., Bennett, V. C., Friend, C. R. L., Van Kranendonk, M. J., Rothacker, L., & Chivas, A. S. (2019). Cross-examining Earth’s oldest stromatolites: Seeing through the effects of heterogeneous deformation, metamorphism and metasomatism affecting Isua (Greenland) ∼3700 Ma sedimentary rocks. Precambrian Research, 331, 105347. https://doi.org/10.1016/j.precamres.2019.105347
- Och, L. M., & Shields-Zhou, G. A. (2012). The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Science Reviews, 110(1–4), 26–57. https://doi.org/10.1016/j.earscirev.2011.09.004
- Peterson, K. J., Lyons, J. B., Nowak, K. S., Takacs, C. M., Wargo, M. J., & McPeek, M. A. (2004). Estimating metazoan divergence times with a molecular clock. Proceedings of the National Academy of Sciences, 101(17), 6536–6541. https://doi.org/10.1073/pnas.0401670101
- Peterson, K. J., Summons, R. E., & Donoghue, P. C. J. (2007). Molecular palaeobiology. Palaeontology, 50(4), 775–809. https://doi.org/10.1111/j.1475-4983.2007.00692.x
- Plummer, P. S. (1980). Circular structures in a Late Precambrian sandstone: Fossil medusoids or evidence of fluidization? Transactions of the Royal Society of South Australia, 104(1), 13–16.
- Plummer, P. S. (2015). Heavitree Quartzite, Amadeus Basin: Its place within the Centralian Superbasin. In G. C. MacDonald (Ed.), Record of abstracts, annual geoscience exploration seminar, Alice Springs (pp. 83–91). Northern Territory Geological Survey, Record 2015-002. https://geoscience.nt.gov.au/gemis/ntgsjspui/handle/1/81542
- Plummer, P. S. (2021). Was the Amadeus Basin of central Australia a crucible for pre-Ediacaran macro-biotic evolutionary trials? Transactions of the Royal Society of South Australia, 145(2), 125–142. https://doi.org/10.1080/03721426.2021.1935585
- Plummer, P. S. (2022). Failed evolutionary trials: Should we expect to find fossil evidence? Academia Letters, 5212. https://doi.org/10.20935/AL5212
- Plummer, P. S., & Gorter, J. D. (2024a). Aspidella and water escape structures from the ca 850 Ma (Tonian) Heavitree Formation, Amadeus Basin, Central Australia. Australian Journal of Earth Sciences, 71(3), 361–369. https://doi.org/10.1080/08120099.2023.2290248
- Plummer, P. S., & Gorter, J. D. (2024b). Were jellyfish stranded on a shoreline sand ca 850 million years ago in the Amadeus Basin of central Australia? Australian Journal of Earth Sciences, 71(5), 639–646. https://doi.org/10.1080/08120099.2024.2352173
- Porter, S. M. (2016). Tiny vampires in ancient seas: Evidence for predation via perforation in fossils from the 780–740 million-year-old Chuar Group, Grand Canyon, USA. Proceedings of the Royal Society B, 283, 20160221. https://doi.org/10.1098/rspb.2016.0221
- Pu, J. P., Bowring, S. A., Ramezani, J., Myrow, P., Raub, T. D., Landing, E., Mills, A., Hodgin, E., & Macdonald, F. A. (2016). Dodging snowballs: Geochronology of the Gaskiers glaciation and the first appearance of the Ediacaran biota. Geology, 44(11), 955–958. https://doi.org/10.1130/G38284.1
- Raup, D. M. (1995). The role of extinction in evolution. In: W. M. F. Francisco & F. J. Ayala (Eds.), Tempo and mode in evolution: Genetics and paleontology 50 years after Simpson. National Academy Press. https://doi.org/10.17226/4910
- Rozhnov, S. V. (2023). Evolutionary-ecological aspects of the origin and early diversification of multicellular animals. Paleontological Journal, 57(11), 1277–1285. https://doi.org/10.1134/S0031030123110114
- Shen, W., Zhu, X., Yan, B., Li, J., Liu, P., & Poulton, S. W. (2022). Secular variation in seawater redox state during the Marinoan Snowball Earth event and implications for eukaryotic evolution. Geology, 50(11), 1239–1244. https://doi.org/10.1130/G50147.1
- Shu, D., Isozaki, Y., Zhang, X., Han, J., & Maruyama, S. (2014). Birth and early evolution of metazoans. Gondwana Research, 25(3), 884–895. https://doi.org/10.1016/j.gr.2013.09.001
- Sperling, E. A., & Stockey, R. G. (2018). The temporal and environmental context of early animal evolution: Considering all the ingredients of an ‘Explosion. Integrative and Comparative Biology, 58(4), 605–622. https://doi.org/10.1093/icb/icy088
- Sprigg, R. G. (1947). Early Cambrian (?) jellyfishes from the Flinders Ranges, South Australia. Transactions of the Royal Society of South Australia, 71(2), 212–224.
- Sprigg, R. G. (1949). Early Cambrian ‘jellyfishes’ of Ediacara, South Australia, and Mount John, Kimberley District, Western Australia. Transactions of the Royal Society of South Australia, 73(1), 72–99.
- Tarlach, G. (2022). The 5 mass extinctions that have swept our planet. Discover: Planet Earth. https://www.discovermagazine.com/planet-earth/mass-extinctions
- Tretkoff, E. (2004). Did gamma rays cause Ordovician mass extinction? APS News, 13(7), 6.
- Turner, E. C. (2021). Possible poriferan body fossils in early Neoproterozoic microbial reefs. Nature, 596(7870), 87–91. https://doi.org/10.1038/s41586-021-03773-z
- Waggoner, B. (2003). The Ediacaran biotas in space and time. Integrative and Comparative Biology, 43(1), 104–113. https://doi.org/10.1093/icb/43.1.104
- Wan, B., Yuan, X., Chen, Z., Guan, C., Pang, K., Tang, Q., & Xiao, S. (2016). Systematic description of putative animal fossils from the early Ediacaran Lantian Formation of South China. Palaeontology, 59(4), 515–532. https://doi.org/10.1111/pala.12242
- Wang, H., Peng, Y., Li, C., Cao, X., Cheng, M., & Bao, H. (2023). Sulfate triple-oxygen-isotope evidence confirming oceanic oxygenation 570 million years ago. Nature Communications, 14(1), 4315. Article https://doi.org/10.1038/s41467-023-39962-9
- Wang, R., Shen, B., Lang, X., Wen, B., Mitchell, R. N., Ma, H., Yin, Z., Peng, Y., Liu, Y., & Zhou, C. (2023). A great late Ediacaran ice age. National Science Review, 10(8), nwad117. https://doi.org/10.1093/nsr/nwad117
- Wells, A. T., Ranford, L. C., Stewart, A. J., Cook, P. J., & Shaw, R. D. (1967). Geology of the north-eastern part of the Amadeus Basin, Northern Territory. Bureau of Mineral Resources, Geology and Geophysics Report, 113.
- Williams, G. E., & Schmidt, P. W. (2003). Possible fossil impression in sandstone from the late Palaeoproterozoic–early Mesoproterozoic Semri Group (lower Vindhyan Supergroup), central India. Alcheringa: An Australasian Journal of Palaeontology, 27(1), 75–76. https://doi.org/10.1080/03115510308619546
- Wood, R., Liu, A. G., Bowyer, F., Wilby, P. R., Dunn, F. S., Kenchington, C. G., Cuthill, J. F. H., Mitchell, E. G., & Penny, A. (2019). Integrated records of environmental change and evolution challenge the Cambrian Explosion. Nature Ecology & Evolution, 3(4), 528–538. https://doi.org/10.1038/s41559-019-0821-6
- Xiao, S., Muscente, A. D., Chen, L., Zhou, C., Schiffbauer, J. D., Wood, A. D., Polys, N. F., & Yuan, X. (2014). The Weng’an biota and the Ediacaran radiation of multicellular eukaryotes. National Science Review, 1(4), 498–520. https://doi.org/10.1093/nsr/nwu061
- Yang, C., Li, Y., Selby, D., Wan, B., Guan, C., Zhou, G., & Li, H-A. (2022). Implications for Ediacaran biological evolution from the ca. 602 Ma Lantian biota in China. Geology, 50(5), 562–566. https://doi.org/10.1130/G49734.1
- Yuan, X., Chen, Z., Xiao, S., Zhou, C., & Hua, H. (2011). An early Ediacaran assemblage of macroscopic and morphologically differentiated eukaryotes. Nature, 470(7334), 390–393. https://doi.org/10.1038/nature09810
- Yuan, Q., Gurnis, M., Asimow, P. D., & Li, Y. (2024). A giant impact origin for the first subduction on Earth. Geophysical Research Letters, 51(9), e2023GL106723. https://doi.org/10.1029/2023GL106723
