ABSTRACT
Clinical relevance
This paper provides eye care practitioners with important information about the potential side effects of 0.01% atropine.
Background
Eye care practitioners routinely administer 0.01% atropine eye drops nightly to slow the progression of myopia, but nobody has assessed accommodative lag or facility, near phoria, intraocular pressure or comfort of drop administration.
Methods
All 21- to 30-year-old adults with no history of accommodative issues or therapy were eligible. During the baseline visit, participants underwent testing related to potential side effects. Participants then administered one drop of 0.01% atropine nightly to both eyes, and all tests were repeated 1 week later.
Results
The average ± standard deviation age of the 31 participants was 23.9 ± 1.6 years, 71% were female, and 81% were Caucasian. The only significant changes were an increase in photopic pupil size from 4.9 ± 0.8 at baseline to 5.1 ± 0.6 mm after 1 week (paired sample t-test, p = 0.002) and an increase of the average intraocular pressure of the two eyes from 15.6 ± 2.7 to 16.7 ± 3.1 mmHg (paired-sample t-test, p = 0.003), but neither of these changes was clinically meaningful. There were no other statistically significant differences before and after 1-week administration of 0.01% atropine for any of the vision, accommodation, reading speed or subjective side effects. When asked how likely they would be to take the atropine drops to delay the onset of myopia on a scale from 1 (definitely not) to 10 (definitely would), participants replied with an average of 8.2 ± 2.0 after taking atropine eye drops for 1 week (paired-sample t-test, p = 0.81).
Conclusion
Nightly administration of 0.01% atropine did not result in any clinically meaningful symptoms, so patients would be very likely to take the drops to delay the onset of myopia.
KEYWORDS:
Introduction
Atropine is a muscarinic receptor antagonist that leads to mydriasis and cycloplegia when administered as an eye drop. Early investigations found substantial slowing of myopia progression with daily administration of 1% atropine eye drops.Citation1–5 However, clinical adoption of 1% atropine myopia control was slow due to the primary side effects of the drug. In 2012, investigators reported slowing of myopia progression with 0.01% atropine.Citation6 Since then, several studies of myopia control using atropine with concentrations lower than 0.1% have reported clinically meaningful slowing of myopia progression,Citation7–14 but the effect on eye growth may be less substantial.Citation6,Citation12 Specifically, the Low-Concentration Atropine for Myopia Progression (LAMP) Study reported that 0.05% atropine provided better myopia control than 0.025% or 0.01% atropine but did not result in additional side effects.Citation12,Citation14 Interestingly, a retrospective chart review also found that only 21% of the children who were administered 0.025% atropine daily became myopic after at least 1 year, compared to 54% of those who received no treatment, effectively delaying the onset of myopia.Citation15
Our research group used a social media platform to survey the parents of children between the ages of 6 and 16 years. When asked, “If your child didn’t currently need glasses or contact lenses, but the doctor said s/he was likely to become nearsighted, would you give your child a treatment every day for two to three years in order to delay the onset of nearsightedness?’ Of the 110 respondents, 83 (75.5%) said they would treat their child. Of those 83 parents who would treat their child, 43.4% preferred one drop in each eye at bedtime, 39.7% preferred 2 hours of outdoor time per day, and a total of 16.9% preferred either specialised glasses or contact lenses. Therefore, it is realistic to believe that parents would prophylactically treat their children to delay the onset of nearsightedness, and they would prefer to use daily eye drops to wearing vision correction (unpublished data).
The myopia control benefits of low concentration (less than 0.1% for the purposes of this paper) atropine have been fairly well documented,Citation7–15 but a comprehensive examination of the side effects associated with low concentration atropine is still lacking. Most studies have reported the effects of low concentration atropine on high contrast distance and near visual acuity, pupil size and accommodative amplitude.Citation6,Citation7,Citation9,Citation12,Citation14,Citation16 In general, neither distance nor near visual acuity is affected by the various low concentrations of atropine.Citation6,Citation7,Citation9,Citation12,Citation14,Citation16 However, pupil size is further reduced by higher concentrations of low concentration atropine.Citation12 Controversy exists related to whether low concentration atropine reduces accommodative amplitudesCitation6,Citation12 or has no effect.Citation16 Many studies measured a variety of questions related to potential side effects of low concentration atropine, but the reporting of results is limited.Citation6,Citation7,Citation9,Citation12,Citation14 Finally, a couple of studies offered photochromic lenses to children who suffer from photophobia and/or progressive addition lenses to children who report difficulty reading. In summary, they found that about one-third of children requested photochromic lenses for light sensitivity, regardless of whether they were in the placebo or atropine group,Citation12 and almost no children requested progressive addition lenses.Citation6,Citation12 A study of side effects reported by adults found that neither the near point of convergence nor reading speed was affected by daily administration of low concentration atropine for 5 days,Citation16 but no other studies on children examined these potential side effects. Two studies reported that atropine had little to no effect on intraocular pressure in children.Citation17,Citation18 Despite the number of studies that have examined the side effects of low concentration atropine,Citation6,Citation7,Citation9,Citation12,Citation14,Citation16–18 none have measured several variables that may be related to the side effects of atropine such as low contrast visual acuity, accommodative lag, accommodative facility, near phoria, photophobia, intraocular pressure or headaches. Furthermore, no study has examined the comfort of atropine eye drops.
The purpose of this study was to provide a thorough assessment of the ocular findings and subjective symptoms associated with nightly administration of 0.01% atropine in young adults and to compare the discomfort related to instillation of 0.01% atropine to artificial tears and 0.5% proparacaine to enable clinicians to compare the comfort 0.01% atropine administration to eye drops they frequently administer during clinical encounters.
Methods
Participants were optometry students between the ages of 21 and 30 years, inclusive, who did not report accommodative issues or a history of accommodative therapy. Adults were included because they are expected to provide a more critical assessment of the side effects related to 0.01% atropine eye drops than children, and the adult participants were better able to provide an assessment of whether they would take the drops to delay the onset of nearsightedness than children. The protocol was approved by the Ohio State University Biomedical Sciences Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent prior to participation. The study was registered in ClinicalTrials.gov: NCT03593044. All participants received $10 for each of two visits.
A compounding pharmacy formulated 0.01% atropine to dispense to participants in 2.5 mL bottles. Subsequent testing by an independent laboratory of a random subset of bottles that were dispensed and returned after 1 week of use indicated a stable formulation of 0.01% atropine eye drops. Participants were taught to instil the drops without touching the tip of the bottle.
The same examiner performed all the visits. At the baseline visit, participants reported their eye colour as blue, green, hazel or brown. The examiner performed a subjective refraction with maximum-plus-to-maximum-vision and binocular blur and placed it into a trial frame for all tests at both visits.
We measured binocular visual acuity with high contrast and low contrast Bailey-Lovie acuity chart at 4 m and a Logarithmic Visual Acuity Chart 2000 ‘New ETDRS’ near visual acuity chart (Precision Vision; LaSalle, IL) at 40 cm, both calibrated to 75–102 cd/m2. All visual acuities are scored by letter and recorded in logMAR notation.
We measured near phoria at 40 cm with Modified Thorington. The participant reported the number that the red line intersected and the side of the card on which it was located. The examiner recorded the magnitude of the phoria in prism dioptres and the direction as eso (positive) or exo (negative).
We measured pupil size of the right eye to the nearest 0.1 mm using a Neuroptics pupillometer (NeurOptics, Inc., Irvine, CA) in photopic and mesopic conditions. For the photopic condition, participants stood with their back towards the visual acuity chart calibrated to 75–102 cd/m2. For mesopic lighting condition, participant stood in the same position with all room lights off except a stand light facing downward at the other end of the room. Under both conditions, we measured the right pupil while the participant looked across the room with the left eye.
We measured the accommodative amplitude of the right eye while the left eye was occluded using the pull-away method until a participant could first identify a 20/40 letter. We averaged three repetitions of the test, using a different letter each time.
We measured accommodative lag using the Grand Seiko WAM-5500 Binocular Autorefractor/Keratometer (AIT Industries, Bensenville, IL) according to protocols used in previous myopia control studies.Citation19–21 The right eye was corrected with trial lenses equal to the manifest refraction, and the left eye was occluded. Participants viewed a 4 × 4 grid of 20/125 size letters illuminated by ambient room light at a distance of 33 cm, and they were constantly told to keep the print clear. A minimum of five readings was averaged.Citation22 To calculate the accommodative lag, the spherical equivalent of the non-cycloplegic autorefraction at distance was subtracted from the spherical equivalent at near (33 cm). The 3D accommodative stimulus minus the difference between the spherical equivalent refractive error at distance and near equalled the accommodative response (negative number equals an accommodative lag and positive number equals an accommodative lead).
Rate of reading was measured using a method outlined by Wilkins et al.Citation23 The test consists of a paragraph of 15 simple words per line, presented in random order, with the same 15 words in each line. Participants were instructed to read a line to ensure that they could read the words used in the test. The participant then read the passage aloud quickly with both eyes open and with as few mistakes as possible for 1 min. The test was then administered again to reduce the learning effect, and the number of words read and mistakes made were recorded only the second time.
Binocular accommodative facility was measured using +2.00/-2.00 D flippers and a near chart at 40 cm. Participants stated when the 20/50 letters on the near chart appeared clear after each flip from +2.00 D to −2.00 D or vice versa. The number of cycles that both the (+) and the (-) side of the flippers were completed in 1 min was recorded to the nearest half-flip.
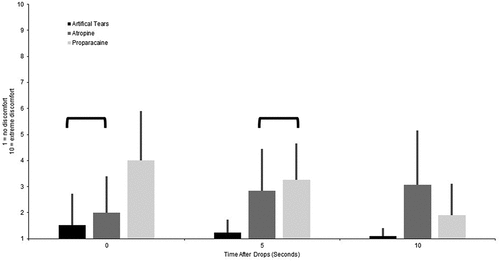
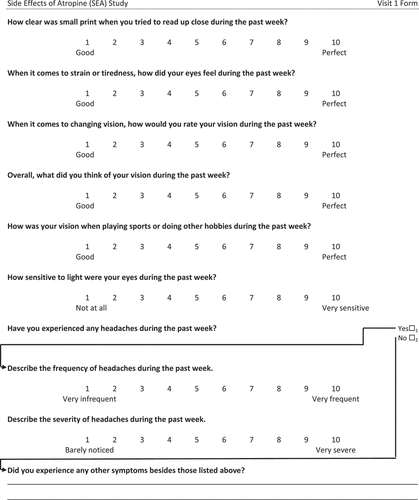
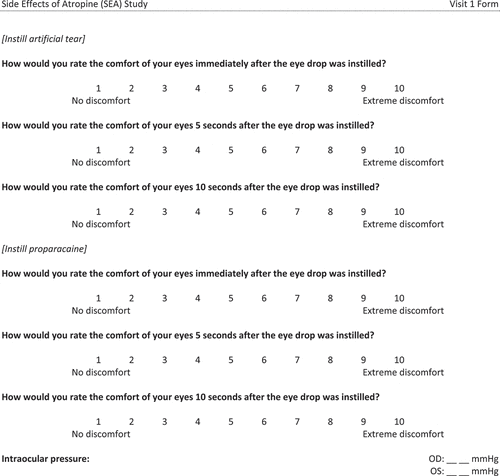
We administered a survey to quantify vision and symptoms as a whole number between 1 and 10. Each type of question had different anchors for the lowest and highest rating (Appendix). Participants were also asked to rate eye comfort on a scale from 1 to 10 immediately, 5 s and 10 s after administering a drop of 0.01% atropine, artificial tear and 0.5% proparacaine to both eyes. We administered the different drops at least 5 min apart in the order presented above so that the numbing effect of proparacaine would not alter the results of the other two drops. The participants were not told what drop was being administered. We evaluated the comfort of these drops so eye care practitioners, who routinely administer artificial tears and proparacaine, would have a scale to which they could compare the comfort of atropine eye drops.
Following corneal anaesthesia with one drop of proparacaine, a single measurement of intraocular pressure with a Tonopen (Reichert; Depew, NY) consisted of the average of four valid measurements with a standard deviation of less than 5%.
We repeated all measurements 1 week after administering 0.01% atropine nightly, except questions pertaining to gender, ethnicity, race, eye colour, eye darkness and undergoing a manifest refraction.
We gave participants a 2.5 mL bottle of 0.01% atropine drops and instructed them to instil one drop in each eye every night for 1 week. At the second visit, participants were asked how many nights the drops were administered, how many nights the drops were not administered and whether or not the participants instilled drops the night before the second visit.
Statistical methods
All comparisons using continuous data before and after 0.01% atropine drop administration were conducted using paired-sample t-tests. Comparisons using categorical data were conducted using one-way and two-way ANOVA. Correlations were assessed using linear regression analyses. Statistical tests of ocular findings were adjusted for multiple comparisons using Bonferroni correction, so p-values must be less than 0.004 (0.05/13) in order to be statistically significant.
Results
Thirty-one participants were enrolled, and all of them completed the study. The majority of participants were young Caucasian females with moderate myopia (). They administered eye drops for an average ± standard deviation of 7.1 ± 0.5 nights (range: 7 to 9 nights) and missed an average of 0.4 ± 0.8 nights (range: 0 to 4). Approximately, 77% of the participants administered the drops every night, and the participants administered drops on 95.9% of the possible nights. Only one participant did not administer atropine the night before visit 2.
Table 1. Demographic and ocular data of the 31 participants.
Ocular findings
Measures of visual acuity, accommodative, near phoria, reading speed and errors did not change significantly after administering 0.01% atropine for 1 week. Although mesopic pupil size did not increase significantly, photopic pupil size increased by an average of 0.2 mm (paired-sample t-test, p = 0.002), which is not clinically meaningful. The average intraocular pressure of the two eyes increased significantly from 15.6 ± 2.7 mmHg to 16.7 ± 3.1 mmHg, although the 1.1 mmHg increase is not clinically meaningful (). The intraocular pressure increased more than 2 mmHg in 8 (25.8%) participants, while it decreased more than 2 mmHg in two participants (6.5%). The maximum increase in IOP over the week was 5.5 mmHg, and the maximum decrease was 2.5 mmHg.
Table 2. Ocular findings before and after nightly administration of 0.01% atropine for 1 week. To adjust for multiple comparisons, Bonferroni correction indicates statistically significant p-values must be less than 0.004*.
Self-reported iris colour was not related to the increase in pupil size in photopic (One-way ANOVA, p = 0.21) or mesopic (One-way ANOVA, p = 0.09) conditions. Iris colour was also not related to the subjective change in comfort when shining the binocular indirect light at the participant (One-way ANOVA, p = 0.71) or the participant’s change in rating of light sensitivity (One-way ANOVA, p = 0.90).
Subjective symptoms
Various aspects of vision were rated from 1 (good) to 10 (perfect). All of the average ratings were above eight, and none changed significantly after 1 week of atropine eye drop administration ().
Table 3. Subjective measures of visual symptoms before and after nightly administration of 0.01% atropine for 1 week (1 = good, 10 = perfect).
When asked to rate how sensitive to light their eyes were during the past week (1 = not at all; 10 = very sensitive), participants reported an average rating of 2.8 ± 2.5 before drops and 3.1 ± 2.5 after administering drops for 1 week (paired-sample t-test, p = 0.65). When a bright light was shined at the participants’ eyes from 1 m away, they reported an average rating (1 = no discomfort; 10 = extreme discomfort) of 2.7 ± 1.9 before drops and 2.0 ± 1.7 after drops (paired-samples t-test, p = 0.002).
There was no significant change in the number of participants who reported headaches, and the frequency or the severity of headaches after 1 week of atropine eye drop administration ().
Table 4. Comparison of the number (proportion) of participants who experienced headaches before and after 0.01% atropine administration for 1 week. By those who experienced headaches, frequency was graded on a scale of 1 (very infrequent) to 10 (very frequent), and severity was graded on a scale of 1 (barely noticed) to 10 (very severe).
When asked how likely the participants would be to take the atropine drops in order to delay the onset of nearsightedness on a scale from 1 to 10 (with 1 being definitely not and 10 being definitely would), participants replied with an average of 8.2 ± 2.1 at the first exam and 8.2 ± 2.0 at the second (paired-sample t-test, p = 0.81).
Comfort during drop administration
A two-way AOVA was conducted to examine the effect of time and drop on the report of comfort. There was a statistically significant interaction between the effects of time and drop on the report of comfort (F[4,270] = 1.015, p < 0.001). Simple main effects showed that immediately, proparacaine was less comfortable than atropine (p < 0.001), proparacaine was less comfortable than artificial tears (p < 0.001) and atropine was less comfortable than artificial tears (p = 0.01). After 5 s, proparacaine was similar to atropine (p = 0.22), proparacaine was less comfortable than artificial tears (p < 0.001) and atropine was less comfortable than artificial tears (p < 0.001). After 10 s, proparacaine was more comfortable than atropine (p < 0.001), proparacaine was less comfortable than artificial tears (p < 0.001), and atropine was less comfortable than artificial tears (p < 0.001, ). There was no significant difference at any time point in the comfort ratings before and after administration of the eye drops.
Discussion
Treatments must weigh the potential benefits with risks. Although the benefits of low concentration atropine myopia control have been thoroughly investigated,Citation6–14 most studies only examine visual acuity, accommodative amplitudes and pupil size to assess risks. This study provided a more thorough investigation of the potential side effects, including accommodative facility, reading speed and discomfort upon drop administration.
Similar to other studies conducted in childrenCitation6,Citation12,Citation24 and adults,Citation16 our study found very little effect on accommodation and pupil size. In addition, we found no change in phoria status, accommodative lag or accommodative facility. Similarly, participants reported no change in subjective vision, and all forms of visual acuity showed no change after administration of low concentration atropine. Other studies of childrenCitation6,Citation12,Citation24 and adultsCitation16 have shown no change of distance or near visual acuity. No other study has measured changes in intraocular pressure following nightly administration of atropine. This study found a statistically significant increase in the average intraocular pressure of the two eyes, but the average increases in intraocular pressure were not clinically meaningful.
Clinically, it is believed that darker irises contain more melanin, which binds with the medication, so darker irises tend to dilate less than lighter irises. However, our results did not show any relationship between iris colour and pupil dilation or between iris colour and light sensitivity.
. These results are in alignment with two studies that showed iris colour is not related to pupil dilation.Citation25,Citation26
Similar to the study conducted by Loughman and Flitcroft, we found no change in reading speed after administering 0.01% atropine for 1 week.Citation16 Because atropine has little effect on accommodation or binocular vision, it is reasonable to believe that reading speed would not be affected, as shown by two independent studies.
Another novel aspect of this study was the comparison of discomfort upon instillation of 0.01% atropine, artificial tears and 0.5% proparacaine eye drops. Initially, atropine provided a level of comfort between artificial tears and proparacaine, but after 10 seconds, atropine resulted in more discomfort than either of the other two drops. However, even after 10 seconds, the discomfort related to atropine was only rated as a 3 on a scale of 1 (no discomfort) to 10 (extreme discomfort), which was less than the initial discomfort related to proparacaine. This comparison of comfort with artificial tears and proparacaine enables eye care practitioners to be able to put the comfort of atropine eye drops into perspective for patients. The discomfort is minimal, initially similar to artificial tears, but may persist for a few seconds.
Before applying low concentration atropine eye drops, participants’ rating of their likelihood of taking the eye drops if they may delay the onset of nearsightedness was 8.2 on a scale of 1 (definitely not) to 10 (definitely would), and it did not change after experiencing 1 week of nightly administration of 0.1% atropine. That indicates experience administering the eye drops that did not shift the risk-to-benefit ratio. Because adults make treatment decisions for their children, this type of question can only be asked of adults and is very pertinent to the potential for prophylactic treatment of children.
Limitations
This study was conducted in adult participants instead of children who would typically undergo atropine treatment for myopia, either to delay the onset or slow the progression. We conducted the study in adults for three reasons: (1) Adults determine whether children undergo low concentration atropine myopia control, (2) adults are not expected to experience side effects related to atropine eye drops differently from children and (3) adults are expected to provide a more critical assessment of the side effects than children. However, studying the side effects of the eye drops in children is a limitation of the study.
At the time we developed the study protocol, only 0.01% atropine had been shown to provide effective myopia control without additional side effects.Citation6 It was not until the LAMP Study investigated various Citation12The Low-Concentration Atropine for Myopia Progression (LAMP) Study indicated better myopia control with similar risk profiles for 0.025% and 0.05% atropine after we completed the protocol, so we could not assess the affects of those slightly higher concentrations.
This study did not examine the long-term effects of 0.01% atropine administration, so other side effects such as allergies were not investigated.
Optometry students were examined in this study, and they have much greater knowledge about myopia than the general population. Although that is unlikely to affect the assessment of potential side effects before and after treatment, it may bias their opinion about taking atropine to delay myopia onset.
Finally, the comfort following atropine eye drops surprisingly did not return to baseline after 10 seconds, so longer assessment of the comfort related to low concentration may be beneficial.
Conclusion
Nightly administration of 0.01% atropine for 1 week did not result in any clinically meaningful side effects. The drops resulted in minimal discomfort upon instillation that lasted for at least 10 seconds but did not result in participants being less willing to administer daily drops of 0.01% atropine to delay the onset of myopia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brodstein RS, Brodstein DE, Olson RJ, et al. The treatment of myopia with atropine and bifocals. A long-term prospective study. Ophthalmology 1984; 91: 1373–1379.
- Yen MY, Liu JH, Kao SC, et al. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989; 21: 180–182, 187.
- Shih YF, Chen CH, Chou AC, et al. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther 1999; 15: 85–90.
- Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006; 113: 2285–2291.
- Yi S, Huang Y, Yu SZ, et al. Therapeutic effect of atropine 1% in children with low myopia. J AAPOS 2015; 19: 426–429.
- Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012; 119: 347–354.
- Chia A, Chua WH, Wen L, et al. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 2014; 157: 451–457 e451.
- Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. J Ocul Pharmacol Ther 2015; 31: 541–545.
- Chia A, Lu Q-S., Tan D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2. Ophthalmology 2016; 123: 391–399.
- Diaz-Llopis M, Pinazo-Durán MD. Superdiluted atropine at 0.01% reduces progression in children and adolescents. A 5 year study of safety and effectiveness. Arch Soc Esp Oftalmol 2018; 93: 182–185.
- Larkin GL, Tahir A, Epley KD, et al. Atropine 0.01% eye drops for myopia control in American children: a multiethnic sample across three US sites. Ophthalmol Ther 2019; 8: 589–598.
- Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 2019; 126: 113–124.
- Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020; 138: 1178.
- Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the Low-Concentration Atropine for Myopia Progression (LAMP) study: phase 2 report. Ophthalmology 2020; 127: 910–919.
- Fang PC, Chung MY, Yu HJ, et al. Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocul Pharmacol Ther 2010; 26: 341–345.
- Loughman J, Flitcroft DI. The acceptability and visual impact of 0.01% atropine in a Caucasian population. Br J Ophthalmol 2016; 100: 1525–1529.
- Yu TC, Wu TE, Wang YS, et al. A STROBE-compliant case-control study: effects of cumulative doses of topical atropine on intraocular pressure and myopia progression. Medicine (Baltimore). 2020; 99: e22745.
- Wu TE, Chen HA, Jhou MJ, et al. Evaluating the effect of topical atropine use for myopia control on intraocular pressure by using machine learning. J Clin Med 2020; 10: 111.
- Walline JJ, Walker MK, Mutti DO, et al. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA 2020; 324: 571–580.
- Berntsen DA, Sinnott LT, Mutti DO, et al. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci 2012; 53: 640–649.
- Mutti DO, Mitchell GL, Hayes JR, et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci 2006; 47: 837–846.
- Mutti DO, Jones LA, Moeschberger ML, et al. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci 2000; 41: 2469–2478.
- Wilkins AJ, Jeanes RJ, Pumfrey PD, et al. Rate of Reading Test(R): its reliability, and its validity in the assessment of the effects of coloured overlays. Ophthalmic Physiol Optics. 1996; 16: 491–497.
- Fu A, Stapleton F, and Wei L, et al. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol 2020 104: 1535–41 .
- Hammond CJ, Snieder H, Spector TD, et al. Factors affecting pupil size after dilatation: the Twin Eye Study. Br J Ophthalmol 2000; 84: 1173–1176.
- Wilcox CS, Heiser JF, Crowder AM, et al. Comparison of the effects on pupil size and accommodation of three regimens of topical dapiprazole. Br J Ophthalmol 1995; 79: 544–548.