?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Clinical relevance
Corneal sensitivity represents an important indicator for corneal health, its innervation and hence also for ocular disease. It is therefore of great interest from a clinical and research perspective to quantify ocular surface sensation.
Background
The aim of this prospective cross-sectional cohort study was to clinically test the within-day and day-to-day repeatability of the new Swiss Liquid Jet Aesthesiometer, employing small droplets of isotonic saline solution for repeatability, and correlate with the Cochet–Bonnet aesthesiometer in a cohort of participants of two different age groups, based on participant feedback (psychophysical method).
Methods
Participants were recruited from two equally, large age groups: group A (18–30 years) and group B (50–70 years). The inclusion criteria were healthy eyes, Ocular Surface Disease Index (OSDI) ≤ 13, and no contact lens wear. Mechanical corneal sensitivity threshold measurements with means of liquid jet and Cochet–Bonnet methods were carried out twice during two visits (a total of four measurements), with a stimulus temperature equal to or slightly above the ocular surface temperature.
Results
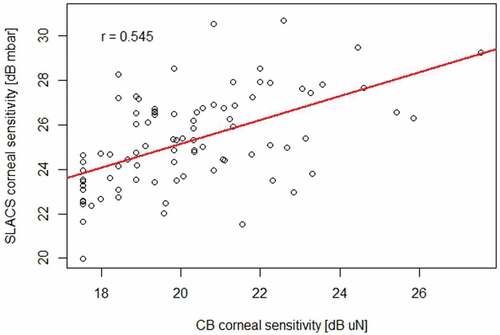
Ninety participants completed the study (n = 45 per age group, average age in group A: 24.2 ± 2.94 years, group B: 58.5 ± 5.71 years). The coefficient of repeatability for the liquid jet method was 2.56 dB within visits and 3.61 between visits. For the Cochet–Bonnet method, it was 2.27 dB within visits and 4.42 dB between visits (Bland Altman with bootstrap analysis). Moderate correlation was observed between the liquid jet and the Cochet–Bonnet method (r = 0.540, p < .001, robust linear regression).
Conclusions
Swiss liquid jet aesthesiometry offers a new examiner independent method for corneal sensitivity measurement with acceptable repeatability and moderate correlation with the Cochet–Bonnet aesthesiometer. It offers a large stimulus pressure range of 100–1500 mbar and a precision of 1 mbar. Stimulus intensity can be tuned more precisely and much smaller sensitivity fluctuations may be potentially detected.
Introduction
The human cornea is innervated by a dense network of sensory nerves that respond to mechanical, chemical, and thermal stimulation.Citation1–4 These sensory nerves serve four functions: detection of foreign bodies or noxious substances,Citation2 detection of tear film thinning to promote tear production,Citation5 detection of changes in the tear film to promote blinking,Citation6,Citation7 and a neurotrophic role in the maintenance of the corneal epithelium.Citation3
Therefore, it is of great interest from a clinical and research perspective, to quantify ocular surface sensation in eyes with altered nerve functions in the sub-basal nerve plexus, such as with advancing age,Citation8–12 in dry eye disease,Citation4 corneal dystrophies (e.g. keratoconus),Citation13 before and/or after refractive surgery,Citation14 in corneal transplants,Citation15,Citation16 with contact lens wearCitation17 or with systemic associations causing peripheral neuropathy (e.g. diabetes).Citation18
Currently, the tactile Cochet–Bonnet aesthesiometer (CB, Luneau Technology, France) is the only commercially available instrument for measuring corneal sensitivity.Citation19 It employs a fine nylon filament (0.08 or 0.12 mm in diameter) that is applied to the cornea with varying pressures, by adjusting its length to produce different stimulus intensities to the ocular surface (max. length 6 cm). Owing to its limitations, it is rarely used in clinical practice. These include the risk of abrasion of the epithelial surface, alignment and precision difficulties, limited stimulus range and hence questionable reliability, and the influence of ambient humidity on how the nylon filament bends.Citation20–23
Chao et al. however reported acceptable repeatability for corneal sensitivity measurement with the Cochet–Bonnet instrument (with a coefficient of repeatability of approximately one-third of the normal corneal threshold).Citation24 These authors used both the 0.12 and 0.08 mm filament diameters, the latter being no longer commercially available.
Various prototypes of non-contact air jet aesthesiometers have been developed to overcome most of these problems,Citation25–29 but only one was shortly commercially available: the Belmonte pain meter, based on the prototype developed by Belmonte et al.Citation25 The airflow impacting on the cornea is supposed to produce a mechanical stimulus by deforming the cornea when its temperature matches the ocular surface temperature. For this purpose, stimulus temperature may be increased or, for a cooling stimulation, it may be kept at room temperature, respectively. A chemical stimulus can be generated by using CO2 gas.Citation25,Citation26
A cooling stimulus will excite the cold temperature-sensitive nerve endings, and a mechanical stimulus should be sensed by the mechano-nociceptors and polymodal nociceptors when a sufficient degree of corneal deformation can be produced by the air jet stimulus. For a true mechanical stimulus, it is hence important to eliminate any thermal component of the stimulus, which has been shown to be difficult, since the air jet will cause a flow rate-dependent evaporative cooling effect on the wet cornea.Citation30,Citation31 It is also problematic that upon arrival at the ocular surface, the air jet stimulus disperses in a lateral motion over the entire corneal surface, creating a stimulus footprint which is difficult to determine.Citation31
In order to overcome the deficiencies, a novel non-invasive liquid jet prototype employing small droplets of isotonic saline solution was presented by Ehrmann et al.Citation32: The liquid jet stimulus exits a microvalve (with 0.1 mm diameter) mounted on a slit lamp and equipped with a heating coil and a temperature sensor, in order to match the ocular surface temperature for the generation of a mechanical stimulus. When a pulsed stimulus is generated, its strength is controlled by switching on and off the microvalve at variable frequencies, with a minimum ‘on’ period of 0.15 ms during a typical stimulus duration of 100 ms, whereby its pressure is fixed (at 300 mbar). Stimulus intensity is quantified as the total volume or corresponding mass.
Recently, a new modified liquid jet prototype aesthesiometer, the Swiss liquid jet aesthesiometer for corneal sensitivity (SLACS), was developed at the School of Engineering, University of Applied Sciences FHNW (Switzerland), employing the same microvalve, heating coil and temperature sensor for stimulus presentation. Stimulus intensity is controlled with variable pressure levels (instead of variable pulse ratio), while stimulus duration is fixed at 40 ms. Stimulus pressure can be set with a precision of 1 mbar, with a range between 100 and 1500 mbar. The working principles and relevant physical properties of this new prototype have been recently described elsewhere.Citation33
The aim of this study was to clinically explore whether and how corneal sensitivity can be determined repeatably with SLACS and to compare its repeatability with the current standard (CB) in a young and older cohort, based on participant feedback (psychophysical method). For this purpose, a total of four corneal sensitivity measurements were carried out: two consecutive measurements during the first visit and two consecutive measurements during the second visit, in order to explore both, within- and day-to-day repeatability.
Methods
This was a prospective cross-sectional cohort study and was approved by the Swiss Ethics Commission and complied with the tenets of the Declaration of Helsinki.
Ninety volunteers were recruited from the patient pool of the optometry department and/or staff members from other departments at the University of Applied Sciences in Olten, Switzerland. They were invited either by email or by personal invitation in the clinic.
Participants were recruited from two equally large age groups, and they represent the same sample as in the previously published article exploring age-related changes in corneal sensitivity.Citation12 The age range for Group A was 18 - 30 years, and for Group B it was 50 - 70 years. It was aimed to balance gender between the two groups. These age groups were determined, such that a clear difference in age between the two groups could be guaranteed and that the age ranges of the younger and older age groups showed a similar spread.
Inclusion criteria were healthy eyes with OSDICitation34≤13 and participant age in either group A or group B. Exclusion criteria were systemic disease that may affect ocular health, injury or history of operations on the anterior segment of the eye, regular application of systemic or ocular medication known to affect the tear film, rigid gas permeable contact lens wear, and soft contact lens wear<48 hours before either study visit.
Measurement procedures
All participants invited to participate in the study were given a participant information sheet explaining the nature of the research before providing signed consent. All measurements were performed on the right eye during two visits and at least 4 hours after awakening to avoid any possible diurnal bias on ocular surface sensitivity. The appointment times did not differ by more than 1 hour between Visits 1 and 2.
During the first visit, all participants completed the OSDI questionnaire to fulfill the inclusion criteria.
The following measurements were carried out twice during the two visits: slit-lamp examination for evaluation of the anterior segment, corneal sensation threshold measurement with the aid of SLACS and subsequently, after a 5-minute break, with CB. After another 5-minute break, the corneal sensitivity measurements were repeated. The second visit took place at least 24 hours thereafter and no later than 14 days later and all measurements were carried out in the same manner as during the first visit. This resulted in a total of four sensory threshold measurements obtained using each method.
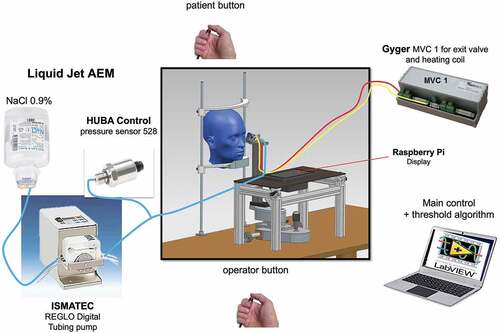
Corneal sensory threshold measurement with SLACS
The head of the participant was positioned on a chin rest (). A liquid jet (Bausch & Lomb balanced salt solution 0.9%, with a pH value of 7,4 and osmolarity of 290.2 mOsm/L, similar to the tear film) at a temperature to match the ocular surface temperature was applied to the ocular surface with low pressure (typically up to 1000 mbar) and low volume (3.29 μL at 1000 mbar and valve opening time of 40 ms). The distance between the ocular surface and the exit valve was set at 15 mm (sufficient to allow free blinking, but close enough to avoid dispersion or deviation of the liquid jet).Citation33
For the determination of the threshold for mechanical stimulation, the pressure of the droplets during a stimulus duration of 40 ms was varied. For corneal sensory threshold measurements, the participant fixates a point light source. Two laterally offset infrared cameras enable the correct centration of the device and its required distancing of 15 mm from the eye. The measurement is carried out in a darkened room so that the participant cannot visually perceive the stimulus, as this may influence the response (felt/not felt). The opening and closing of the exit valve result in a sound noticeable to the participant. However, the participant is instructed that the exit valve can be activated without actual presentation of a liquid stimulus.
The test procedure for the determination of corneal sensitivity involves a software algorithm that randomly presents liquid jet stimuli above or below threshold, without any input of the examiner on the choice of stimulus intensities presented. Upon release of a liquid jet stimulus, the participant indicated whether the stimulus was felt, by pushing a button.
Corneal sensory threshold measurement with CB
Before commencement of the study, the CB aesthesiometer was calibrated with use of a laboratory analytical balance (AB54-S; Mettler Toledo, Greifensee, Switzerland, precision ±1 µg, repeatability ±0.1 mg/0.1 mg).Citation30 The measurements obtained were converted to force (µN).
Participants were instructed to direct their gaze in the upward direction, and the nylon thread of the CB aesthesiometer was moved slowly towards the peripheral inferior cornea (approximately 3 mm superior to the lower limbus) of the right eye until the tip of the filaments contacted the corneal surface perpendicularly, and a slight bend of the nylon filament was observed. The threshold for stimulation was determined using the ascending limit method.
Four stimulus presentations were made at each intensity level, at approximately the same location, and the threshold was determined as the level at which two positive responses to the stimulus were recorded. Stimulus intensity was increased in 0.5 cm steps starting at 6.0 cm. The filament length was recorded as a threshold, which was then converted to a force in µN. Both, the 0.08 diameter and 0.12 mm diameter filaments were used, whereby the 0.08 mm diameter filament with the lower intensity stimulus range was used first. The 0.12 mm diameter filament was used if corneal sensitivity was below the stimulus range offered by the 0.08 mm diameter filament.
If corneal sensitivity was above the stimulus range of the 0.08 mm diameter filament, the lowest available corneal sensory threshold with the 0.08 mm filament was included in the statistical analysis, i.e. truncated measurements were included in the study. In the study by Chao et al., the inclusion of truncated measurements did not affect repeatability.Citation24
Statistical analysis
All measurements were transformed to a logarithmic scale, and the outcome of the transformation formula was called “dB”. This also reflects the fact that, like many psychophysical entities, pressure and force stimuli are essentially scaled logarithmically. The transformation was conducted according to the following formula:
Corneal sensory threshold (CST) represents the threshold, i.e. minimal stimulus intensity to detect a signal: as CST increases, corneal sensitivity decreases.
For the determination of the sample size, an approximate confidence interval of 0.80–1.25 was aimed at for the coefficient of repeatability (CoR). This means that the CoR obtained in this study should have confidence interval limits neither lower than 80% nor higher than 125% of the estimated value. With a sample size of n = 45 (per age group), a relative confidence interval of 0.83–1.26 was obtained and deemed suitable.
The statistical analysis was carried out with the software R (Version 4.2.1). Test–retest reliability was investigated using the Bland–Altman method, which is a key measure for repeatability. For non-parametric data, a bootstrap analogy of the Bland-Altman analysis including non-parametric tests and resampling methods was applied, in order to test for significant differences or dependencies. In addition, the correlation between the two methods was explored, with means of Pearson (or robust linear regression models for non-parametric data).
Results
Descriptive data
Ninety participants completed the study (n = 45 per age group), of which 46 were males. Average age in group A was 24.2 ± 2.94 years (23 males), and in group B it was 58.5 ± 5.71 years (23 males). The mean OSDI score for the overall group was 5.41 ± 4.47 (Group A: 4.85 ± 4.45, Group B: 6.05 ± 4.45). The data were found to be not normally distributed, hence non-parametric statistical tests were applied.
The results of the corneal sensory thresholds are summarised in (medians/interquartile ranges). For a total of 360 measurements (90 participants with each of the four measurements), the 0.12 mm filament of the Cochet–Bonnet instrument was used 54 times (i.e. corneal sensitivity was higher than the stimulus range offered by this filament in 306 measurements). In 94 measurements corneal sensitivity exceeded the stimulus range with the 0.08 mm filament and the truncated thresholds were included in the statistical analysis.
Table 1. Descriptive data for overall corneal sensitivity threshold measurements with SLACS and CB: medians/interquartile ranges.
Table 2. Descriptive data for individual corneal sensitivity threshold measurements with SLACS and CB: medians/interquartile ranges.
No statistically significant difference was observed for sex, neither with SLACS nor with CB (for SLACS, p = 0.830, for CB, p = 0.800).
Repeatability
The data were tested for variability differences between the two measurements within each visit, between the first measurements of each visit, between the second measurements of each visit, as well as between the combined measurements of each visit, using resampling (bootstrap) methods. This analysis was carried out for both methods and for the overall group, as well as for the age groups separately.
Variability within visits did not differ to a statistically significant degree between the two visits (for SLACS: p = 0.093, mean difference = −0.20 dB, CI [−0.45, 0.04]; for CB: p = 0.505, mean difference = −0.12 dB, CI [−0.47, 0.23]). Variability within visits was lower than between visits (for SLACS: p = 0.009, mean difference = 0.39 dB, CI [0.06, 0.73 dB]; for CB: p < 0.001, mean difference = 1.12 dB, CI [0.64, 1.65]). Age Group B showed a statistically non-significant trend for a larger between-visit variability with SLACS (p = 0.132, mean difference = 0.46 dB, CI [−0.17, 1.11]); however, no between-visit variability differences between age groups were noted for CB (p = 0.652, mean difference = 0.38, CI [−0.60, 1.39]).
A bootstrap analogy of the Bland Altman analysis was carried out, showing within- and between-visit repeatability. as well as show the estimated means and coefficients of repeatability (CoR), as well as the lower and upper limits with their 95% confidence intervals for SLACS and CB. The results are displayed for the overall group () as well as for each age group separately ().
Figure 2. Bland Altman with bootstrap limits of agreement plots showing within visit (to the left) and between visit repeatability (to the right) for SLACS: bold lines for means; thin lines for 95% confidence intervals of the means; dotted lines for the lower and upper limits of CoR with their 95% confidence intervals.
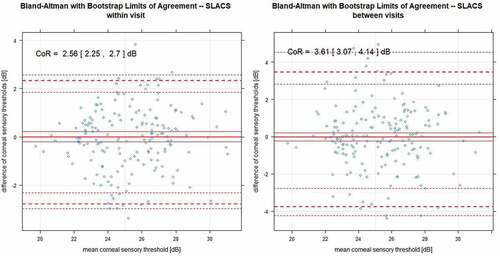
Figure 3. Bland Altman with bootstrap limits of agreement plots showing within visit (to the left) and between visit repeatability (to the right) for CB: bold lines for means; dotted lines for the lower and upper limits of CoR with their 95% confidence intervals.
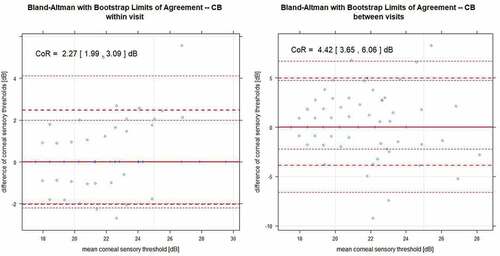
Table 3. Bland Altman analysis for the differences in measurements [dB] within and between visits (type) for groups A and B, as well as for the overall group: mean [CI], CoR [CI], lower limit [CI] and upper limit [CI].
For CB, there are fewer measurement points in comparison, as there are a small number of stimulus increments in comparison to the SLACS method, that is, each measurement point represents multiple measurements. For both methods, between-visit variability was considerably higher than within-visit variability.
As mentioned above, in 94 measurements corneal sensitivity exceeded the stimulus range with the 0.08 mm filament, resulting in truncated thresholds for these measurements. When excluding these truncated thresholds from the analysis, CoR increased for both, within- and between visit repeatability to 2.37 [2.06, 3.71] dB and 4.74 [3.60, 5.88] dB, respectively.
Differences in corneal sensitivity between visits and individual measurements
The median corneal sensory thresholds and Hodge-Lehman 95% confidence intervals were estimated for all four measurements and both (SLACS and CB) methods, with the application of a bootstrap method (). No statistically significant difference in corneal sensory thresholds between visits 1 and 2 was noted for SLACS (p = 0.890; Wilcoxon signed-rank test); however, CST increased to a statistically significant amount during visit 2 for CB, with a median difference of 0.67 dB (p = 0.04; Wilcoxon signed-rank test). This means that corneal sensitivity was estimated to be lower during visit 2 with CB.
Figure 4. Estimated median values and Hodge Lehman 95% confidence intervals for corneal sensory thresholds for SLACS and CB for the overall group for all four measurements separately, obtained with a bootstrap method.
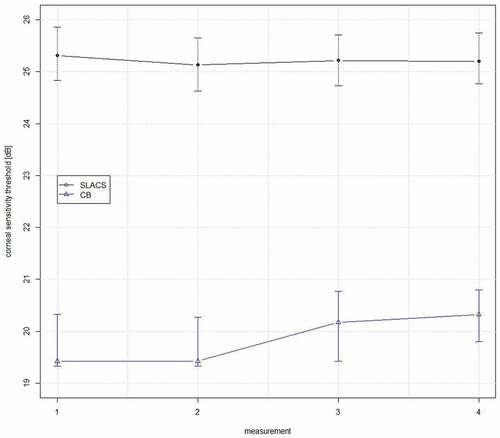
For the liquid jet measurements, there were no statistically significant differences between measurements, neither within visits (measurement 1 versus 2: p = 0.112, mean difference = −0.19 dB, CI [−0.44, 0.05]; measurement 3 versus 4: p = 0.899, mean difference = 0.16 dB, CI [−0.28, 0.31]), nor between the first and second measurements of the two visits (measurement 1 versus 3: p = 0.462, mean difference = −0.12 dB, CI [−0.46, 0.23]; measurement 2 versus 4: p = 0.639, mean difference = 0.09 dB, CI [−0.27, 0.44]). No significant differences for SLACS were found between any of the measurements within or between visits for neither age group. However, corneal sensitivity was significantly lower in age group B than in age group A, and this difference was described elsewhere.Citation12
For the Cochet–Bonnet measurements, no significant differences in corneal sensitivity were found between measurements 1 and 2 (p = 0.334, mean difference = −0.11 dB, CI [−0.34, 0.13]), and between measurements 1 and 3 (p = 0.280, mean difference = 0.27 dB, CI [−0.22, 0.74]). However, significantly lower levels of corneal sensitivity were observed during measurement 4 compared to measurement 2 (p = 0.006, mean difference = 0.71 dB, CI [0.21, 1.21]), as well as during measurement 4 compared to measurement 3 (p = 0.012, mean difference = 0.33 dB, CI [0.07, 0.60]). Similar differences were observed when considering the two age groups separately, when the CB method was applied.
Correlations for sensory thresholds with SLACS and CB sensory thresholds
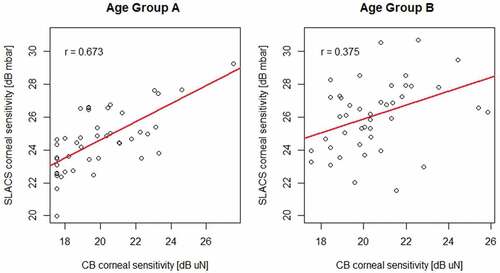
Corneal sensory thresholds with SLACS and CB were moderately correlated (r = 0.54; p < 0.001; robust linear regression; ). The correlation between the two methods was higher for the younger group A than for the older group B (r = 0.67 and p < 0.001 for group A; r = 0.38 and p = 0.03 for group B; robust linear regression; ).
Discussion
This study examines whether and how corneal sensitivity can be determined reliably and repeatedly with the new liquid jet aesthesiometer prototype (SLACS) in a cohort of participants of two different age groups, based on participant feedback (psychophysical method). The technique employs small droplets of isotonic saline solution with a pH similar to that of the human tear film. Stimulus intensity is controlled by variable pressure levels and its working principle, as well as relevant physical properties, were recently described elsewhere.Citation33
Corneal sensory thresholds with means of SLACS were determined without any input from the examiner, by employing a software algorithm that randomly presents stimuli above or below threshold. The corneal sensitivity measurements were compared to those obtained with the only commercially available aesthesiometer – the Cochet–Bonnet (CB) aesthesiometer – which applies a mechanical stimulus in the form of a fine nylon filament to the cornea with varying pressures by adjusting its length to produce different stimulus intensities on the ocular surface. Although the liquid jet stimulus differs in nature from the nylon filament, it was heated to match the ocular surface temperature to eliminate any thermal components and to generate a stimulus of mechanical nature.
Psychophysical measurements are always affected by some degree of variability, and this was also observed in this study. Both methods hence showed considerable levels of variability in corneal sensitivity. It must also be noted that CB provides a much smaller number of only 12 stimulus increments as well as a truncated intensity range,Citation28 which may result in an overestimation of measurement reliability. In this study, the stimulus range of the only commercially available 0.12 mm diameter nylon thread was applied in only 54 of the 360 measurements, and corneal sensitivity was above the stimulus range of the commercially no longer available 0.08 mm diameter filament in 94 measurements. This means that in 26.1% of these measurements, corneal sensitivity was underestimated with CB. In addition, when excluding truncating thresholds, CoR increased for both, within- and between-visit repeatability, resulting in an overestimation of repeatability.
With SLACS, the wide stimulus range was sufficient to successfully determine corneal sensory thresholds for all participants, i.e. no truncated measurements were obtained for this instrument in the present study. It is indeed however difficult to know if the variability noted for the SLACS method is caused by psychophysical noise or indeed the method itself, as there is no gold standard: The only commercially available method for mechanical corneal sensitivity measurement represents the CB with its known limitations.
With both methods, SLACS and CB, higher coefficients of repeatability were noted for the older age Group B. This may be explained by the age-related decrease in corneal sensitivity.Citation8–12 The reproducibility of SLACS was found to be good, as no change in sensitivity measurements was observed between the two visits. Interestingly, corneal sensory thresholds were significantly higher (i.e. corneal sensitivity was lower) during the second visit obtained with the CB method. Corneal sensitivity was lower during visit 2 and this reached statistical significance when comparing measurements 4 and 2, as well as when comparing measurements 4 and 3. However, between measurements 1 and 3 there was only one trend that was not statistically significant.
One possible explanation for this apparent psychological learning effect may be that the participants were apprehensive and almost fearful of the nylon thread approaching their eye during the first visit. Having realised that the measurement was indeed harmless and knowing what to expect during a repeat visit, they may have been more relaxed and hence less attentive during visit 2, resulting in a higher threshold. This emphasises the subjective nature of this method. For both methods, however, between-visit variability was considerably higher than within-visit variability. This was not unexpected, as measurements on different days are more likely to be influenced by participant-specific factors caused by individual fluctuations due to the mental and/or emotional status, the environment, and/or variations in the hormonal status.
The correlation between the two methods, SLACS and CB, was moderate in the overall group and considerably better for the younger age Group A. This confirms a relationship between the new liquid jet and the CB method, despite their different modes of stimulus and psychophysical techniques. The larger variability in Group B may have contributed to the weaker correlation between the two methods in this group.
For better comparison with repeatability measures in a previously published study,Citation35 corneal sensitivity results obtained with the CRCERT-Belmonte aesthesiometer were converted to a logarithmic scale and replotted as a Bland Altman bootstrap analysis. Compared to SLACS in this study, a much lower estimated coefficient of repeatability value of 0.81 with a confidence interval of [0.36, 0.98] dB was obtained. The air jet is likely to additionally stimulate the highly sensitive cold fibres,Citation30,Citation31 which may result in a higher level of corneal sensitivity.
Higher levels of sensitivity are likely to show less variability, when the precision of the detection capability of participants increases, as was previously shown by psychophysical perimetric threshold testing.Citation36 Lower levels of corneal sensitivity are accompanied by increased neuronal noise. This could also be shown in the present study, where variability was higher in the older age group (exhibiting lower levels of corneal sensitivity). Variability was also noted to be higher, when truncated measurements of high corneal sensitivity obtained with CB, were excluded. It can be hypothesised that this may in part explain the lower variability in psychophysical responses with air jet aesthesiometry. In addition, as the air jet stimulus disperses in a lateral motion over the entire corneal surface, it can be hypothesised that this increases the chance of stimulus detection.Citation31 The liquid jet stimulus applied in this study was heated, in order to avoid stimulus induced tear film evaporation, hence leading to a different psychophysical response.
Different corneal locations were stimulated with the two methods compared in this study, and this represents a limitation of this study. The liquid jet stimulus was sent at the corneal apex, whereas the nylon thread of the CB instrument was placed at the cornea inferiorly, whilst the gaze of the participants was directed in a superior direction. Due to the large and overlapping receptive fields of corneal nerve endings in the subbasal nerve plexus,Citation1,Citation3 the ability to localise stimuli on the cornea is affected. There is no agreement in the literature if there is a difference in corneal sensitivity at different corneal locations.Citation11,Citation26,Citation37–39 However, nerve fibre density is greatest at the corneal apex and it decreases towards the limbus,Citation40 which supports the hypothesis that corneal sensitivity should be lower in the peripheral than central cornea.
It is unclear, if such potentially small sensitivity differences are detectable. It is therefore possible, that corneal locations of different levels of corneal sensitivity were addressed with the two methods applied in the present study, possibly resulting in an underestimation of corneal sensitivity with CB. Yet, the peripheral corneal location chosen for the CB stimulus was preferred, in order to reduce any apprehension of the participant, caused by the visual awareness of an approaching nylon thread. This approach was considered as preferable, as the CB was already found to have a limited stimulus range that was confirmed in this study. In 94 of the 360 measurements, corneal sensitivity exceeded the stimulus range with the 0.08 mm filament in this study. For the liquid jet stimulus application, the corneal apex was preferred, as this assures a perpendicular arrival of the stimulus at the ocular surface.
Another limitation of this study represents the difference in psychophysical techniques between the two methods, that could not be accounted for: The test procedure for SLACS involves a software algorithm that randomly presents liquid jet stimuli above or below threshold, without any examiner input on the choice of stimulus intensities presented. This examiner-independent approach was unfortunately not possible with the Cochet–Bonnet method. This may have affected the precision of its measurements, leading to either higher or lower corneal sensitivity measurements, ultimately increasing variability.
Due to the risk of a small abrasion of the epithelial surface with the nylon filament of the CB instrument, it was decided not to randomise the order in which the instruments were tested. This could have potentially affected corneal sensitivity measurements with CB in both directions. A decrease due to sensory adaptation and/or patient fatigue, or indeed an increase due to a training effect, resulting in increased attention by the participant and hence higher corneal sensitivity measurements. Either way, this potential effect was not controlled for and must therefore be considered as another limitation of this study.
In conclusion, acceptable repeatability was obtained with the new liquid jet aesthesiometer in this prospective clinical study. Moderate correlation was observed with the only commercially available Cochet–Bonnet aesthesiometer. With a precision of 1 mbar and a large pressure range of 100–1500 mbar, stimulus intensity can be tuned more precisely and potentially detect much smaller sensitivity fluctuations with the new liquid jet aesthesiometer prototype.
Ethics registration
Swissethics (SNCTP) ID 2019-01252; clinicaltrials.gov NCT04045509.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rózsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982; 14: 105–120. doi:10.1016/0304-3959(82)90092-6
- Acosta MC, Tan ME, Belmonte C, et al. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. IOVS 2001; 42: 2063–2067.
- Müller LJ, Marfurt CF, Kruse F, et al. Corneal nerves: structure, contents and function. Exp Eye Res 2003; 76: 521–542. doi:10.1016/S0014-4835(03)00050-2
- Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf 2017; 15: 404–437. doi:10.1016/j.jtos.2017.05.002
- Jordan A, Baum J. Basic tear flow. Does it exist? Ophthalmology 1980; 87: 920–930. doi:10.1016/S0161-6420(80)35143-9
- Doane MG. Blinking and the mechanics of the lacrimal drainage system. Ophthalmology 1981; 88: 844–851. doi:10.1016/S0161-6420(81)34940-9
- Wu Z, Begley CG, Situ P, et al. The effects of increasing ocular surface stimulation on blinking and sensation. IOVS 2014; 55: 1555–1563. doi:10.1167/iovs.13-13780
- Roszkowska AM, Colosi P, Ferreri FMB, et al. Age-related modifications of corneal sensitivity. Ophthalmologica 2004; 218: 350–355. doi:10.1159/000079478
- Acosta MC, Alfaro ML, Borrás F, et al. Influence of age, gender and iris color on mechanical and chemical sensitivity of the cornea and conjunctiva. Exp Eye Res 2006; 83: 932–938. doi:10.1016/j.exer.2006.04.018
- Murphy PJ, Patel S, Kong N, et al. Noninvasive assessment of corneal sensitivity in young and elderly diabetic and nondiabetic subjects. IOVS 2004; 45: 1737–1742. doi:10.1167/iovs.03-0689
- Golebiowski B, Papas EB, Stapleton F. Factors affecting corneal and conjunctival sensitivity measurement. OVS 2008; 85: E241–246. doi:10.1097/OPX.0b013e3181694f96
- Nosch DS, Käser E, Bracher T, et al. Age-related changes in corneal sensitivity. Cornea 2022; online ahead of print.
- Spadea L, Salvatore S, Vingolo EM. Corneal sensitivity in keratoconus: a review of the literature. Sci World J 2013; 2013: 1–7. doi:10.1155/2013/683090
- Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf 2014; 12: 32–45. doi:10.1016/j.jtos.2013.09.001
- Mathers WD, Jester JV, Lemp MA. Return of human corneal sensitivity after penetrating keratoplasty. Arch Ophthalmol 1988; 106: 210–211. doi:10.1001/archopht.1988.01060130220030
- Al-Aqaba MA, Otri AM, Fares U, et al. Organization of the regenerated nerves in human corneal grafts. Am J Ophtalmol 2012; 153: 29–37.e4. doi:10.1016/j.ajo.2011.06.006
- Stapleton F, Marfurt C, Golebiowski B, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on neurobiology. IOVS 2013; 54: TFOS71. doi:10.1167/iovs.13-13226
- Tavakoli M, Petropoulos IN, Malik RA. Assessing corneal nerve structure and function in diabetic neuropathy: cornea and diabetic neuropathy: a review. Clin Exp Optom 2012; 95: 338–347. doi:10.1111/j.1444-0938.2012.00743.x
- Cochet P, Bonnet R. L’esthéseiométrie cornéenne. Réalisation et intérêt pratique. Bulletin Societés d’Ophtalmologie de France. 1961; 6: 541–550.
- Murphy PJ, Lawrenson JG, Patel S, et al. Reliability of the Non-Contact Corneal Aesthesiometer and its comparison with the Cochet–Bonnet aesthesiometer. OPO 1998; 18: 532–539. doi:10.1016/S0275-5408(98)00021-0
- Millodot M, Larson W. Effect of bending of the nylon thread of the Cochet-Bonnet aesthesiometer upon the recorded pressure. The Contact Lens 1967; 1: 5–6.
- Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: implications of use of a non-contact air jet aesthesiometer versus the Cochet–Bonnet aesthesiometer. Exp Eye Res 2011; 92: 408–413. doi:10.1016/j.exer.2011.02.016
- Lum E, Murphy PJ. Effects of ambient humidity on the Cochet–Bonnet aesthesiometer. Eye 2018; 32: 1644–1651. doi:10.1038/s41433-018-0150-z
- Chao C, Stapleton F, Badarudin E, et al. Ocular Surface Sensitivity Repeatability with Cochet-Bonnet Esthesiometer. OVS 2015; 92: 183–189. doi:10.1097/OPX.0000000000000472
- Belmonte C, Acosta M, Schmelz M, et al. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Investigative Ophthalmology & Visual Science 1999; 40: 513–519.
- Stapleton F. Corneal and conjunctival sensitivity to air stimuli. BJO 2004; 88: 1547–1551. doi:10.1136/bjo.2004.044024
- Feng Y, Simpson TL. Nociceptive sensation and sensitivity evoked from human cornea and conjunctiva stimulated by CO 2. IOVS 2003; 44: 529–532. doi:10.1167/iovs.02-0003
- Murphy P, Morgan P, Patel S, et al. A new non-contact corneal aesthesiometer. OPO 1996; 16: 101–107. doi:10.1046/j.1475-1313.1996.95001026.x
- Vega JA, Simpson TL, Fonn D. A noncontact pneumatic esthesiometer for measurement of ocular sensitivity: a preliminary report. Cornea 1999; 18: 675–681. doi:10.1097/00003226-199911000-00009
- Nosch DS, Pult H, Albon J, et al. Does air gas aesthesiometry generate a true mechanical stimulus for corneal sensitivity measurement? Clin Exp Optom 2018; 101: 193–199. doi:10.1111/cxo.12603
- Golebiowski B, Lim M, Papas E, et al. Understanding the stimulus of an air-jet aesthesiometer: computerised modelling and subjective interpretation. OPO 2013; 33: 104–113. doi:10.1111/opo.12025
- Ehrmann K, Saha M, Falk D. A novel method to stimulate mechanoreceptors and quantify their threshold values. Biomed Phys Eng Express 2018; 4: 025004. doi:10.1088/2057-1976/aa9b8d
- Nosch DS, Oscity M, Steigmeier P, et al. Working principle and relevant physical properties of the Swiss Liquid Jet Aesthesiometer for Corneal Sensitivity (SLACS) evaluation. OPO 2022; 42: 609–618. doi:10.1111/opo.12962
- Schiffman RM. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000; 118: 615–621. doi:10.1001/archopht.118.5.615
- Golebiowski B, Papas E, Stapleton F. Corneal mechanical sensitivity measurement using a staircase technique. OPO 2005; 25: 246–253. doi:10.1111/j.1475-1313.2005.00295.x
- Russell RA, Crabb DP, Malik R, et al. The relationship between variability and sensitivity in large-scale longitudinal visual field data. IOVS 2012; 53: 5985–5990. doi:10.1167/iovs.12-10428
- Murphy PJ, Armstrong E, Woods LA. A comparison of corneal and conjunctival sensitivity to a thermally cooling stimulus. In: Sullivan D, Stern M Tsubota K, et al., editors. Lacrimal gland, tear film, and dry eye syndromes 3. Vol. 506. Advances in experimental medicine and biology. Boston, MA: Springer US; 2002. p. 719–722.
- Situ P, Simpson TL, Fonn D. Eccentric variation of corneal sensitivity to pneumatic stimulation at different temperatures and with CO2. Exp Eye Res 2007; 85: 400–405. doi:10.1016/j.exer.2007.06.006
- Patel DV, Tavakoli M, Craig JP, et al. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea 2009; 28: 735–740. doi:10.1097/ICO.0b013e318193e0e3
- Golebiowski B, Chao C, Stapleton F, et al. Corneal nerve morphology, sensitivity, and tear neuropeptides in contact lens wear. OVS 2017; 94: 534–542. doi:10.1097/OPX.0000000000001063