ABSTRACT
Clinical relevance
Vision-related problems can be part of longstanding sequelae after COVID-19 and hamper the return to work and daily activities. Knowledge about symptoms, visual, and oculomotor dysfunctions is however scarce, particularly for non-hospitalised patients. Clinically applicable tools are needed as support in the assessment and determination of intervention needs.
Background
The purpose of this study was to evaluate vision-related symptoms, assess visual and oculomotor function, and to test the clinical assessment of saccadic eye movements and sensitivity to visual motion in non-hospitalised post-COVID-19 outpatients. The patients (n = 38) in this observational cohort study were recruited from a post-COVID-19 clinic and had been referred for neurocognitive assessment.
Methods
Patients who reported vision-related symptoms reading problems and intolerance to movement in the environment were examined. A structured symptom assessment and a comprehensive vision examination were undertaken, and saccadic eye movements and visual motion sensitivity were assessed.
Results
High symptom scores (26–60%) and prevalence of visual function impairments were observed. An increased symptom score when reading was associated with less efficient saccadic eye movement behaviour (p < 0.001) and binocular dysfunction (p = 0.029). Patients with severe symptoms in visually busy places scored significantly higher on the Visual Motion Sensitivity Clinical Test Protocol (p = 0.029).
Conclusion
Vision-related symptoms and impairments were prevalent in the study group. The Developmental Eye Movement Test and the Visual Motion Sensitivity Clinical Test Protocol showed promise for clinical assessment of saccadic performance and sensitivity to movement in the environment. Further study will be required to explore the utility of these tools.
Introduction
Specific eye-related symptoms may be manifested during and after COVID-19 and have been described in ophthalmic research that includes hospitalised and non-hospitalised patients. The most common symptoms include eye itching, dry eye, eye tearing, blurred vision, and photosensitivity.Citation1 The most associated ocular condition is conjunctivitis, which may precede the COVID-19 infection or develop during or after.Citation2 Other ocular and neuro-ophthalmic complications have also been described, most commonly in conjunction with severe acute disease and intensive care.Citation2–6
Other vision-related problems after COVID-19 pertain to less specific symptoms that may affect daily life. A study that addressed potential rehabilitation needs and symptoms with an impact on daily life 4 months after hospitalisation found symptoms such as blurred vision (10%), light sensitivity (9%), difficulty or discomfort when altering focus or gaze (4%), and discomfort when exposed to visual motion (6–12%).Citation7
A subsequent study that specifically targeted vision-related problems in patients with rehabilitation needs after hospitalisation found a high prevalence of reading-related symptoms, blurry vision, and light sensitivity.Citation8 The study also found visual function deficits in over 80% of the patients who reported vision-related symptoms, mostly related to binocular function and eye movements. Binocular function and eye movements are essential for maintaining a clear, single and stable visual percept when interacting, ambulating and performing tasks in daily life.
About two thirds had symptoms of dizziness, of whom a considerable percentage experienced that motion in the environment provoked the symptoms. This suggests the possible presence of visual vertigo, a phenomenon seen in other neurological conditions such as vestibular disorders, brain injury, and migraineCitation9,Citation10 and which may co-exist with eye movement or binocular vision problems.Citation11 Eye movement and visuo-vestibular dysfunctions have since been reported as being more common in patients with post-covid and as correlating with neuro-behavioural symptoms.Citation12
During the pandemic, a considerable share of patients with a COVID-19 infection did not require hospital treatment. Still, a substantial percentage of these patients reports long-standing symptoms of general tiredness,Citation13 fatigue,Citation14 and cognitive symptoms.Citation15,Citation16 These symptoms may be more common in non-hospitalised than in hospital-treated patients.Citation15 These disabling complications will likely continue to be a challenge to public health, health care systems, and socioeconomic structures.
Considering the potential impact of vision-related factors that affect everyday activities, even in patients who did not require hospital treatment, further knowledge about symptoms to consider and their possible associations are of interest. Due to the limited availability of highly specialised rehabilitation resources and the trend where patients are referred to primary care for assessment, it is of interest to identify tools that can be applied in a clinical setting for assessment and documentation.
The purpose of this observational study was to study vision-related symptoms, assess visual and oculomotor function, and test the clinical assessment of saccadic eye movements and sensitivity to visual motion in non-hospitalised post-COVID-19 patients.
Methods
Process
Patients were recruited consecutively from a post-COVID-19 clinic at a regional rehabilitation unit during the period January-December 2022. The patients had been referred from primary care for a neurocognitive assessment to provide a basis for decisions about the need for further rehabilitation and/or extended sick leave. The patients had not been hospitalised but had gone through a COVID-19 infection that was either laboratory confirmed or diagnosed through a medical examination.
The patients were asked about visual symptoms at the initial medical examination, and those who reported any symptoms concerning impaired clarity of vision, double vision, reading-related problems, photosensitivity, or intolerance to movement in the environment, tired or gritty eyes, or headaches were offered a vision examination at the clinic. The vision examination was performed by a licenced optometrist and included symptom assessment, a vision examination, and the clinical assessment of saccadic eye movements and visual motion sensitivity.
The study was approved by the Swedish Ethical Review Authority and all patients provided written consent before inclusion in the study.
Assessments
General symptoms were assessed with the Brain Injury Vision Symptom Survey (BIVSS).Citation17 This is originally intended for symptom assessment in patients with mild to moderate brain injury and includes 28 questions that cover seven areas of symptoms: visual clarity/acuity, visual comfort, double vision, photosensitivity, dry eyes, depth perception, visual field, and reading. BIVSS was administered as an interview and for each question the patient was asked to grade how often the symptom occurred: Never (0), Seldom (1), Occasionally (2), Frequently (3), or Always (4). A cut-off score of 31 is considered useful for symptom screening in TBI patients.Citation17
Symptoms while reading and near activities were assessed with the Convergence Insufficiency Symptom Survey (CISS).Citation18 The CISS is intended for symptom assessment in the presence of convergence insufficiency, which is an insufficient alignment of the eyes for near viewing. It includes 15 questions on symptoms and the patient is asked to grade how frequently each symptom occurs: Never (0), Infrequently (1), Sometimes (2), Fairly often (3), or Always (4). A score of 21 is considered useful as a cut-off.Citation18
The vision examination included visual acuity (Snellen Chart, 4 m), near acuity (near chart), stereo acuity (Lang II test), eye motility, and clinical assessment of fixation, pursuit eye movements, and saccadic eye movements (on cue and self-paced). The patient was instructed to keep the head still during eye movement testing and only move the eyes. One or two pens were used as targets and were presented 60 cm from the eyes of the patients. When testing fixation one target was held at 7 positions for 3–4 seconds: primary position, to the left of the patients, up-left, down-left, right, right-up, and right-down. The patient was instructed to maintain fixation on the target as steadily and carefully as possible.
When testing pursuit eye movements one target was moved in 4 sweeps horizontally and 4 sweeps vertically. The length of a sweep was approximately 60 cm (30 cm in each direction from centre) and took approximately 2 seconds from endpoint to endpoint. The examiner aimed at performing the sweeps in a sinusoidal movement and instructed the patient to maintain fixation on the target as carefully as possible.
When testing saccades on cue, two pens were held in front of the patient. The separation between the pens was approximately 60 cm (30 cm in each direction from centre). The patient was instructed to fixate on the nose of the examiner and to be prepared to re-fixate to the pen that the examiner wiggled. It was emphasised that the refixation should be as quick and accurate as possible.
When testing self-paced saccades, the targets were presented in the same way as in the previous saccade test but this time the targets were held still at all times. The patient was instructed to make as many re-fixations as possible between the targets while the examiner counted the saccades. The aim was to perform 15 saccades.
Binocular function and accommodative functions were examined; this included heterophoria (prism cover test), fusional vergences (prism bar), near point of convergence (Royal Air Force ruler), near point of accommodation (Royal Air Force ruler), and vergence facility (3 base in, 12 base out prism). Criteria for clinical signs were derived from the literature and are presented in .
Table 1. List of functions examined, assessment methods, and assessment criteria.
Saccadic eye movements were assessed with the Developmental Eye Movement Test.Citation19 In the first step, the patient read (aloud) 40 one-digit numbers arranged in two columns while the examiner measured time duration and monitored any reading errors. This was then repeated with a new set of numbers. Next, the patient read 80 numbers distributed over 16 horizontal lines. There are 5 irregularly spaced numbers per line, intended to mimic the way the eyes make saccadic movements during reading. The total time duration for reading numbers horizontally was divided by the total time for reading numbers vertically to get a ratio.
Sensitivity to movement in the environment was assessed according to the Visual Motion Sensitivity Clinical Test Protocol.Citation20 The patient was asked to stand and gaze straight ahead at a fixation mark on the wall at eye level at 4 m distance. An OKN (Opto Kinetic Nystagmus) drum was held at 25 cm from the face so that much of the peripheral visual field patient’s of the patient was encompassed. The drum was rotated at slow-medium velocity while the patient was asked to grade any induced symptoms on a scale of 0–10. Zero meant the patient was not bothered at all; 10 meant there is a strong effect where the patient felt nauseous or experienced some other somatic or visual sensation. The test was performed sequentially: right, left, lower, and upper visual fields. A total score was calculated (max 40).
Statistical analysis
IBM SPSS Statistics 28 was used for statistical analysis. Multiple linear regression was performed with the Enter method (default method in SPSS). In the event of skewed data for independent variables, a logarithmic transformation was performed. Welch’s t-test was used for comparing means. Normality tests were performed with the Kolmogorov-Smirnov method. A P value of <.05 was set as the level of significance.
Results
A total of 46 patients were invited, of whom 38 accepted and were included in the analysis (29 women and 9 men, mean age 46.8 (9.3) years, range 27–62 years). The median time since the infection was 22 months, min 12 and max 29 months.
General vision symptoms according to BIVSS
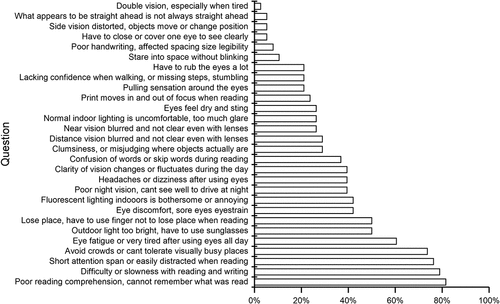
The BIVSS is intended for brain injury patients but was used in this setting since it covers a wide array of potential vision-related symptoms. Some questions were left unanswered by some of the patients because they said they could not relate to the question or did not know. The questions that were most left unanswered were poor handwriting (9 patients), poor night vision (7 patients), and staring into space without blinking (6 patients).
The mean total symptom score was 46.2 (16.8) and all but three patients scored above the cut-off of 31. The top three symptoms reported as frequently or always occurring were related to reading (76.3–81.6%) followed by difficulties tolerating visually busy places (73.7%) ().
Vision symptoms when reading or doing near activities
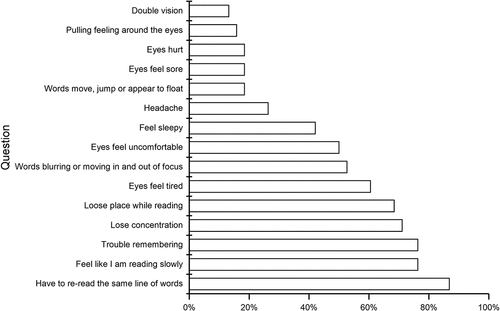
All patients responded to the CISS and only one patient left a question unanswered. The mean total symptom score was 33.6 (9.2). All but three patients scored above the cut-off of 21. The top four symptoms that occurred fairly often or always were frequent re-reading, trouble remembering, slow reading, and loss of concentration while reading (71.1–86.8%) (). No significant difference in scores was found between patients with and without diagnosed convergence or accommodative insufficiency.
Vision examination
Twenty-nine patients (74.4%) had between 1–3 clinical signs concerning visual acuity, visual field, eye movements, accommodative or binocular functions (). Seven patients met the criteria for convergence insufficiency and two patients for accommodative insufficiency.
Table 2. Clinical signs found during the vision examination.
Saccadic eye movements
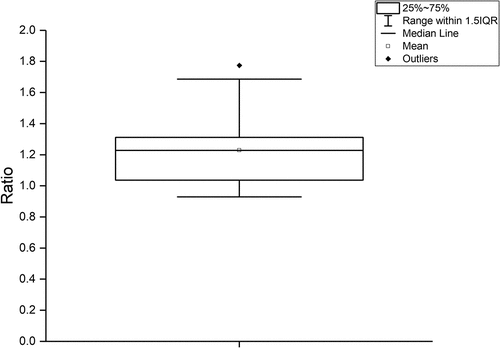
Thirty-two patients were able to perform the test in full. Six patients (15.8%) did not complete the test due to fatigue or discomfort. A total duration was extrapolated for the five patients who performed at least half of test C (8 out of 16 lines of horizontal reading). The mean ratio was 1.23 (0,21) and 21 patients had a ratio exceeding 1.2 ().
Symptoms versus clinical findings
A linear regression analysis was performed to investigate what clinical findings might contribute to the symptom level according to CISS. The CISS total score was set as the dependent variable and binocular functions and DEM ratio were included as independent variables according to the Enter method. Non-significant variables were removed. The final model was significant (p < 0.001) and explained 35.7% of the variation (adjusted R square). The two variables included in the model were the Developmental Eye Movement Test ratio (B = 23.520, t = 3.988, p < 0.001) and near point of convergence (B = 14.712, t = 2.275, p = 0.029). According to the model, an increased CISS score was associated with an increased Developmental Eye Movement Test ratio and receded near point of convergence (difficulty aligning the eyes for near viewing).
Visual Motion Sensitivity Clinical Test Protocol
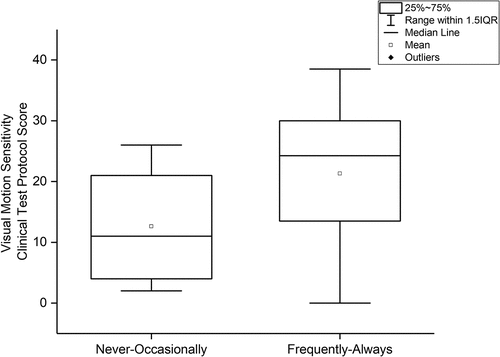
Patients who responded Always or Frequently (73.6%, n = 28) on item 23 in BIVSS (Avoid crowds/cannot tolerate “visually busy” places) had a significantly higher total score on the Visual Motion Sensitivity Clinical Test Protocol (p = 0.029) than those who responded Never/Seldom/Occasionally (23.8%, n = 9) (). One patient answered that they did not know.
Discussion
The purpose of this study was to study vision-related symptoms, assess visual and oculomotor function, and test the clinical assessment of saccadic eye movements and sensitivity to visual motion in post-COVID patients.
General vision symptoms, as assessed with the BIVSS, found a wide array of frequent symptoms. Reading-related symptoms were most common, with the top three symptoms being short attention span/easily distracted, poor comprehension, and difficulty/slowness with reading and writing. The mechanism behind these symptoms is probably significantly related to cognitive function. Research has indeed found affected domains of cognitive function, including concentration, memory, attention, and executive function.Citation15,Citation16
On the other hand, the saccadic test (Developmental Eye Movement Test) showed that a considerable number of patients struggled. Furthermore, the regression analysis found that performance in the saccadic test contributed to the level of symptoms. From the findings of this study, it is not possible to differentiate whether basic oculomotor dysfunctions are a direct contributing cause of reading-related symptoms or whether this indicates other cognitive impairments, such as slowed processing speed, affected memory function, or attention.
Based on findings regarding basic oculomotor dysfunctions in post-COVID-19 patientsCitation12 and other patient groups,Citation21,Citation22 it may be necessary to consider oculomotor inefficiency as part of the underlying reasons for reading-related symptoms. If so, these problems could be targeted with appropriate spectacle correction, guidance on visual ergonomics, or vision therapy.
Close behind the top three reading-related symptoms came another type of symptom; Avoiding crowds/cannot tolerate “visually busy” places. This is a complex symptom where the mechanism is not clear. Research findings raise the possibility that the neural substrate involves cortical areas V6 and V6a which are involved in higher-level visual motion computation.Citation23
The Visual Motion Sensitivity Clinical Test Protocol showed a significantly higher score in the subgroup who reported frequently or always having difficulty tolerating visually intense places. It thus appears that the Visual Motion Sensitivity Clinical Test Protocol is sensitive to pronounced difficulties. Since the test was performed in an otherwise quiet and still examination room, it is plausible that the symptoms were provoked by the external visual motion (Gibsonian optic flow) created by the rotating striped OKN drum.
The mechanism behind the symptom is not clear, but it has been observed in neurologic and vestibular disorders.Citation9,Citation10 Theories include increased visual dependence due to vestibular disorders, altered visuo-vestibular cortical interactions, and disruptions or conflict in the brain’s processing of motion in the environment in relation to self-motion.Citation9,Citation10,Citation20
In a study of balance and visual reliance in post-COVID syndrome patients, Gervasoni et alCitation24 made a striking observation when comparing body sway with open and closed eyes. It was found that body sway parameters were more distant from normality values (worse) with the eyes open, which is when the patients integrated vision, proprioceptive, and vestibular information. Further investigation will be required to understand the mechanisms behind visual motion sensitivity.
The findings suggest that the Visual Motion Sensitivity Clinical Test Protocol can be used to estimate and document the extent of the symptom. This may be useful for follow-ups and patient insight. It may also provide guidance if multifocal spectacles, which may exacerbate symptoms, should be discouraged. From a rehabilitation perspective, hypersensitivity to visual motion may be targeted with compensatory measures and habituating therapy.Citation20
Direct eye- and vision-related symptoms were common (26.3–60.5%) and included eye fatigue, sore or strained eyes, headaches, or dizziness after using the eyes, blurred vision at distance or near, and fluctuating clarity of vision. These symptoms suggest binocular dysfunction, accommodative problems, or possibly inadequate refractive correction. The vision examination found an increased prevalence of these visual dysfunctions that was in line with observations in hospital-treated patientsCitation8 and patients with post-COVID syndrome.Citation12
Statistical analysis did not show direct associations between the symptoms and visual dysfunctions, possibly due to the limited number of participants, making the study underpowered, or that the cause of the symptoms is multifactorial where for example mental or physical fatigue may contribute to the problems indirectly. Future research should aim to control for these factors. The visual function problems found in this study can, based on clinical experience, be targeted with appropriate spectacles and vision therapy.
Symptoms included those indicating dry eyes (21.1–26.3%); eyes feel dry and sting, and have to rub the eyes a lot. Similar findings have been shown in previous research.Citation1,Citation13,Citation25 Examinations have shown increased dry eye disease in post-COVID patients and thus underlined the need for ocular surface assessment and treatment where required.Citation25
Increased light sensitivity has been reported by 42.1–50.0% of subjects, with outdoor as well as normal indoor light causing discomfort and specifically indoor fluorescent lighting being perceived as bothersome or annoying (42.1%). Light sensitivity is a common persisting symptom after COVID-19Citation1,Citation8,Citation26,Citation27 and has been proposed as part of the diagnostic criteria for neurologic post-acute sequelae of SARS-CoV-2 infection.Citation28
The mechanism of light sensitivity may be of central or peripheral origin and ocular causes are not uncommon.Citation29–31 A suggested approach to the diagnosis of light sensitivity and photophobia comprises the assessment of ocular and neurologic causes.Citation32 Treatment options to relieve symptoms include intense treatment of dry eye, coloured tints, and sunglasses outdoors.Citation32
Study limitations
The study includes a limited number of participants, aged 27–62, who were recruited from a rehabilitation clinic to which they had been referred for neurocognitive assessment. This means there are limitations to how the findings can be generalised to other age groups or patients without the needs for neurocognitive rehabilitation. It is possible that some of the visual and oculomotor problems identified in this study were present before the infection. Future research may benefit from a control group.
Conclusion
The BIVSS appears to be applicable to post-COVID patients and is advantageous because it covers a broad range of symptoms, from ocular to central causes. Further evaluation will be required to identify cut-off scores and possible adjustments to questions. For example, headaches are covered only briefly, and this is a frequent symptom in this patient group.
The CISS is a complementary test that focuses more specifically on reading and near activities. It appears to capture those patients who experience and demonstrate strains when doing reading and similar tasks. The vision examination found high levels of visual dysfunctions, similar to previous findings in hospitalised patients. It thus appears necessary to consider these problems also in patients who did not require hospital treatment.
The Developmental Eye Movement Test and the Visual Motion Sensitivity Clinical Test Protocol appear to be useful and easily implemented clinical tools for the assessment and documentation of saccadic performance and visual motion sensitivity, respectively. Further study will be required to establish normal values and cut-off scores.
Due to the close interactions between visual and cognitive functions and the subsequent difficulty in differentiating between the associated symptoms, future research should aim to address the relationship between visual and neuropsychological findings. An improved understanding of these interactions will help to shape individualised rehabilitation interventions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Luo X, Lv M, Zhang X et al. Clinical manifestations of COVID-19: an overview of 102 systematic reviews with evidence mapping. J Evid Based Med 2022; 15: 201–215. doi:10.1111/jebm.12483.
- Sen M, Honavar SG, Sharma N et al. COVID-19 and Eye: a review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol 2021; 69: 488–509. doi:10.4103/ijo.IJO_297_21.
- Forouhari A, Mansouri V, Safi S et al. A systematic literature review and bibliometric analysis of ophthalmology and COVID-19 research. J Ophthalmol 2022; 2022: 8195228. doi:10.1155/2022/8195228.
- Petzold A. Neuro-ophthalmic implications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) related infection and vaccination. Asia Pac J Ophthalmol (Phila) 2022; 11: 196–207. doi:10.1097/APO.0000000000000519.
- Tisdale AK, Dinkin M, Chwalisz BK. Afferent and efferent neuro-ophthalmic complications of coronavirus disease 19. J Neuroophthalmol 2021; 41: 154–165. doi:10.1097/WNO.0000000000001276.
- Teo KY, Invernizzi A, Staurenghi G et al. COVID-19 related retinal micro-vasculopathy - a review of current evidence: COVID-19 related retinal micro-vasculopathy. Am J Ophthalmol 2021; 235: 98–110. doi:10.1016/j.ajo.2021.09.019.
- Divanoglou A, Samuelsson APK, Sjodahl PER et al. Rehabilitation needs and mortality associated with the Covid-19 pandemic: a population-based study of all hospitalised and home-healthcare individuals in a Swedish healthcare region. EClinicalMedicine 2021; 36: 100920. doi:10.1016/j.eclinm.2021.100920.
- Johansson J, Levi R, Jakobsson M et al. Multi-professional neurorehabilitation after Covid-19 infection should include assessment of visual function. Arch Rehabil Res Clin Transl 2022; 4 : 100184. doi:10.1016/j.arrct.2022.100184.
- Bronstein AM. The visual vertigo syndrome. Acta Otolaryngol Suppl 1995; 520 Pt : 45–48. doi:10.3109/00016489509125186.
- Bednarczuk NF, Bonsu A, Ortega MC et al. Abnormal visuo-vestibular interactions in vestibular migraine: a cross sectional study. Brain 2019; 142: 606–616. doi:10.1093/brain/awy355.
- Winkler PA, Ciuffreda KJ. Ocular fixation, vestibular dysfunction, and visual motion hypersensitivity. Optometry 2009; 80: 502–512. doi:10.1016/j.optm.2009.01.014.
- Kelly KM, Anghinah R, Kullmann A et al. Oculomotor, vestibular, reaction time, and cognitive tests as objective measures of neural deficits in patients post COVID-19 infection. Front Neurol 2022; 13: 919596. doi:10.3389/fneur.2022.919596.
- Ballering AV, van Zon SKR, Olde Hartman TC et al. Lifelines corona research I. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022; 400: 452–461. doi:10.1016/S0140-6736(22)01214-4.
- Townsend L, Dyer AH, Jones K et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLos One 2020; 15 : e0240784. doi:10.1371/journal.pone.0240784.
- Holdsworth DA, Chamley R, Barker-Davies R et al. Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLos One 2022; 17 : e0267392. doi:10.1371/journal.pone.0267392.
- Tavares-Junior JWL, de Souza ACC, Borges JWP et al. COVID-19 associated cognitive impairment: a systematic review. Cortex 2022; 152: 77–97. doi:10.1016/j.cortex.2022.04.006.
- Laukkanen H, Scheiman M, Hayes JR. Brain injury vision symptom survey (BIVSS) questionnaire. Optom Vis Sci 2017; 94: 43–50. doi:10.1097/OPX.0000000000000940.
- Rouse MW, Borsting EJ, Mitchell GL et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalmic Physiol Opt 2004; 24: 384–390. doi:10.1111/j.1475-1313.2004.00202.x.
- Garzia RP, Richman JE, Nicholson SB et al. A new visual-verbal saccade test: the development eye movement test (DEM). J Am Optom Assoc 1990; 61: 124–135.
- Tannen B, Bagavandoss S, Ciuffreda KJ et al. Clinical quantitative assessment of visual motion sensitivity in concussion/mild traumatic brain injury. Vis Dev Rehabil 2020; 6: 264–268.
- Moller MC, Johansson J, Matuseviciene G et al. An observational study of trait and state fatigue, and their relation to cognitive fatigability and saccade performance. Concussion 2019; 4 : CNC62. doi:10.2217/cnc-2019-0003.
- Kerkhoff G. Neurovisual rehabilitation: recent developments and future directions. J Neurol Neurosurg Psychiatry 2000; 68: 691–706. doi:10.1136/jnnp.68.6.691.
- Pitzalis S, Fattori P, Galletti C. The human cortical areas V6 and V6A. Vis Neurosci 2015; 32: E007. doi:10.1017/S0952523815000048.
- Gervasoni F, LoMauro A, Ricci V et al. Balance and visual reliance in post-COVID syndrome patients assessed with a robotic system: a multi-sensory integration deficit. Neurol Sci 2022; 43: 85–88. doi:10.1007/s10072-021-05647-8.
- Gambini G, Savastano MC, Savastano A et al. Ocular surface impairment after COVID-19: a cohort study. Cornea 2021; 40: 477–483. doi:10.1097/ICO.0000000000002643.
- McHarg M, Wang Y, Yakin M et al. Ocular symptoms in COVID-19 infection: a survey study. J Ophthalmic Inflamm Infect 2022; 12: 42. doi:10.1186/s12348-022-00319-w.
- Hellgren L, Levi R, Divanoglou A et al. Seven domains of persisting problems after hospital-treated Covid-19 indicate a need for a multiprofessional rehabilitation approach. J Rehabil Med 2022; 54: jrm00301. doi:10.2340/jrm.v54.2434.
- Moghimi N, Di Napoli M, Biller J et al. The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep 2021; 21: 44. doi:10.1007/s11910-021-01130-1.
- Diel RJ, Mehra D, Kardon R et al. Photophobia: shared pathophysiology underlying dry eye disease, migraine and traumatic brain injury leading to central neuroplasticity of the trigeminothalamic pathway. Br J Ophthalmol 2021; 105: 751–760. doi:10.1136/bjophthalmol-2020-316417.
- Buchanan TM, Digre KB, Warner JEA et al. The unmet challenge of diagnosing and treating photophobia. J Neuroophthalmol 2022; 42: 372–377. doi:10.1097/WNO.0000000000001556.
- Chang TT, Ciuffreda KJ, Kapoor N. Critical flicker frequency and related symptoms in mild traumatic brain injury. Brain Inj 2007; 21: 1055–1062. doi:10.1080/02699050701591437.
- Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol 2016; 61: 466–477. doi:10.1016/j.survophthal.2016.02.001.