 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Clinical Relevance
Realistic benchmarks can serve as comparators for optometrists wishing to engage in clinical practice audits of their glaucoma care.
Background
The iCareTrack study established the appropriateness of glaucoma care delivery through clinical record audits of Australian optometry practices. Benchmarks required for monitoring and improving glaucoma care delivery do not exist. This study developed realistic benchmarks for glaucoma care and then benchmarked the performance of practices from the iCareTrack study to establish aspects of care that warrant attention from quality improvement initiatives.
Methods
Benchmarks were developed from the pre-existing iCareTrack dataset using the Achievable Benchmarks of Care (ABC) method. The iCareTrack study had audited the appropriateness of glaucoma care delivery against 37 clinical indicators for 420 randomly sampled glaucoma patient records from 42 Australian optometry practices. The four-step ABC method calculates benchmarks based on the top 10% of best-performing practices adjusted for low patient encounter numbers. iCareTrack results were compared to the benchmarks to explore the distribution of practices that were at, above or below benchmark.
Results
Benchmarks were developed for 34 of 37 iCareTrack indicators. For 26 (of 34) indicators, the benchmarks were at or above 90% appropriateness. The benchmarks for 14 (of 34) iCareTrack indicators were met by more than 80% of eligible practices, indicating excellent performance. Some aspects of glaucoma care such as peripheral anterior angle assessment, applanation tonometry, and visual field assessment appeared to be delivered sub-optimally by optometrists when compared to the benchmarks.
Conclusion
This study established benchmarks for glaucoma care delivery in optometry practices that reflect realistic and top achievable performance. The large number of indicators with benchmarks above 90% confirmed that glaucoma care can and should be delivered by optometrists at very high levels of appropriateness. Benchmarking identified pockets of sub-optimal performance that can now be targeted by quality improvement initiatives.
Introduction
As a leading cause of irreversible vision impairment globally, glaucoma is a considerable public health concern in part due to its insidious, often asymptomatic presentation that makes case detection challenging.Citation1 Approximately half of the estimated 200 000 Australians with glaucoma are undiagnosed.Citation2 Provision of appropriate glaucoma care that is in line with evidence is desirable.Citation3 For example, early diagnosis and appropriate management of glaucoma can slow down or prevent irreversible vision lossCitation4,Citation5 as well as improve patients’ vision-related quality of life and reduce the societal economic burden of disease.Citation6 With growing evidence showing that provision of appropriate care can improve patient outcomes and reduce health expenditure,Citation7 there is interest in measuring and evaluating the appropriateness of care delivery in glaucoma.
The delivery of appropriate glaucoma care varies, with appropriateness reported to range from 2% to 100%.Citation8 To establish the appropriateness of glaucoma care in Australia, the iCareTrack study, a cross-sectional clinical record audit of a nationally representative sample of optometry practices, assessed glaucoma care against validated clinical indicators derived from guideline recommendations. Glaucoma care was appropriate in 63% (95% CI 61%, 64%) of patient encounters.Citation9 As optometrists are the major providers of primary eye care, including glaucoma care, it is important to determine if these iCareTrack appropriateness scores represent a good standard of glaucoma care or if improvement is required.
Benchmarking is a continuous quality improvement approach that compares current performance to an established target level (benchmark).Citation10 In health care, benchmarking involves assessing care provided against quality or clinical indicators and providing feedback to practitioners in the form of comparison to the established benchmark. Benchmarking is beneficial as it raises practitioners’ awareness and allows for self-reflection that can assist in improving the appropriateness of care provided.Citation11 To be effective, benchmarks should be realistic and attainable,Citation12 as practitioners will reject unachievable benchmarks and be discouraged from modifying their behaviour.Citation13
Benchmarks can either be developed via consensus, derived from established national or international standards or published data or developed from existing data.Citation12,Citation14,Citation15 It is important that benchmarks and performance scores are comparable,Citation16 that is, measuring the same aspects of care by the same methods. Thus, to develop valid benchmarks for use as comparators for the iCareTrack results, the benchmarks should be derived or developed from clinical record audit results.
As a quality improvement activity, benchmarking has been shown to be effective for a range of health care disciplines,Citation14 and superior to audit and feedback alone.Citation17 As contemporary benchmarks for glaucoma care delivery by optometrists do not exist, this study aimed to develop benchmarks for glaucoma care delivery and to explore the iCareTrack results from the context of those benchmarks.
Methods
Benchmarks for glaucoma care were established via the Achievable Benchmarks of Care (ABC) method, a data-driven method for deriving benchmarks from the existing dataset.Citation12,Citation18
Appropriateness scores from the iCareTrack study were used to calculate the benchmarks. The justification for and the methods used to measure the appropriateness of glaucoma care in the iCareTrack study are reported elsewhere.Citation9 The iCareTrack study determined the appropriateness of glaucoma care by randomly sampling 420 glaucoma patient clinical records from 42 nationally representative optometry practices in Australia.Citation9 A randomised stratified sample of optometry practices was generated from a list containing the details of 2870 optometry practices from four states (New South Wales, Queensland, South Australia, and Victoria) that account for 84.3% of Australians. Ten sets of random alphabetical letter combinations were used as the starting point to search for eligible clinical records. Appropriate care was defined as care delivered in line with the clinical indicators which are the measurable components of guideline recommendations.Citation3,Citation19 The clinical indicators were derived from evidence-based glaucoma guidelines and refined by experts via Delphi process. Data existed for 37 iCareTrack clinical indicators that were stratified into the domains of history taking (17 indicators), physical examinations (12 indicators), recall (7 indicators) and referral (1 indicators). For each iCareTrack clinical indicator, the number of eligible patient encounters, number of encounters that both met and did not meet the clinical indicator criteria, and the percentage appropriateness score were extracted for each practice location into Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA).
Benchmarks were calculated using the four-step ABC method that establishes realistic and attainable benchmarks based on top performers.Citation12,Citation18 The ABC method is outlined in .
Table 1. Steps involved in calculating Achievable Benchmarks of Care.Citation12.
In step 1, a Bayesian estimator technique was used to adjust the iCareTrack appropriateness levels to limit the undue influence high-appropriateness scores based on small eligible patient encounter numbers might have on a final benchmark.Citation12,Citation18 This adjusted appropriateness level was the adjusted performance fraction (APF) which was calculated using the equation (x + 1)/(d + 2) where x is the number of patient encounters where the indicators were met, and d was the total number of eligible patient encounters.Citation12 For example, if a practice had 100% appropriateness from only one eligible patient encounter, the APF would be (1 + 1)/(1 + 2) = 0.67, while for another practice with 100% appropriateness derived from 10 patient encounters, the APF would be (10 + 1)/(10 + 2) = 0.92. shows the APF calculations for one example clinical indicator across the 42 practices assessed in the iCareTrack study.
Table 2. Ranking of locations by APF proportion (step 2) for iCaretrack clinical indicator of Peripheral anterior chamber assessment by gonioscopy or van Herick’s. Cumulative total of sequential addition of eligible patient encounters to determine a subset of 10% eligible patient encounters (shown in grey). ‘Yes’ indicates number of patient encounters that met indicator criteria and ‘No’ indicates number of eligible patient encounters that did not meet the indicator criteria for that indicator (step 2).
In step 2, for each clinical indicator, practice results were ranked in descending order of the APF. Starting with the best-performing practice, eligible patient encounters were sequentially totalled until the subset represented at least 10% of eligible patient encounters for that clinical indicator’s entire dataset. The benchmark percentage was calculated from the subset using the formula provided in step 4 of .
shows the results of ranking by APF proportion for one iCareTrack clinical indicator (P24, peripheral anterior chamber assessment by gonioscopy or van Herick’s). There were 420 eligible patient encounters in this dataset; thus, the subset must contain a minimum of 42 patient encounters. In this example, after adding the top five practices based on their performance in descending order, there were 50 patient encounters which met the minimum patient encounter requirement. Of these 50 patient encounters (4th column), 46 met the criteria (2nd column). Thus, the ABC is calculated as 46/50, which gives a benchmark of 92% appropriateness (final column).
Benchmarking iCareTrack results
The iCareTrack appropriateness scores for each practice location (n = 42) were compared to the developed benchmarks to examine the performance distribution. For comparison to the benchmarks, the practice locations were sorted into three categories based on their appropriateness scores: 1) at or better than the benchmark, 2) at or better than the average appropriateness score but worse than benchmark, and 3) worse than the average appropriateness score.
Results
describes the characteristics of the participating optometry practices and glaucoma patient records in the iCareTrack study.Citation9 Most practices were located in major cities (69%), were corporate practices (71%), and used electronic records (78%).Citation9
Table 3. Characteristics of the optometry practices (n = 42) and glaucoma patients (n = 42) in the iCaretrack study.Citation9.
Thirty-seven of 38 clinical indicators investigated in the iCareTrack study had data available for analysis. Benchmarks were calculated for 34 iCareTrack clinical indicators as two history taking clinical indicators (H2 Ethnicity, H13 Raynaud’s syndrome, see ) had a zero percent appropriateness score (that is, nil compliance) and a recall clinical indicator (RC32 Recall for high-risk glaucoma suspect who is treated and not at target IOP) had insufficient patient encounters to enable calculations (). The benchmarks for eight clinical indicators (RC30, RC31, RC32, RC33, RC34, RC35, RC36, RF37, see ) were calculated from small eligible patient encounter numbers. To limit the effect of small eligible patient encounter numbers, these seven recall indicators were combined to give a more robust combined recall benchmark indicator (RCC) (). The benchmarks for 31 (of 34) iCareTrack indicators were at or above 60% appropriateness with 26 indicators at or above 90% appropriateness (, last column). The benchmark for overall appropriateness indicated that glaucoma care could be realistically delivered at 74% appropriateness ().
Table 4. The iCaretrack appropriateness levels with corresponding Achievable Benchmarks of Care for glaucoma care delivery in Australia.
Benchmarking the iCaretrack clinical indicators
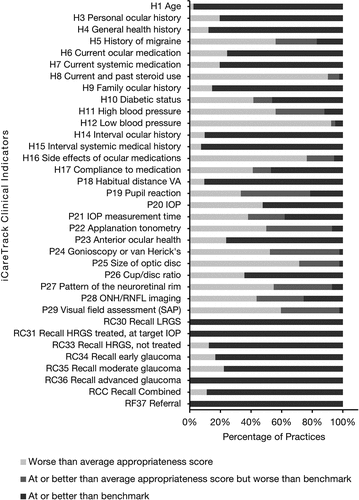
The benchmarks for 14 (of 34) iCareTrack indicators were met by at least 80% of eligible practices, while 18 (of 34) indicators were met by at least 60% of eligible practices (). However, for 10 iCareTrack indicators more than 80% of the eligible practices did not meet benchmarks (, bars H5 history of migraine, H8 current and past steroid use, H11 high blood pressure status, H12 low blood pressure status, H16 side effects of ocular medication, P22 applanation tonometry, P24 gonioscopy or van Herick’s, P25 size of optic disc, P27 pattern of neuroretinal rim, and P29 visual field assessment by standard automated perimetry (SAP)).
Figure 1. iCaretrack practice appropriateness scores benchmarked against the benchmarks for glaucoma care clinical indicators. The percentage of practices are shown for at or better than benchmarks (black), at or better than average appropriateness score but worse than the benchmark (dark grey), and worse than average appropriateness score (light grey).
Discussion
This study established benchmarks for 34 of the iCareTrack clinical indicators from a nationally representative sample of optometry practices using a valid and established method. The calculated benchmarks suggest that an improvement target for overall glaucoma care of an 11-percentage point increase on the iCareTrack results to an appropriateness of 74% is realistic and achievable for Australian optometry.
The large number of indicators (26 of 34) with benchmarks calculated at or above 90% indicates that substantial components of glaucoma care are already delivered by top-performing optometry practices at very high levels of appropriateness. Most (17 of 26) indicators with high benchmarks had correspondingly high iCareTrack appropriateness scores, indicating that a majority of optometry practices performed similarly to the top 10% of practices. Several indicators with low (<50%) iCareTrack appropriateness scores (e.g., applanation tonometry (P22), gonioscopy or van Herick’s (P24), and pattern of neuroretinal rim (P27)) had benchmarks calculated above 60%, indicating that the top 10% of optometry practices performed at a much higher level compared to the rest of the practices for these indicators. For indicators with extremely low appropriateness scores such as current and past steroid use (H8) (2%) and low blood pressure status (H12) (2%) benchmarks were calculated at an incremental increase (15% and 19%, respectively), making the benchmarks more realistic and attainable compared to a blanket approach that might set all benchmarks at an arbitrarily selected level. Establishing realistic and attainable benchmarks is necessary for quality improvement initiatives as practitioners may reject unachievable benchmarks and be discouraged from modifying their behaviour.Citation12,Citation13
Most (>80%) optometry practices met the benchmarks for 14 of the iCareTrack indicators whilst about two-thirds (>60%) of optometry practices met the benchmarks for 18 indicators. There were 10 iCareTrack indicators where most (>80%) optometry practices did not meet the benchmarks (H5, H8, H11, H12, H16, P22, P24, P25, P27, P29). These indicators related to identifying risk (H5 history of migraine, H8 current and past steroid use, H11 high blood pressure status, H12 low blood pressure status), identify side effects of treatment (H16 side effects of ocular medication) and detecting and monitoring glaucoma (P22 applanation tonometry, P24 gonioscopy or van Herick’s, P25 size of optic disc, P27 pattern of neuroretinal rim, P29 visual field assessment with SAP). Establishing realistic benchmarks for the iCareTrack clinical indicators and benchmarking performance is an important step in evaluating current glaucoma care delivery and setting goals for planning and assessing interventions to improve the appropriateness of glaucoma care. This study identified that several important glaucoma clinical indicators such as side effects of glaucoma medications (H16), gonioscopy or van Herick’s (P24), optic nerve head assessment (P25, P27), applanation tonometry (P22), and visual field assessment by SAP (P29) should be targeted for improvement.
Similar to this study, benchmarks established for Spanish ophthalmologists for eight glaucoma clinical indicators using the ABC method were all above 80% with five at 100%; the indicators with the benchmarks at 100% were for assessing IOP, gonioscopy, optic nerve head assessment, visual field assessments, and selecting monotherapy as first-line management.Citation20 Likewise, this study found comparably high benchmarks for IOP measurement (100%), cup–disc ratio assessment (100%), and gonioscopy or van Herick’s assessment (92%). However, the benchmark for visual field assessment was markedly lower in this study (62%) compared to the benchmark previously set for Spanish ophthalmologists (100%).Citation20 This difference could be attributed to the iCareTrack clinical indicator criteria that mandated that visual field assessment must be performed by SAP whilst this was not a requirement of the Spanish study. A fifth of Australian optometrists report not having access to SAP instead using frequency doubling technology instruments.Citation21 As a result, the average iCareTrack appropriateness score for visual field assessment by SAP was only 31%.
The strength of this study lies in the use of the ABC approach, which is a proven objective method of creating realistic, attainable, and clinically relevant benchmarks while limiting the effect of high-performers with low eligible patient encounters.Citation17,Citation20 In addition, the benchmarks were developed from a nationally representative sample, increasing the credibility of the benchmarks. Lastly, calculating benchmarks from real-world data and comparing the performance of the representative sample against the benchmarks pinpointed areas of opportunity to enhance glaucoma care delivery by Australian optometrists.
Limitations of this study must also be acknowledged. The accuracy of the ABC approach is affected when calculations are made using small eligible patient samples.Citation12 The benchmarks for seven clinical indicators in this study (six recall, one referral) were calculated from small samples (<20) and therefore should be viewed with caution. The small number of eligible patient encounters is a common issue in performance measurement studiesCitation22,Citation23 that may result in benchmarks that are not entirely realistic or attainable. To overcome this limitation for the recall domain, the data was pooled. Another limitation is that the iCareTrack clinical indicators are predominantly for open-angle glaucoma care. While less prevalent than open-angle glaucoma,Citation24 angle closure glaucoma accounts for nearly half of all vision impairment associated with glaucoma.Citation25 Furthermore, the glaucoma care clinical indicators were developed for the iCareTrack study period of 2013–2014, and as a result, a few clinical indicators may not be aligned with current international standards for evidence-based glaucoma care. For example, the referral indicator for early glaucoma (RC34) specifies recall within 12 months and this is no longer in line with contemporary guideline recommendations. Current guidance recommends that recall frequency should be determined by the rate of progression, which is established by conducting three visual field assessments with SAP per year for the first 2 years following diagnosis.Citation26 An update of the iCareTrack clinical indicators to include specific indicators for angle closure glaucoma care and to reflect contemporary glaucoma care is warranted. Finally, the benchmarks calculated in this study reflect glaucoma care provided by optometrists in an Australian context and may not be generalisable to optometrists in other countries.
This benchmarking exercise identified variations in glaucoma care delivery where opportunity exists to undertake remedial action. Due to the essential role optometrists play in glaucoma primary care in Australia, optimising the appropriateness of glaucoma care delivery at this point of the care cycle will lead to improvements in detection rates and facilitate timely initiation of treatment. These, in turn, will improve patients’ vision-related quality of life and reduce the societal economic burden of glaucoma.Citation6 Benchmarks can be incorporated into audit and feedback quality improvement interventions,Citation27,Citation28 as the addition of benchmarking leads to results superior to audit and feedback alone.Citation17 The use of a benchmark simplifies the feedback message as it provides an explicit level of care to attain and indicates if remedial action is required.Citation13
The realistic benchmarks established in this study will be used as comparators in a bespoke online glaucoma care self-audit tool where optometrists would complete several self-audit cycles over a defined time period. The tool would encourage optometrists to reflect on the level of glaucoma care they provide compared to the benchmarks and to set improvement goals. As quality improvement should be a continuous activity, it is expected that once optometrists meet the benchmark they would strive to exceed it. These benchmarks should also be updated periodically to reflect improvements made in glaucoma care delivery as a result of optometrists engaging in the quality improvement initiatives and to remain relevant to contemporary practice.
In conclusion, the developed benchmarks served as comparators that provided a greater understanding of appropriateness of glaucoma care by identifying large areas of excellence and small pockets of sub-optimal care where improvements would be desirable. The high number of iCareTrack clinical indicators with benchmarks at or above 90% confirmed that glaucoma care can be delivered by optometrists at very high levels of appropriateness. These benchmarks represent realistic and attainable goals for glaucoma care that optometrist can use as comparators to their own performance and to determine if and where remedial action may be required.
Acknowledgements
Melinda Toomey was supported through an Australian Government Research Training Program Scholarship and a University of New South Wales (UNSW) Scientia PhD Scholarship. Rajendra Gyawali was supported through a UNSW Scientia PhD Scholarship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Flaxman SR, Bourne RRA, Resnikoff S et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017; 5: e1221–1234. doi:10.1016/S2214-109X(17)30393-5.
- Keel S, Xie J, Foreman J et al. Prevalence of glaucoma in the Australian National eye health survey. Br J Ophthalmol 2019; 103: 191–195. doi:10.1136/bjophthalmol-2017-311786.
- Hunt TD, Ramanathan SA, Hannaford NA et al. CareTrack Australia: assessing the appropriateness of adult healthcare: protocol for a retrospective medical record review. BMJ Open 2012; 2: e000665. doi:10.1136/bmjopen-2011-000665.
- Kass MA, Heuer DK, Higginbotham EJ et al. The Ocular Hypertension Treatment Study: a randomised trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 701–713. doi:10.1001/archopht.120.6.701.
- Heijl A, Leske M, Bengtsson B et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120: 1268–1279. doi:10.1001/archopht.120.10.1268.
- Varma R, Lee PP, Goldberg I et al. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol 2011; 152: 515–522. doi:10.1016/j.ajo.2011.06.004.
- Fritz JM, Cleland JA, Brennan GP. Does adherence to the guideline recommendation for active treatments improve the quality of care for patients with acute low back pain delivered by physical therapists? Med Care 2007; 45: 973–980. doi:10.1097/MLR.0b013e318070c6cd.
- Ho KC, Stapleton F, Wiles L et al. Systematic review of the appropriateness of eye care delivery in eye care practice. BMC Health Serv Res 2019; 19: 646. doi:10.1186/s12913-019-4493-3.
- Ho KC, Stapleton F, Wiles L et al. iCaretrack: measuring the appropriateness of eyecare delivery in Australia. Ophthalmic Physiol Opt 2020; 40: 433–441. doi:10.1111/opo.12699.
- Joint Commission Resources. Benchmarking in health care. 2nd ed. Oakbrook Terrace (USA): Joint Commission Resources; 2012.
- Shepherd N, Meehan TJ, Davidson F et al. An evaluation of a benchmarking initiative in extended treatment mental health services. Aust Health Rev 2010; 34: 328–333. doi:10.1071/AH09698.
- Kiefe CI, Weissman NW, Allison JJ et al. Methodology matters-XII. Identifying achievable benchmarks of care: concepts and methodology. Int J Qual Health Care 1998; 10: 443–447. doi:10.1093/intqhc/10.5.443.
- Gude WT, Brown B, van der Veer SN, et al. Clinical performance comparators in audit and feedback: a review of theory and evidence. Implement Sci 2019; 14: 39. [accessed 2021 Nov 13]. implementationscience.biomedcentral.com/article/10.1186/s13012-13019-10887-13011.
- Thonon F, Watson J, Saghatchian M. Benchmarking facilities providing care: an international overview of initiatives. SAGE Open Med 2015; 3: 2050312115601692. doi:10.1177/2050312115601692.
- Ellis J. Sharing the evidence: clinical practice benchmarking to improve continuously the quality of care. J Adv Nurs 2000; 32: 215–225. doi:10.1046/j.1365-2648.2000.01429.x.
- Vogelezang F, Heeringen H. Benchmarking: comparing apples to apples. In: Sadowski C Zimmermann T, editors. Rethinking productivity in software engineering. Berkeley (CA): Apress; 2019. p. 205–217.
- Kiefe CI, Allison JJ, Williams OD et al. Improving quality improvement using achievable benchmarks for physician feedbackA randomized controlled trial. JAMA 2001; 285: 2871–2879. doi:10.1001/jama.285.22.2871.
- Weissman NW, Allison JJ, Kiefe CI et al. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract 1999; 5: 269–281. doi:10.1046/j.1365-2753.1999.00203.x.
- Runciman WB, Coiera EW, Day RO et al. Towards the delivery of appropriate health care in Australia. Med J Aust 2012; 197: 78–81. doi:10.5694/mja12.10799.
- Castejón-Cervero MA, Jiménez-Parras R, Fernandez-Arias I et al. Evaluation of compliance with the EGS guidelines in Spain, using Achievable Benchmarks of Care (ABC®) methodology: the IMCA Study. Eur J Ophthalmol 2011; 21: 149–155. doi:10.5301/EJO.2010.5973.
- Jamous KF, Kalloniatis M, Hayen A et al. Application of clinical techniques relevant for glaucoma assessment by optometrists: concordance with guidelines. Ophthalmic Physiol Opt 2014; 34: 580–591. doi:10.1111/opo.12146.
- O’Brien SM, Delong ER, Peterson ED. Impact of case volume on hospital performance assessment. Arch Intern Med 2008; 168: 1277–1284. doi:10.1001/archinte.168.12.1277.
- Arling G, Reeves M, Ross J et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration Medical Centers. Circ Cardiovasc Qual Outcomes 2012; 5: 44–51. doi:10.1161/CIRCOUTCOMES.111.961474.
- Tham YC, Li X, Wong TY et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014; 121: 2018–2090. doi:10.1016/j.ophtha.2014.05.013.
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006; 90: 262–267. doi:10.1136/bjo.2005.081224.
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol 2021; 105: 1. doi:10.1136/bjophthalmol-2021-egsguidelines.
- Ivers N, Sales A, Colquhoun HL et al. No more ‘business as usual’ with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implement Sci 2014; 9: 14. doi:10.1186/1748-5908-9-14.
- Gude WT, Roos-Blom M-J, van der Veer SN et al. Health professionals’ perceptions about their clinical performance and the influence of audit and feedback on their intentions to improve practice: a theory-based study in Dutch intensive care units. Implement Sci 2018; 13: 33. doi:10.1186/s13012-018-0727-8.
