ABSTRACT
Clinical relevance
Patients prescribed pilocarpine ophthalmic solution are advised to be cautious when driving at night, but studies evaluating the effects of pilocarpine hydrochloride ophthalmic solution 1.25% (pilo), approved to treat presbyopia, on driving at night are lacking.
Background
This double-masked, crossover, phase 3b study evaluated night-driving performance with pilo or the placebo once daily.
Methods
Forty-three adults (40–55 years) with presbyopia impacting daily activities and mesopic, high-contrast, binocular distance-corrected near vision 6/12–6/30 were randomised to bilateral treatment with pilo followed by placebo or placebo followed by pilo (with a ≥7-day washout between interventions). Night-driving performance was evaluated at twilight at a closed-circuit course. Primary efficacy endpoint: overall composite night-driving performance Z score at the end of the 7–14-day intervention period, 1 hour post-instillation. Pilo was considered non-inferior if the lower limit of the 95% confidence interval (CI) for the least squares mean difference (LSMD, pilo minus placebo) was >–0.25. Other efficacy endpoints: individual components of the night-driving performance test (hazard avoidance rate; road sign recognition rate and distance; pedestrians recognition distance; overall driving and lane-keeping times) and night-driving experience questionnaire. Safety included treatment-emergent adverse events (TEAEs).
Results
The mean overall composite Z scores were −0.121 (pilo) and 0.118 (placebo). The LSMD (pilo minus placebo) was −0.224 (95% CI, −0.346, −0.103), with 3 of the 7 individual tasks being significantly better with the placebo. The questionnaire did not reveal significant differences between pilo and the placebo. There were no serious or severe TEAEs and no TEAE-related discontinuations. The most common ocular TEAEs were headache and visual impairment with pilo (both 27.9%), and dry eye (7.0%) with the placebo.
Conclusion
The overall performance of night driving was inferior with pilo, compared with placebo. The study findings are consistent with the current class labelling and provide evidence to inform regulators and assist clinicians considering prescribing pilo to adults who seek treatment of presbyopia symptoms and drive at night.
ClinicalTrials.gov identifier: NCT04837482.
Introduction
Presbyopia is an age-related condition with progressive reduction in lens accommodation that leads to near-vision impairment (blurred vision)Citation1 and affects daily activities such as checking a cell phone screen or watch, reading labels on various products, and recognising people in photos.Citation2 As it progresses, presbyopia eventually impairs intermediate vision as well,Citation3 affecting activities such as working on a computer screen or in the kitchen.Citation4 Considering that the world population continues to ageCitation5 and presbyopia is inevitable,Citation2 projections indicate that 1 in 5 individuals will have presbyopia by 2050.Citation6
Until recently, there were no pharmacologic treatments specifically designed for treating presbyopia. In October 2021, an ophthalmic solution of pilocarpine hydrochloride (HCl) 1.25% in a proprietary (pHastCitation7) formulation (Vuity®; Allergan, an AbbVie company) was approved by the United States Food and Drug Administration for once-daily use. In March 2023, pilocarpine HCl 1.25% was approved for the option of twice-daily use.Citation8,Citation9 Approval of once-daily use (relevant to the current study) was based on results from the randomised, double-masked, controlled, phase 3Citation10 GEMINI 1Citation11 and GEMINI 2 (ClinicalTrials.gov identifier: NCT03857542) studies, which established the safety and efficacy of pilocarpine HCl 1.25% dosed bilaterally, once daily for 30 days in participants with presbyopia. Findings showed significantly greater proportions of participants gaining ≥3 lines in mesopic, high-contrast, binocular distance-corrected near visual acuity (DCNVA) at Day 30, Hours 3 (primary endpoint) and 6 (key secondary endpoint) with pilocarpine HCl 1.25% once daily than the placebo once daily. Notably, pilocarpine HCl 1.25% once daily was also shown to reduce pupil size, consistent with its mechanism of action,Citation11 and to have an acceptable safety profile, without any reports of serious adverse events.Citation8,Citation11
Constricting the pupil with pilocarpine or other miotic agent reduces retinal illumination and may potentially affect activities performed in dim lighting, such as driving at night. Patients using miotics to treat glaucoma are thus advised to exercise caution when driving at night and performing other potentially hazardous activities/occupations in poor illumination.Citation12 Per requirement by the United States Food and Drug Administration, the label of pilocarpine HCl 1.25% also includes these warnings and precautions.Citation8 However, studies evaluating the actual impact of pilocarpine on night-driving performance have not been conducted (to our knowledge).
This study evaluated night-driving performance in real-world driving conditions (without artificial ambient lighting), as well as safety in participants with presbyopia using pilocarpine HCl 1.25% bilaterally once daily, compared with the placebo once daily.
Methods
Study design
This randomised, double-masked, single-centre, crossover, phase 3b study (ClinicalTrials.gov identifier: NCT04837482) was conducted in Australia between 14 May 2021 and 7 December 2021, in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines; applicable regulations and guidelines governing clinical study conduct (including the Good Clinical Practice guidelines); and ethical principles of the Declaration of Helsinki.
The study protocol was approved by an institutional review board/independent ethics committee prior to study start, and all participants provided written informed consent before screening or initiation of any study-related procedures.
Study population
The complete list of eligibility criteria is available in Supplemental Table S1. At screening (visit 1, 1‒30 days before randomisation), eligible participants (40–55 years of age) with a valid driver’s licence (per local regulations) were in good general health and had objective and subjective evidence of presbyopia; complaints of poor near vision (without near correction) impacting daily activities (ie, score ≥3 on at least 1 question among questions 5‒7 of the main National Eye Institute Visual Function questionnaire 25, or questions A3‒A5 of the National Eye Institute Visual Function questionnaire 25 near-vision subscale); photopic (≥251 lux at target), high-contrast habitual distance visual acuity of 6/9.5 or better bilaterally; mesopic (10–11 lux at target), high-contrast DCNVA of 6/12 to 6/30 (measured at 40 cm); photopic, near visual acuity correctable to 6/12 or better bilaterally; and were willing to wear monofocal correction (either spectacles or contact lenses) to achieve photopic, binocular, corrected distance visual acuity (CDVA) of 6/9.5 or better during the study.
Key exclusion criteria included presence of severe dry eye disease; abnormal or clinically significant results (per the investigator or designee) on dilated ophthalmic examination (eg, retinal findings) or medical history; history of intraocular surgery, except photorefractive keratectomy or laser-assisted in situ keratomileusis; diagnosis of ocular hypertension or any type of glaucoma; and anisocoria >1 mm between pupils under mesopic conditions.
Randomisation and masking
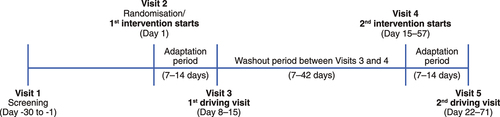
As shown in , on Day 1 (visit 2), participants were randomised in a 1:1 ratio to one of 2 study intervention sequences. Intervention sequence #1 involved use of pilocarpine HCl 1.25% once daily first, followed by a minimum washout period of 1 week and then use of the placebo once daily. Intervention sequence #2 involved use of the placebo once daily first, followed by a minimum washout period of 1 week and then use of pilocarpine HCl 1.25% once daily. The randomisation sequence was computer generated, and an automated interactive electronic response system was used to assign participants to study interventions centrally. Both interventions were provided in identically appearing bottles, masking both participants and study investigators/staff to the intervention assignment.
Intervention administration and study visits
After randomisation on Day 1 (visit 2; ), the first dose of the first intervention of each patient was administered bilaterally by site personnel. Participants were instructed to continue bilateral once-daily dosing (between 8 AM and 10 AM) at home for 7–14 days, until the first driving test visit (visit 3, which could occur on any day from Day 8 to 15 of the overall study). After the washout period (which could span 7 to 42 days), the first dose of the second intervention of each patient was administered bilaterally by site personnel on visit 4 (which could occur on any day from Day 15 to 57 of the overall study). Participants were again instructed to continue bilateral dosing at home (as described above) for 7–14 days, until the second driving test visit (visit 5, which could occur on any day from Day 22 to 71 of the overall study).
For each intervention, the time between the first dose and driving test was 7 to 14 days. On the day of the driving tests, the eyedrops were administered by the site staff 1 hour (±15 minutes) before the test, based on previous findings showing peak pupil constriction 1 hour after administration of pilocarpine HCl 1.25%.Citation11
Assessments
At screening (visit 1) and both driving visits (visits 3 and 5, before the driving tests), binocular habitual distance visual acuity was assessed at 4 metres (pre- and post-dosing if applicable), using logMar charts in mesopic and photopic conditions under normal room lighting. Testing was conducted with the optimal distance refractive correction. Forced choice letter-by-letter scoring was used for each test and the total number of letters correctly identified was recorded for each eye. Intraocular pressure was measured in both eyes at screening and on the first day of dosing of each intervention (ie, visits 2 and 4). A slit lamp biomicroscopy examination was performed at each visit (including pre- and post-dosing on driving visits). Adverse events were also recorded at each visit.
On driving visits, 1 hour (±15 min) following instillation of the study intervention, visual function was assessed to ensure that habitual distance visual acuity was 6/9.5 or better; participants who did not have monofocal correction of 6/9.5 or better in both eyes were provided with monofocal spectacles by the study site. Progressive addition lenses were prohibited.
The driving tests were then conducted under real-world, night-light conditions at a closed-road driving circuit that was representative of a rural roadCitation13 and consisted of hills, bends, curves, intersections, straight sections, standard road signs, and lane marking (Supplemental Figure S1). Both driving tests were conducted when the road surface was dry (not on rainy days). The driving circuit did not include artificial ambient lighting and night-driving performance was assessed after nautical twilight (beginning at sunset and ending when the centre of the sun is 12° below the horizon).
Participants were provided with standard instructions regarding the driving circuit and tasks to be completed, and were allowed to familiarise themselves with the instrumented vehicle for 5 min before initiating each test. The night-driving performance metrics included road sign recognition, driving time, lane-keeping, pedestrian recognition, overall composite night-driving performance Z score, and nightdriving experience questionnaire. A detailed description of each assessment is presented in Supplemental Table S2.
Outcome measures
The primary efficacy endpoint was the overall composite night-driving performance Z scoreCitation13–16 at approximately 1 hour post-instillation of the study intervention, calculated using the average of Z scores for the following components: hazards hit (%), road sign recognition (%) and recognition distance, pedestrian recognition distances (pedestrians 1 and 2), overall driving time, and time outside of the driving lane (%). A higher Z score indicated better driving performance.Citation14 For each participant, the Z score for each component/task of the driving test was the difference between his/her value and mean value of the assessments of all participants from the pooled data of both periods combined, divided by the standard deviation of all corresponding assessment values.
Because a lower percentage of hazards hit, lower percentage of time outside of the driving lane, and a shorter lap duration indicate better driving performance, the Z scores of these components were reversed (ie, multiplied by −1) before computing the overall driving Z score.Citation17 Non-inferiority of pilocarpine HCl 1.25% was claimed if the lower limit of the 95% confidence interval of the least squares mean difference (pilocarpine HCl 1.25% ‒ placebo) was above −0.25.
Other efficacy endpoints included each individual component of the night-driving performance test, expressed as percentages or distances, as well as response to a night-driving experience questionnaire, expressed in terms of number and frequency per category for each question.
Safety measures included the incidence of adverse events, mesopic and photopic habitual distance visual acuity over time, and the proportion of participants with a severity increase in biomicroscopy findings from baseline >1 grade (for findings typically graded) or a change from absence at baseline to presence post-baseline (for findings not graded).
Statistical analysis
The sample size calculation was based on the assumption that there was no difference in the primary efficacy endpoint (defined above) between intervention groups; a variance of 0.11; and an anticipated dropout rate ≤25%. Considering that at least 40 participants completing the study was required to obtain 90% power in a 1-sided non-inferiority test of the mean overall composite night-driving performance Z score with a type-1 error of 0.025 and non-inferiority margin of −0.25 units, enrolment of 54 participants was planned. The non-inferiority test for the primary endpoint was the only formally specified hypothesis testing in the study. P values for other comparisons were provided for reference only, without adjustment for multiplicity.
The intent-to-treat population (ie, all randomised participants) was used for efficacy analyses, including an ad hoc analysis of the overall composite night-driving performance Z score and individual components by age (≤50 vs >50 years). The safety population included all participants who received ≥1 dose of study intervention.
Analyses were performed without imputation of missing values. Categorical variables were summarised by frequency and percentage. Continuous variables were summarised by mean, standard deviation, and median. The composite night-driving performance Z score was analysed using linear mixed-effects model with repeated measures, using study intervention group, intervention sequence, and study period as fixed effects, and participant nested within sequence as random effect. An unstructured covariance matrix was used to model the within-participant correlation error.
Results
Study population disposition, demographics, and baseline characteristics
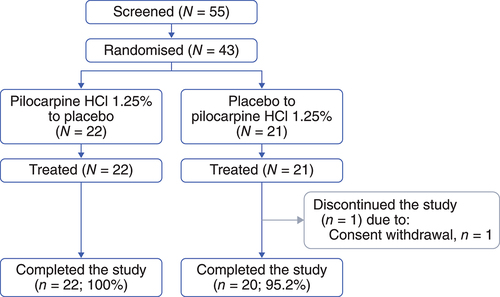
Due to the low dropout rate, 55 individuals were screened, and enrolment was stopped at 43 participants who were randomised (pilocarpine HCl 1.25% to placebo, N = 22; placebo to pilocarpine HCl 1.25%, N = 21) and treated. Forty-two (97.7%) completed the study. One (2.3%) participant withdrew consent and discontinued the study without completing treatment with pilocarpine HCl 1.25% once daily ().
At baseline, 42 (97.7%) participants had photopic, high-contrast, binocular DCNVA of 6/19 or better, and 29 (67.4%) had mesopic, high-contrast DCNVA of 6/19 or better. Overall, patient demographics and baseline characteristics were balanced between the pilocarpine HCl 1.25% to placebo and placebo to pilocarpine HCl 1.25% groups ().
Table 1. Participant demographics and characteristics at baseline.
Intervention duration
The mean (standard deviation) adaptation period between the start of the pilocarpine HCl 1.25% intervention and driving test was 9.8 (2.9) and 9.1 (2.0) days in the first (pilocarpine HCl 1.25% to placebo) and second (placebo to pilocarpine HCl 1.25%) intervention sequence, respectively. The mean (standard deviation) adaptation period between the start of the placebo and driving test was 10.2 (3.1) and 10.4 (3.5) days in the first (pilocarpine HCl 1.25% to placebo) and second (placebo to pilocarpine HCl 1.25%) intervention sequence, respectively. Overall, pilocarpine HCl 1.25% and the placebo were used for 9.5 (2.5) and 10.3 (3.3) days, respectively, before the driving tests.
Primary efficacy endpoint
The mean (standard deviation) overall composite night-driving performance Z score was −0.121 (0.450) with pilocarpine HCl 1.25% and 0.118 (0.401) with the placebo (). The least squares mean difference between pilocarpine HCl 1.25% and the placebo was −0.224, and the lower limit of the 95% confidence interval was below −0.25, at −0.346 (). This finding indicated that participants did not perform as well in the night-driving performance test after using pilocarpine HCl 1.25%, compared with the placebo.
Table 2. Overall composite night-driving performance driving Z score following use of pilocarpine HCl 1.25% once daily or the placebo.
Other efficacy endpoints
When analysing each component of the overall night-driving performance test individually, numerical differences between pilocarpine HCl 1.25% and the placebo were noted (), with the rate of road sign recognition, recognition distance of pedestrian #2, and total driving time being significantly better with the placebo than pilocarpine HCl 1.25% (P = 0.0304, P = 0.0231, and P = 0.0016, respectively).
Table 3. Individual components of the night-driving performance test following use of pilocarpine HCl 1.25% once daily or the placebo.
For the night-driving experience questionnaire, measures of the frequency of double images, and difficulties seeing hazards on the road, reading road signs, and judging distances were numerically greater with the placebo, whereas reading car gauges (eg, speedometer) without eye straining, and adjusting vision when looking from the road to the speedometer and back were numerically greater with pilocarpine HCl 1.25% (Supplemental Table S3). However, none of the differences were significant (P ≥ 0.0747). Overall, 76.2% (pilocarpine HCl 1.25%) and 83.7% (placebo) of participants reported that it was not difficult at all to see during the night-driving test, 14.3% (pilocarpine HCl 1.25%) and 14.0% (placebo) of participants reported that it was a little difficult, and 9.5% (pilocarpine HCl 1.25%) and 2.3% (placebo) of participants reported that it was moderately difficult, respectively (P = 0.1866; Supplemental Table S3).
Ad hoc analysis of night-driving performance by age group
When analysing the overall composite night-driving performance Z score in subgroups of participants who were ≤50 vs >50 years of age, a significant difference favouring the placebo was observed in the ≤50 subgroup (mean [standard deviation], −0.300 [0.084]; 95% confidence interval, −0.475, −0.125; P = 0.0019), not the >50 subgroup (mean [standard deviation], −0.136 [0.086]; 95% confidence interval, −0.316, 0.044; P = 0.1307).
When analysing each individual component of the night-driving performance test in the aforementioned age subgroups, the pilocarpine HCl 1.25% minus placebo difference in road sign recognition distance was significantly different, favouring the placebo, in participants who were ≤50 years of age (mean [standard deviation], −17.9 [4.7]; 95% confidence interval, −27.8, −8.0; P = 0.0012), whereas the between-intervention differences in recognition distance of pedestrian #2 (mean [standard deviation], −37.0 [15.7]; 95% confidence interval, −70.0, −3.9; P = 0.0304) and total driving time (mean [standard deviation], 20.3 [6.1]; 95% confidence interval, 7.5, 33.1; P = 0.0038) were significantly different, favouring the placebo, in participants who were >50 years of age.
Findings of the night-driving experience questionnaire did not reveal any significant differences between pilocarpine HCl 1.25% and the placebo in either age subgroup (P ≥ 0.1035).
Safety
Overall, treatment-emergent adverse events were reported in 74.4% (pilocarpine HCl 1.25%) and 18.6% (placebo) of participants (). The majority of participants had treatment-emergent adverse events that were ocular in nature (pilocarpine HCl 1.25%, 90.6%; placebo, 75.0%) and mild in severity (pilocarpine HCl 1.25%, 62.5%; placebo, 100%). None were severe or serious and none led to discontinuation of the study intervention.
Table 4. TEAEs overall by category (upper portion) and listing of TEAEs reported in >1 participant in one group (lower portion).
With pilocarpine HCl 1.25%, the most commonly reported treatment-emergent adverse events were headache (27.9%), visual impairment (27.9%), eye pain (18.6%), and vision blurred (16.3%), all of which had a reasonable possibility of being intervention-related (per investigator judgement). With the placebo, no headaches were reported, all ocular treatment-emergent adverse events had a reasonable possibility of being intervention-related (per investigator judgement), except vision blurred and blepharitis (n = 1 each), and the only treatment-emergent adverse events reported in more than 1 participant was dry eye (7.0%) ().
As a treatment-emergent adverse event of special interest, all headaches reported in participants using pilocarpine HCl 1.25% were mild (75.0%, n = 8/12) or moderate (25.0%, n = 4/12) in severity, all but 1 started on Day 1 and were intermittent, 75.0% did not require medication, all were resolved without sequalae at study end, and none led to discontinuation of the intervention (Supplemental Table S4).
The second treatment-emergent adverse event of special interest, visual disturbances (eg, dimming, distortion, and jumping), was reported in 46.5% (pilocarpine HCl 1.25%) and 4.7% (placebo) of participants (Supplemental Table S4). Overall, 86.4% were mild (none were severe), 77.3% were intermittent, none required medication, all were resolved without sequalae at study end, and none led to discontinuation of the intervention (Supplemental Table S4).
Notably, the mean (standard deviation) pre-dose to post-dose change in monofocal habitual distance visual acuity was not clinically meaningful following intervention with pilocarpine HCl 1.25% (mesopic, −0.9 [4.9] letters; photopic, −1.5 [4.5] letters) or the placebo (mesopic, 1.0 [2.5] letters; photopic, 0.6 [1.8] letters). Two participants (4.7%) had a clinically significant finding (corneal staining) on slit lamp biomicroscopy with pilocarpine HCl 1.25%. There were no clinically significant biomicroscopy findings following intervention with the placebo, and no clinically significant fundoscopy findings with either intervention.
Discussion
In this study, the primary efficacy endpoint was not met and the overall driving performance at night in real-world conditions was inferior with pilocarpine HCl 1.25%, compared with the placebo. This finding has significance for adults who drive at night while using pilocarpine HCl 1.25% for treatment of symptoms associated with presbyopia.
Overall, the study findings are clinically important as this is the first study evaluating night-driving performance of the only eye drop currently approved to treat presbyopia. In the absence of a comparator eye drop, standards previously established by the American National Standards Institute to assess night-driving performance of multifocal vs monofocal intraocular lensesCitation18 were used as guidelines to interpret our findings. The American National Standards Institute indeed set the non-inferiority margin at 25%, meaning that the performance difference between the multifocal and monofocal intraocular lenses was deemed acceptable if ≤25% for recognition distances.Citation18 Considering these historical data, and vision loss ranging from 0.2% to 13.3% (vs placebo) for the individual tasks of road sign and pedestrian recognition distances, night-driving performance while using pilocarpine HCl 1.25% was well within the acceptable, American National Standards Institute-defined margin.
Whether presbyopia is corrected surgically or not, different optical means represent different opportunities and limitations/compromises that can impact night-driving performance (among other activities).Citation19–24 Since pilocarpine HCl 1.25% works by constricting the pupil,Citation8,Citation11 some loss of light perception was expected following dosing, and our findings confirmed it in a live driving test conducted in real-world conditions. However, the loss resulted in differences (pilocarpine HCl 1.25% minus placebo) that were considerably smaller than 25%. The study findings thus provide evidence to inform regulators and assist clinicians who are considering prescribing pilocarpine HCl 1.25% to adults who seek treatment of presbyopia symptoms and drive at night, while being consistent with the current warning on the product label.Citation8
When analysing each task included in the overall night-driving performance test individually, significant differences between pilocarpine HCl 1.25% and the placebo were observed for 3 of the 7 tasks. In addition, the night-driving performance questionnaire did not reveal any significant differences between interventions. It is also worth noting that the night-driving performance test was conducted 1 hour following intervention administration, ie, peak constriction time for pilocarpine HCl 1.25%. Had the test been conducted at Hour 3 (primary timepoint in the GEMINI 1 studyCitation11), the differences between pilocarpine HCl 1.25% and placebo reported herein for the individual driving-related tasks and overall night-driving performance score would likely have been reduced.
A potential limitation of the study is that the intervention period leading to each driving test was not fixed. Although the time between first administration of pilocarpine HCl 1.25% and driving at night is likely to vary among users in real life, the numerically shorter adaptation period with pilocarpine HCl 1.25% observed herein (compared with the placebo) could have negatively impacted outcomes, based on GEMINI 1 study findings indicating that the proportion of participants gaining ≥3 lines in mesopic, high-contrast, binocular DCNVA at Hour 1 increased between Day 7 (39.5%) and Day 14 (50.6%).Citation25 In other words, if vision improves between Day 7 and Day 14 when participants use pilocarpine HCl 1.25%, using it for at least 1 more day (eg, 10.5 days instead of the reported 9.5 days) might have resulted in better outcomes with pilocarpine HCl 1.25% and thus smaller differences, compared with the placebo.
Since most cars are now equipped with a navigation system with digital display, it would have been informative to question the study participants about the ease of use of a car navigation system. However, the answer to such question would likely be similar to that regarding the ease of reading car gauges without eye straining (for example), as both tasks would involve the same back-and-forth between the instruments and the road.
Conclusion
The overall driving performance at night in real-world conditions was reduced when the study participants used pilocarpine HCl 1.25% to manage their presbyopia (compared with the placebo) and the driving test was performed 1 hour post-administration (peak time for pupil constriction induced by pilocarpine HCl 1.25%). This has implications for presbyopic adults driving at night after using pilocarpine HCl 1.25% to manage their presbyopia, in line with the current label advising pilocarpine HCl 1.25% users to exercise caution when driving at night.
The road sign and pedestrian recognition distances, however, were well below the American National Standards Institute-defined, historic 25% cut-off within which differences are deemed acceptable. Similarly, the night-driving performance questionnaire showed no significant differences between pilocarpine HCl 1.25% and the placebo. Pilocarpine HCl 1.25% was also safe and well tolerated, consistent with a previous report.Citation11
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) authorship criteria, take responsibility for the integrity of the work as a whole, and have given approval for this version to be published.
Compliance with ethics guidelines
The Independent Ethics Committee or Institutional Review Board approved the study protocol, informed consent forms, and recruitment materials before patient enrolment. The study was conducted in accordance with the International Council for Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki. All patients provided written informed consent before screening.
Figure S1
Download PDF (125.5 KB)Table S1
Download PDF (117.2 KB)Table S2
Download PDF (84.8 KB)Table S3
Download PDF (136.7 KB)Table S4
Download PDF (79.2 KB)Acknowledgements
We acknowledge the following researchers (institutions) for their contribution to the study: Prof. Joanne Wood (Centre for Accident Research and Road Safety in the School of Optometry and Vision Science, Queensland University of Technology in Australia) and Associate Prof. Alex Black (School of Optometry and Vision Science, Queensland University of Technology in Australia). The authors would also like to thank the study participants. Medical writing support was provided by Michele Jacob, PhD, of Evidence Scientific Solutions, Inc (Philadelphia, PA, USA) and funded by AbbVie.
Disclosure statement
Financial arrangements of the authors with companies whose products may be related to the present report follow, as declared by the authors. George O Waring IV: consultant for Allergan (an AbbVie company). Mile Brujic: speaker, consultant, researcher, and/or advisor for ABB Optical, Alcon, Allergan (an AbbVie company), Art Optical, Bausch + Lomb Health, Blephex, Contamac, CooperVision, CSEye, Euclid, Eyevance, Horizon Therapeutics, Johnson & Johnson Vision Care, Kala Pharmaceuticals, Luneau, Novartis, Optovue, Oyster Point, RVL, Sight Sciences, Sun Pharmaceutical, Tangible Science, Tarsus, Walman Optical, and Zea Vision. Selina McGee: consultant for Allergan (an AbbVie company), Bausch + Lomb, Bruder, Cynosure, Dompe, EyeVance, Horizon Therapeutics, Kala Pharmaceuticals, Lumenis, Novartis, Ocuphire, Optovue, Osmotica, Oyster Point, Sun Pharmaceutical, Tarsus, Thea, and Versant. J Morgan Micheletti: consultant and/or speaker for Alcon, Allergan (an AbbVie company), Avelino, Bausch + Lomb, BVI, Centricity, Diamatrix, Glaukos, Johnson & Johnson, Lenstec, New World Medical, RXSight, STAAR, Tarsus, Visus Therapeutics, and Zeiss; research grants from Alcon and Johnson & Johnson; patent rights with Diamatrix. Cathy Zhao, Scott Schachter, Haixia Liu, and Eleonora Safyan are employees of AbbVie and may hold AbbVie stock/options.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymised, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicenced products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08164622.2023.2279189.
Additional information
Funding
References
- Grzybowski A, Ruamviboonsuk V. Pharmacological treatment in presbyopia. J Clin Med 2022; 11: 1385. doi:10.3390/jcm11051385.
- Institute for Quality and Efficiency in Health Care (IQWiG). Presbyopia: overview. [accessed 2023 Aug 14]. https://www.ncbi.nlm.nih.gov/books/NBK423833/?report=reader#_NBK423833_pubdet.
- American Optometric Association. Optometric clinical practice guideline - care of the patient with presbyopia. [ accessed 2023 Aug 14]. https://www.sdeyes.org/docs/CPG-17.pdf.
- Institute for Quality and Efficiency in Health Care (IQWiG). How can presbyopia be corrected? [accessed 2023 Aug 14]. https://www.ncbi.nlm.nih.gov/books/NBK423827/?report=reader.
- United Nations Department of Economic and Social Affairs. World population ageing 2020 highlights. [accessed 2023 Aug 14]. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Sep/un_pop_2020_pf_ageing_10_key_messages.pdf.
- Fricke TR, Tahhan N, Resnikoff S et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology 2018; 125: 1492–1499. doi:10.1016/j.ophtha.2018.04.013.
- McGee S, Waring GO IV. Pilocarpine hydrochloride ophthalmic solution 1.25%: an innovative prescription eye drop for the treatment of presbyopia. touchREVIEWS Ophthalmol 2022; 16: 54–62. doi:10.17925/USOR.2022.16.2.54.
- Allergan. VUITYTM (pilocarpine hydrochloride ophthalmic solution) 1.25%, for topical ophthalmic use [prescribing info]. [accessed 2023 Aug 14]. https://www.rxabbvie.com/pdf/vuity_pi.pdf.
- AbbVie. VUITY™ (pilocarpine HCI ophthalmic solution) 1.25%, the first and only FDA-approved eye drop to treat age-related blurry near vision (presbyopia), is now available. [accessed 2023 Aug 14]. https://news.abbvie.com/news/press-releases/vuity-pilocarpine-hci-ophthalmic-solution-125-first-and-only-fda-approved-eye-drop-to-treat-age-related-blurry-near-vision-presbyopia-is-now-available.htm.
- National Library of Medicine. ClinicalTrials.Gov glossary terms. [accessed 2023 Jun 21]. https://clinicaltrials.gov/study-basics/glossary.
- Waring GO IV, Price FW Jr, FW P Jr, Wirta D et al. Safety and efficacy of AGN-190584 in individuals with presbyopia: the GEMINI 1 phase 3 randomized clinical trial. JAMA Ophthalmol 2022; 140: 363–371. doi:10.1001/jamaophthalmol.2022.0059.
- Alcon Laboratories. Isopto® carpine (pilocarpine hydrochloride ophthalmic solution) 1%, 2% and 4% [prescribing info]. [accessed 2023 Aug 14]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/200890s001lbl.pdf.
- Wood JM. Age and visual impairment decrease driving performance as measured on a closed-road circuit. Hum Factors 2002; 44: 482–494. doi:10.1518/0018720024497664.
- Wood JM, Collins MJ, Chaparro A et al. Differential effects of refractive blur on day and nighttime driving performance. Invest Ophthalmol Vis Sci 2014; 55: 2284–2289. doi:10.1167/iovs.13-13369.
- Wood J, Chaparro A, Carberry T et al. Effect of simulated visual impairment on nighttime driving performance. Optom Vis Sci 2010; 87: 379–386. doi:10.1097/OPX.0b013e3181d95b0d.
- Wood J, Chaparro A, Hickson L. Interaction between visual status, driver age and distracters on daytime driving performance. Vision Res 2009; 49: 2225–2231. doi:10.1016/j.visres.2009.06.017.
- Buch JR, Toubouti Y, Cannon J. Randomized crossover trial evaluating the impact of senofilcon A photochromic lens on driving performance. Optom Vis Sci 2020; 97: 15–23. doi:10.1097/opx.0000000000001466.
- American National Standards Institute (ANSI). 2017. ANSI requirements on night driving performance for multifocal IOL. ANSI Z80.12-2007 (R2017).
- Bamdad S, Ahmad Razavizadegan S, Farvardin M et al. Vision-related quality of life after bilateral implantation of monofocal and multifocal intraocular lenses. J Ophthalmic Vis Res 2022; 17: 19–26. doi:10.18502/jovr.v17i1.10166.
- Ozulken K, Kiziltoprak H, Yuksel E et al. A comparative evaluation of diffractive trifocal and new refractive/extended depth of focus intraocular lenses for refractive lens exchange. Curr Eye Res 2021; 46: 811–817. doi:10.1080/02713683.2020.1833347.
- Mai EL, Lian IB, Chang DC. Assessment of contrast sensitivity loss after intrastromal femtosecond laser and LASIK procedure. Int J Ophthalmol 2016; 9: 1798–1801. doi:10.18240/ijo.2016.12.16.
- Chu BS, Wood JM, Collins MJ. The effect of presbyopic vision corrections on nighttime driving performance. Invest Ophthalmol Vis Sci 2010; 51: 4861–4866. doi:10.1167/iovs.10-5154.
- Chu BS, Wood JM, Collins MJ. Effect of presbyopic vision corrections on perceptions of driving difficulty. Eye Contact Lens 2009; 35: 133–143. doi:10.1097/ICL.0b013e3181a1435e.
- Berry S, Mangione CM, Lindblad AS et al. Development of the National Eye Institute refractive error correction quality of life questionnaire: focus groups. Ophthalmology 2003; 110: 2285–2291. doi:10.1016/j.ophtha.2003.08.021.
- Johnston J, Feulner L, Davidson J et al. Pilocarpine HCl ophthalmic solution 1.25% in moderate presbyopia: a post hoc analysis of the GEMINI phase 3 trials. American Academy of Optometry (AAOpt) 2022 Annual Meeting; 2022; San Diego, CA, USA.