ABSTRACT
Clinical relevance
Pupil size evaluation using clinical examination may be important for detecting and monitoring individuals at risk of neurotoxic effects from chemical exposure, as it may enable early intervention and the implementation of preventive measures.
Background
This work aimed to investigate the association between pesticide exposure and pupil size. Pupil size is regulated by muscarinic and nicotinic receptors, and it is well-established that common pesticide chemicals disrupt this regulation.
Methods
Twenty agricultural workers exposed to pesticides, and twenty participants not exposed, underwent visual screening, and pupil size evaluation under mesopic and photopic conditions. Additionally, signs of neurotoxicity and pesticide exposure in both groups were evaluated using the modified version of the neurotoxic symptoms questionnaire (Q16) and measuring cholinesterase (AChE) levels in blood, respectively.
Results
Agricultural workers exposed to pesticides had a score indicating medium–high level of neurotoxicity (49.85 (SD ± 8.94)) which was significantly higher (t (36) = 7.659, p ≤ 0.0001) than non-exposed participants who had low levels of neurotoxicity (27.25 SD ± 8.86). There was a significant difference in pupil size (mm) under mesopic (t (19) 4.42 p = 0.003) and scotopic (t (19) 4.63, p = 0.0002) conditions between the two groups. Additionally, there was a significant difference in AChE blood levels (t (19) 2.94 p = 0.008) between exposed and non-exposed participants, indicating that exposed workers had low levels of this enzyme (average exposed group 3381 U/L (SD ± 1306)) compared to the non-exposed group (average non-exposed group 4765 U/L (SD ± 1300)). A significant negative correlation between AChE levels, years of exposure, and pupil size was found. The latter finding importantly showed that smaller pupils are associated with the accumulation of acetylcholine or a decrease in the activity of the enzyme AChE.
Conclusion
Pupil size of agricultural workers exposed to pesticides can be abnormal and is associated with neurotoxicity as indicated by symptomatology and cholinesterase levels. Evaluation of pupil size may be useful for clinically detecting neurotoxicity.
Introduction
Pesticides are chemical substances used to prevent, destroy, or control plant and animal pests. They can be classified according to their chemical family (organochlorines, organophosphates, carbamates, pyrethroids, dipyridyl compounds, and inorganic salts).Citation1 Pesticides can cause toxicity and harm in humans if the level of exposure is sufficiently high.Citation2,Citation3 It has been estimated that annually there are 385 million cases of unintentional, acute pesticide poisoning worldwide, including around 11,000 fatalities.Citation4
Agricultural populations exposed to pesticides have been reported to suffer various health problems, mainly neurological disorders, and respiratory, reproductive, endocrinological, and dermal problems.Citation5–7 For example, organophosphorus compounds inhibit enzymes like acetylcholinesterase (AChE) and neuropathy target esterase (NTE)Citation8,Citation9; this results in the accumulation of acetylcholine (ACh) and phosphatidylcholine, respectively which may lead to acute or intermediate poisoning due to the cholinergic effect and organophosphate-induced delayed polyneuropathy (OPIDP).Citation9,Citation10
The main manifestations of acute intoxication are excessive overstimulation of muscarinic and nicotinic cholinergic receptors, which can lead to changes in consciousness, muscle weakness, and excessive secretory activity.Citation11,Citation12 In addition, organophosphate and carbamate exposure is associated with neurodegenerative diseases.Citation13–15
Given the wide range of pesticide exposure on physiological and cognitive functions,Citation16,Citation17 one possible sensitive diagnostic test to quantify the signs and symptoms of neurotoxicity, is the evaluation of vision and visual function, which previous studies have shown to be highly susceptible to neurotoxic damage.Citation18–20 A candidate measure is the pupil size,Citation21–23 which has experimentally shown reversible changes due to exposure to organophosphorus.Citation24–26 Importantly, changes in pupil size have occurred without other overt signs of toxicity and suggest that miosis may be an early sign of pesticide exposure.Citation22,Citation27
Pupillary miosis is the function of the sphincter of the pupil, which is innervated by parasympathetic fibres. At the same time, mydriasis is the function of the dilator pupillae and is controlled by sympathetic fibres.Citation22,Citation28 In this way, the pupil size reflects the function of the autonomic nervous system, which regulates the antagonistic actions of the iris sphincter and the dilator muscles of the eye and controls the amount of light entering the eye.Citation29
Synaptic transmission in the neural pathways regulating pupil size involves AChE, which, as mentioned, is inhibited by pesticide-related organophosphates.Citation8 Organophosphates in the eye can cause significant AChE inhibition and muscarinic receptor downregulation which may lead to abnormal presentations of pupil size, particularly miosis.Citation27,Citation30 Indeed, most visual and ocular signs and symptoms of acute organophosphate poisoning are reflected in different types of muscarinic manifestations (generally the parasympathetic system), such as pupillary contraction (miosis), stimulation of the different endings of the lacrimal gland and blurred vision (due to accommodation).Citation30 The miotic effect may be reduced after repeated exposure to organophosphates because of the development of tolerance to cholinergic agonists and desensitisation of muscarinic AChE receptors on the pupillary sphincter.Citation31
Accordingly, miosis may be a potential marker for pesticide agents in the body.Citation22,Citation27 Given the strong action of the pesticide on the neuronal system that supports miosis, the assessment of pupil size in individuals exposed to pesticides may provide a sensitive and efficient indication of neurotoxicity, as its measurement is non-invasive fast and easy to perform.
The present study aimed to establish whether exposure to occupational levels of pesticides in agricultural workers is associated with altered pupil sizes, particularly signs of miosis. In addition, pupil size with neurotoxic symptoms (using a modified version of the Neurotoxic Symptoms Q16 questionnaire) was associated with the level of blood AChE activity which is a commonly used indicator of pesticide exposure.Citation32
Recent research (conducted on farm workers) has shown that regular exposure to pesticides can lead to neurotoxicity resulting in deficits in visual function, particularly in contrast and colour perception, and elevated inflammatory markers (Substance p) in tears.Citation33 This finding suggests that measuring changes in vision may be a useful way of quantifying the impact on the brain. The present study seeks to add to this understanding and by investigating the relationship between neurotoxicity and pupil size in workers exposed to occupational levels of pesticides.
Methods
This research followed the principles of the Declaration of Helsinki for medical research in human beings. Each participant was informed of the benefits and risks of the study and a written informed consent was signed. The study was approved by the ethics committee of the Faculty of Health Sciences of the University of La Salle (Memorandum 029 17,042,017).
An observational cross-sectional analytical study was conducted to quantify the pupil size in agricultural workers exposed to pesticides, their relationship with neurotoxic symptoms, and blood cholinesterase levels. These results were compared to non-agricultural workers not exposed to pesticides. There were 38 participants in the present study. The non-exposed group comprised 20 subjects (8 women and 12 men) with a mean age of 27 years (SD ± 7.05), and the exposed group was 18 male subjects with a mean age of 31 (SD ± 5.68).
Agricultural workers in the exposed group were selected from potato farms that used pesticides such as organophosphates (OPs), carbamates (CAs), pyrethroids (PIs): Bifenthrin (2-methylbiphenyl-3-ylmethyl(1RS,3RS)-3-[(Z)-2-chloro-3,3,3-trifluoroprop-1-enyl]-2,2-dimethylcyclopropanecarboxylate), Profenofos (O-4-bromo-2-chlorophenyl O-ethyl S-propyl phosphorothioate), cypermethrin ((RS)-a-cyano-3-phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichloro vinyl)-2,2- dimethyl cyclopropane carboxylate) and Carbosulfan (2,3-dihydro-2,2-dimethylbenzofuran-7-yl (dibutylaminothio) methylcarbamate).
Potato farms in Colombia use between 1.0 and 2.5 kg/ha of pesticides annually. Over 4 weeks, the type and frequency of use of pesticides by applicators and operators collaborating on farms who participated in the present study were: OPs: 22 times; PIs: 16 times; CAs: 22 times. The population under study was located geographically in the municipality of Belén, Boyacá, aged between 20 and 40 years, and worked as farmers for at least one year. The average number of years of exposure was 12.6 (SD ± 5.10) and ranged from 4 to 22 years.
Participants of the same age group who were not exposed to occupational levels of pesticides comprised the non-exposed group. The sample size used in the present study was estimated with a power of 80% and a type I error of 5%, based on two previous studies and assuming an effect size of 0.61 (Glass’s Delta). This effect size was derived from previously reportedCitation18,Citation34 means and standard deviations of the neurotoxic symptoms questionnaire (Q16) scores for participants exposed and not exposed to pesticides.
The exclusion criteria for both groups were those who reported an alteration or were previously diagnosed with alterations in the pupillary response and neurobehavioral deficiencies, systemic diseases, macular diseases, and opacities in the cornea and/or lens. Another exclusion criterion for the non-exposed group was living with someone who worked or was exposed to pesticides. The inclusion criteria for the non-exposed group were individuals who had never worked or were exposed for long periods to any pesticide or suffered from any disease related to changes in pupil size.
The neurotoxic symptoms questionnaire Q16 is commonly used to monitor the early effects of neurotoxic exposures in the working population and has been internationally recognised as the gold standard test for the detection of neurotoxic symptoms by the World Health Organization.Citation35 This questionnaire has been modified and validated,Citation36 to be used in different studies for screening of neurotoxicity due to exposure to organic solvents and their impacts on vision.Citation18,Citation33,Citation34
The questionnaire contained 16 short questions about the symptoms commonly described by workers exposed to solvents, such as: ‘I have a short memory’, Sometimes I have a painful tingling sensation in some part of my body”, or ‘I feel that I have less sensitivity or a complete loss of sensation in some parts of the arms or legs’. These questions are evaluated using a 5-level Likert scale ranging from totally disagree (1) to agree (5). Once the subject completed the questionnaire, the total sum of the scores is used to indicate the level of neurotoxicity, which can be categorised as Low (16–32), Medium-low (33–48), Medium high (49–64) and high (65–80).
Instrumentation and procedures
An optometric examination includes a battery of tests such as visual acuity assessment, anterior segment examination, direct ophthalmoscopy (for retinal fundus assessment), refraction test, and evaluation of the pupils. All tests were assessed binocularly, under standardised conditions, and by the same examiner. Participants were first visually screened, including a comprehensive anamnesis and distance and near visual acuity assessment using the Good-Lite ETDRS and for near vision and near visual chart, respectively. After examination, participants who displayed abnormalities in vision were excluded from the study. All participants filled out a baseline survey that included questions related to alcohol consumption, smoking, medications, metabolic diseases, or neurological diseases.
Biomicroscopy was assessed using the Ezer Slit lamp®, while direct ophthalmoscopy and retinoscopy were assessed using the Welch Allyn® diagnostic kit. Participants who required refractive correction were corrected at that time with a frame trial and lenses, this frame was used during the examination to take the different visual tests, and at the end, the final optical prescription was given to each participant.
After the visual screening, pupil size was assessed with the Autorefractor keratometer (Huvitz Wavefront Ref/Keratometer HRK −7000, version 5.02.00A made in Korea). This system comprises various features that provide measurements, including the pupillary diameter. To measure the pupil diameter, the subject was asked to observe the target without trying to make any visual effort to see the image but rather to be completely relaxed, and three measurements for each eye were obtained to provide an estimate of the average pupil size of each eye. This procedure was performed under mesopic and scotopic targets (0.80 lux for mesopic and 0.08 lux for scotopic) as these were the measurement conditions of the device. The distance between the corneal apex of the subject and the equipment was 40 mm.
The level of AChE in blood was measured by taking a venous blood sample from participants at the same visit. This sample was centrifugated (1200 rpm x 10 min), and the serum was separated. The serum was transported to a laboratory in an ice chamber at a temperature between 6.8°C and 7.4°C, and the kinetic method was used at 405 nm to determine the amount of cholinesterase in the serum. The Wiener lab® brand kit and procedure Fixed ΔT was used. Reagent A was reconstituted with 3 ml of reagent B and pre-incubated for 1 min at 25°C, 20 µl of the sample was added and mixed and immediately the absorbance at 405 nm was recorded, and immediately the stopwatch was triggered, it was reread at 30, 60 and 90 s. The average difference was determined every 30 according to ΔA/30) by subtracting the reading from the previous one and averaging the values. This was repeated with each of the samples. The reference values established by the Wiener lab® laboratory for the kit used and the temperature used are 3200–9000 U/L.
Statistical analysis
The Shapiro–Wilk test was used to verify that the data was normally distributed. Depending on the type of data and comparison between groups or conditions, either a t-test or Mann–Whitney test was used. A two-way ANOVA with post hoc comparison (Tukey’s test, corrected for multiple comparisons) was also performed to establish whether pupil size was significantly different between exposed and non-exposed individuals for different lighting conditions. Correlation analysis between two variables was assessed using Spearman’s Rho. In all cases, a p < 0.05 value was considered statistically significant. The analysis was conducted with the Graph Pad software, version 9.
Results
Indicators of neurotoxicity and pesticide exposure
There was a statistically significant difference between non-exposed and exposed groups for AChE activity (t (36) 2.99, p = 0.008). In particular, the group of exposed workers had lower activity of AChE 3381 U/L SD ± 1306 U/L than the non-exposed group 4765 U/L SD ± 1300 U/L. Cohen’s d, was 1.06, indicating a moderate to large effect size. Modified Q16 neurotoxic symptoms questionnaire scores were also significantly different between the two groups (t (36) = 7.659, p ≤ 0.0001), with the exposed group reporting on average higher symptom scores of 49.85 SD ± 8.94 (which indicated medium-high neurotoxicity levels) than the non-exposed group 27.25 SD ± 8.86. The average duration of exposure was 12.6 years (SD ± 5.10). Cohen’s d, was 2.53, indicating a large effect size.
There was no correlation between the modified neurotoxic symptoms questionnaire Q16 score and years of exposure (rho = 0.10 (p = 0.65)) for the exposed group (see ). However, modified Q16 neurotoxic symptoms questionnaire scores were negatively correlated with AChE activity in bloodrho = −0.56 (p = 0.0009) for the exposed group but not for the non-exposed group p = 0.39. This result suggests that higher neurotoxicity symptoms may be associated with lower AChE activity (see discussion) in individuals exposed to pesticides.Citation8,Citation32
Table 1. Correlation matrix between pupil size and neurotoxicity measures for the exposed group.
Pupil size measurements
The average pupil size for the exposed group for mesopic and scotopic light levels was smaller than the non-exposed group (). The Shapiro–Wilk normality test showed that all pupil size data were normally distributed. A two-way ANOVA was conducted with Lighting Conditions (mesopic and scotopic) and Group (non-exposed and exposed) as factors. This analysis showed a significant effect of Lighting Condition (F (3, 57) = 43.50, p = 0.0136, η2 = 0.69) with pupil size on average larger under scotopic than mesopic conditions. A main effect of Group was also observed, with pupil sizes on average smaller for the exposed group than the non-exposed group (F (19, 55) = 2.152, p = <0.0001, η2 = 0.42). There was no significant interaction effect.
Figure 1. Average pupil size differences for the exposed and non-exposed group for both mesopic and scotopic light levels. Tukey’s post hoc comparison tests ***p < 0.002.
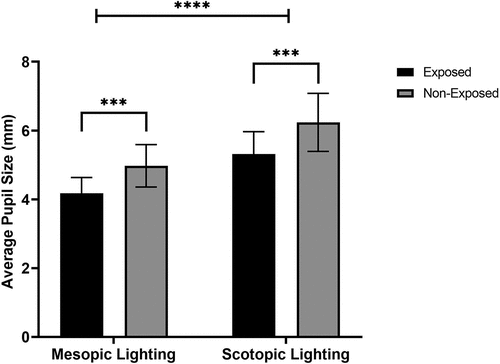
Tukey’s post hoc comparison tests showed that pupil size for the exposed group was significantly smaller than the non-exposed group for Mesopic (p < 0.002) and Scotopic (p < 0.003) conditions (). Years of pesticide exposure were negatively correlated with pupil size under mesopic and scotopic conditions (p < 0.05).
Significant correlations were observed between AChE activity and pupil size for mesopic (rho = 0.589, p = 0.010) and scotopic conditions (rho = 0.504, p = 0.033), such that lower AChE activity is associated with smaller pupil sizes. There was no significant correlation between modified Q16 scores and pupil size regardless of the lighting condition rho = −0.100 (p = 0.650) mesopic and rho = −0.110 (p = 0.610) scotopic ().
Discussion
The present study quantified the relationship between pesticide exposure and pupil size and associated pupil size with neurotoxic symptoms (as measured by the modified Q16) and AChE in blood. Exposure to pesticides was associated with significant alterations in pupil size, which was significantly smaller (in both mesopic and scotopic conditions) for the exposed group compared to the non-exposed group.
The exposed group, on average, reported significantly more neurotoxic symptoms and lower levels of AChE in blood, indicating overstimulation of the nervous system that manifests in a spectrum of neurotoxic symptoms, several of which are assessed using the modified Q16 questionnaire. These symptoms associated with AChE inhibition encompass involuntary muscle twitching and weakness (Questions 11–15), cognitive impairment, including confusion or other neurological deficits (Questions 1–8), and in severe cases, organ failure, particularly respiratory failure (Question 10).
Pesticides inhibit AChE levels which can impact nicotinic and muscarinic processes, including pupil size.Citation11 As reported in the present study, the exposed group had smaller pupil size, and this may occur due to the accumulation of ACh at nerve synapses due to the inhibition of the AChE enzyme. The excessive amounts of ACh produced result in symptoms or signs of cholinergic poisoning.Citation9,Citation11 Previous studies on the effects of these chemical compounds on the eye suggest that the inhibition of AChE is a direct result of the vapours on ocular tissues rather than systemic absorption and distribution of the pesticide.Citation19,Citation24,Citation31
The localised increase in ACh leads to the contraction of the pupillary sphincter muscle, resulting in dose-dependent miosis.Citation24,Citation27 In patients intoxicated with Sarin vapours (one of the most toxic organophosphorus compounds), after 24 h of exposure, the acetylcholinesterase activity was significantly lower in patients with miotic pupils than those with normal-sized pupils.Citation37 In summary, the findings of a negative medium association between AChE levels and pupil size indicate that miosis may be a sensitive index of exposure to pesticides at lower levels that can lead to other identifiable effects or poisoning. However, future research is needed to further establish and quantify this relationship.
Years of pesticide exposure were negatively correlated with pupil size under mesopic and scotopic conditions. These results are consistent with signs of acute poisoning from exposure to organophosphates, previously described as a direct consequence of the inhibition of AChE activity. Whilst AChE levels are correlated with pupil size, modified Q16 symptom scores were not (). This finding may be because the modified neurotoxic symptoms Q16 questionnaire registers more general symptoms of neurotoxicity that are not necessarily related to vision or visual impairment.
Perhaps another contributing factor is that it is well-known that significant individual differences exist in how people subjectively report their perception of pain and discomfort. Accordingly, the subjective nature and variability of individual reports of neurotoxic symptoms will likely affect the correlation strength with a specific outcome measure. Note, however, that the modified Q16 was correlated with AChE, which suggests that AChE is related to general neurotoxicity symptoms.
It is important to highlight that the exposed group in this study exclusively consisted of males, in contrast to the non-exposed group, which included 12 males and 8 females. Consequently, gender differences, along with gender-related factors such as body mass index (BMI) may be contributing factors to pupil size. Although previous studies have demonstrated the independence of pupil size from gender,Citation38,Citation39 a further analysis (t-test) was performed to determine whether the pupil size and levels of neurotoxicity of female control participants differed from their male control counterparts. This analysis revealed no statistical difference in pupil size under both mesopic (p = 0.21) and scotopic conditions (p = 0.45), AChE activity (p = 0.49) as well and Q16 neurotoxicity levels (p = 0.32), suggesting that gender may not be a contributing factor in accounting for the present findings.
It is important to consider age-related changes in pupil size, which tend to be more pronounced in significantly older individuals, with pupil size diminishing by 0.6 to 1 mm every 20 years.Citation38,Citation39 In the current study, both groups were approximately age-matched, with the exposed group averaging 31 years and the non-exposed group 27 years. The modest 4-year difference (estimated to be 0.12–0.2 mm) is anticipated to have minimal impact on the findings of this study, given the gradual rate of pupil size change associated with age.
This study demonstrates that pupil size may be used as an important biomarker or indicator for pesticide exposure; specifically, pupils size measurement can be used to detect and monitor the presence of a pesticide in the body and serve as a sign of adverse health effects caused by this exposure. This highlights the utility of pupil size measures in the broader context of other clinical tests and the medical history of the patient. As such, pupil size measurement could potentially guide clinicians towards improved diagnosis and better case management.Citation22,Citation27
The use of cost-effective and easy-to-measure biomarkers like these might help evaluate potential pesticide exposures and allow for decisions to be made about the regulation of the use of pesticides to protect human health and the prevention of pesticide toxicity. Pupil size measures can be used in conjunction with other measures of neurotoxicity (e.g., modified neurotoxic symptoms Q16) to contribute to a more comprehensive assessment of the consequences of pesticide exposure and used as a means of monitoring recovery.
Acknowledgements
This work was supported by Universidad de La Salle, Bogotá, Colombia, in collaboration with The University of New South Wales. We express our sincere gratitude to the potato and strawberry farmworkers from Belén Boyacá, Colombia. We also acknowledge the research group named Critical Zone from the La Salle Environmental Engineering program for providing information regarding the pesticides used in the geographical area of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abubakar Y, Tijjani H, Egbuna C et al. Pesticides, history, and classification. In: Natural remedies for pest, disease and weed control. Elsevier; 2020. p. 29–42.
- Sharma A, Shukla A, Attri K et al. Global trends in pesticides: a looming threat and viable alternatives. Ecotoxicol Environ Saf 2020; 201: 110812. doi:10.1016/j.ecoenv.2020.110812.
- FAO, WHO. International code of conduct on pesticide management guidelines on highly hazardous pesticides. Rome, Italy; 2016. www.fao.org/publications.
- Boedeker W, Watts M, Clausing P et al. The global distribution of acute unintentional pesticide poisoning: estimations based on a systematic review. BMC Public Health 2020; 20: 1875. doi:10.1186/s12889-020-09939-0.
- Kim KH, Kabir E, Jahan SA. Exposure to pesticides and the associated human health effects. Sci Total Environ 2017; 575: 525–535. doi:10.1016/j.scitotenv.2016.09.009.
- Eddleston M. Poisoning by pesticides. Medicine 2020; 48: 214–217. doi:10.1016/j.mpmed.2019.12.019.
- Jett DA. Neurotoxic pesticides and neurologic effects. Neurol Clin 2011; 29: 667–677. doi:10.1016/j.ncl.2011.06.002.
- Tsai YH, Lein PJ. Mechanisms of organophosphate neurotoxicity. Curr Opin Toxicol 2021; 26: 49–60. doi:10.1016/j.cotox.2021.04.002.
- Mukherjee S, Gupta RD. Organophosphorus nerve agents: types, toxicity, and treatments. J Toxicol 2020; 2020: 16. doi:10.1155/2020/3007984.
- Ganie SY, Javaid D, Hajam YA et al. Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: a critical appraisal. Toxicology 2022; 472: 153181. doi:10.1016/j.tox.2022.153181.
- King AM, Aaron CK. Organophosphate and carbamate poisoning. Emerg Med Clin North Am 2015; 33: 133–151. doi:10.1016/j.emc.2014.09.010.
- Naughton SX, Terry AV. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018; 408: 101–112. doi:10.1016/j.tox.2018.08.011.
- Voorhees JR, Rohlman DS, Lein PJ et al. Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds. Front Neurosci 2017; 10: 590. doi:10.3389/fnins.2016.00590.
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 2015; 10: 124. doi:10.3389/fncel.2015.00124.
- Moretto A, Colosio C. Biochemical and toxicological evidence of neurological effects of pesticides: the example of Parkinson’s disease. Neurotoxicology 2011; 32: 383–391. doi:10.1016/j.neuro.2011.03.004.
- Aloizou AM, Siokas V, Vogiatzi C et al. Pesticides, cognitive functions and dementia: a review. Toxicol Lett 2020; 326: 31–51. doi:10.1016/j.toxlet.2020.03.005.
- Muñoz-Quezada MT, Lucero BA, Iglesias VP et al. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. Int J Occup Environ Health 2016; 22: 68–79. doi:10.1080/10773525.2015.1123848.
- Jiménez-Barbosa IA, Boon MY, Khuu SK et al. Exposure to organic solvents used in dry cleaning reduces low and high level visual function. PLoS One 2015; 10: 1–23. doi:10.1371/journal.pone.0121422.
- Ples˘tina R, Piuković-Ples˘tina M, Roberts DV. Effect of anticholinesterase pesticides on the eye and on vision. CRC Crit Rev Toxicol 1978; 6: 1–23. doi:10.3109/10408447809029332.
- Fareed M, Kesavachandran CN, Pathak MK et al. Visual disturbances with cholinesterase depletion due to exposure of agricultural pesticides among farm workers. Toxicol Environ Chem 2012; 94: 1601–1609. doi:10.1080/02772248.2012.718780.
- Ferencova N, Visnovcova Z, Olexova LB et al. Eye pupil – a window into central autonomic regulation via emotional/cognitive processing. Physiol Res 2021; 70: 669–682. doi:10.33549/physiolres.934749.
- Hall CA, Chilcott RP. Eyeing up the future of the pupillary light reflex in neurodiagnostics. Diagnostics 2018; 8: 19. doi:10.3390/diagnostics8010019.
- Jaga K, Dharmani C. Ocular toxicity from pesticide exposure: a recent review. Environ Health Prev Med 2006; 11: 102–107. doi:10.1265/ehpm.11.102.
- Hulet SW, Sommerville DR, Crosier RB et al. Comparison of low-level sarin and cyclosarin vapor exposure on pupil size of the Gottingen minipig: effects of exposure concentration and duration. Inhal Toxicol 2006; 18: 143–153. doi:10.1080/08958370500306131.
- Taylor JT, Davis E, Dabisch P et al. Alterations in autonomic function in the guinea pig eye following exposure to dichlorvos vapor. J Ocular Pharmacol Therapeut 2008; 24: 473–479. doi:10.1089/jop.2008.0020.
- Dabisch PA, Horsmon MS, Taylor JT et al. Gender difference in the miotic potency of soman vapor in rats. Cutan Ocul Toxicol 2008; 27: 123–133. doi:10.1080/15569520802064376.
- Genovese RF, Benton BJ, Oubre JL et al. Evaluation of miosis, behavior and cholinesterase inhibition from low-level, whole-body vapor exposure to soman in African green monkeys (Chlorocebus sabeus). J Med Primatol 2010; 39: 318–327. doi:10.1111/j.1600-0684.2010.00413.x.
- McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol 2015; 5: 439–473.
- Bouffard MA. The pupil. Continuum 2019; 25: 1194–1214. doi:10.1212/CON.0000000000000771.
- Rengstoflt RH. Vision and ocular changes following accidental exposure to organophosphates. J Appl Toxicol 1994; 14: 115–118. doi:10.1002/jat.2550140213.
- Dabisch PA, Miller DB, Reutter SA et al. Miotic tolerance to sarin vapor exposure: role of the sympathetic and parasympathetic nervous systems. Toxicol Sci 2005; 85: 1041–1047. doi:10.1093/toxsci/kfi151.
- Lionetto MG, Caricato R, Calisi A et al. Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. Biomed Res Int 2013; 2013: 321213. doi:10.1155/2013/321213.
- Jiménez Barbosa IA, Rodríguez Alvarez MF, Bernal Bechara LC et al. Impairment of visual and neurologic functions associated with agrochemical use. PLoS One 2023; 18: 0290263. doi:10.1371/journal.pone.0290263.
- Jimenez-Barbosa IA, Rodriguez MF, Dussan GA et al. Ocular surface and tear film changes in workers exposed to organic solvents used in the dry-cleaning industry. PLoS One 2019; 14: 0226042. doi:10.1371/journal.pone.0226042.
- WHO, Nordic Council of Ministers Working Group. Chronic effects of organic solvents on the central nervous system and diagnostic criteria. Copenhagen; 1985 Jun 10.
- Jimenez I, Khuu S, Ying M. Modified Q16 Neurotoxic Symptoms Questionnaire. Ciencia y Tecnología para la Salud Visual y Ocular 2011; 9: 19–37.
- Nozaki H, Hori S, Shinozawa Y et al. Relationship between pupil size and acetylcholinesterase activity in patients exposed to sarin vapor. Intensive Care Med 1997; 23: 1005–1007. doi:10.1007/s001340050447.
- Bieniek MM, Frei LS, Rousselet GA. Early ERPs to faces: aging, luminance, and individual differences. Front Psychol 2013; 4: 268. doi:10.3389/fpsyg.2013.00268.
- Winn B, Whitaker D, David -F et al. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci 1944; 35: 1132–1137.
