ABSTRACT
Clinical relevance
Clinical assessment of age-related macular degeneration (AMD) relies on biomarkers that do not necessarily reflect the contributions of vascular dysfunction. Validation of clinically accessible methods of measuring retinal vascular integrity could provide a more holistic understanding of AMD-related changes to facilitate appropriate care.
Background
There is conflicting evidence if retinal vessel calibre is significantly altered in the early stages of AMD. This study examined the outer and inner diameters of first order retinal vessels in intermediate AMD eyes using en face optical coherence tomography (OCT).
Methods
Retinal en face (6 × 6 mm) OCT images were examined in a single eye of participants with intermediate AMD (n = 46) versus normal macula (n = 43) for arterioles (all identifiable) and venules (40/46 and 39/43 identifiable). All participants were aged ≥50 years without diabetes mellitus, hypertension, or other systemic vascular disease.
Results
Intra- and inter-grader agreement was good-to-excellent for all en face OCT measurements of arteriole and venule diameters (intraclass correlation coefficient = 0.87 to 0.99). Arteriolar outer diameters (82.3 ± 19.8 µm vs 73.8 ± 16.1 µm; p < 0.05) and inner diameters (35.1 ± 8.4 µm vs 31.5 ± 8.1 µm; p < 0.05) were significantly greater in AMD eyes compared to normal eyes. Venular inner diameter was significantly greater (43.1 ± 9.5 µm vs 39.2 ± 10.1 µm; p < 0.05), but outer diameter remained unchanged (p = 0.17) in AMD eyes compared to normal eyes.
Conclusion
Arteriolar dilation and altered venular inner diameter were observed in intermediate AMD eyes. These results support further investigation of vascular contributions to AMD in the early stages of disease, possibly using the en face OCT imaging modality.
Introduction
Age-related macular degeneration (AMD) is a major cause of vision loss worldwide.Citation1 Although the exact pathogenesis of the disease is unknown, there is strong evidence for a vascular component, as degenerative changes in the choriocapillaris have been observed in all stages of AMD using both in vivo and post-mortem techniques.Citation2–4 Loss of retinal vessel density and perfusion in vivo have also been described in the early stages of AMD,Citation5–7 and have been associated with prognostic markers of AMD including reticular pseudodrusen and nascent geographic atrophy.Citation5
The AMD-associated changes which occur in individual retinal blood vessels, however, are less clear. Measurements of retinal vessel calibre using central retinal arteriolar and venular equivalent have provided conflicting results, including significant increase,Citation8,Citation9 decrease,Citation9 or no changeCitation8,Citation10,Citation11 in the early stages of AMD relative to normal eyes. Other measures of vessel calibre, such as arteriovenous nickingCitation10 and focal arteriolar narrowing,Citation12 have demonstrated associations with AMD severity, albeit in isolated studies.
These discrepancies may be due to various reasons including heterogeneity within and between study populations, and current measures of vessel calibre being too generalised (such as central retinal arteriolar and venular equivalent) and/or less repeatable (using colour fundus photography) than other newer retinal imaging modalities.Citation13 The latter is particularly relevant considering the growing number of high-resolution ocular imaging technologies with improved tissue penetrance now available in clinical practice and promoted for accurate assessment of AMD.Citation14
More broadly, validation of a more precise measure of retinal vessel calibre may also provide further insight into other diseases where the practice is more commonplace, such as diagnosing hypertensive retinopathy and assessing cardiovascular risk.Citation15
En face optical coherence tomography (OCT) imaging presents a high-resolution image from which retinal vessels can be assessed. Case studies of macular choroidal macrovessels,Citation16 para-vascular inner retinal defects,Citation17 and even naevi,Citation18 have highlighted the improved diagnostic ability of using en face OCT versus colour fundus photography. Thus far, there has been no assessment of outer and inner retinal vessel calibre in intermediate AMD using en face OCT imaging.
The primary aim of this study was to use en face OCT to determine if retinal vessel calibre is significantly altered in eyes with intermediate AMD, potentially validating a newer method of measuring retinal vessel calibre and reinforcing pathophysiological understanding of vascular changes in the early stages of AMD.
Methods
Study population
Participant eyes for this study were identified through retrospective analysis of patient records from the Centre for Eye Health (CFEH) Sydney, Australia, from 1 January 2016 to 31 December 2020. CFEH is a referral-only, diagnostic, and management eye clinic run by specially trained optometrists and ophthalmologists.Citation19 The majority of patients referred to CFEH have or are suspected to have ocular disease including AMD.
Approximately 23% of patients have normal ocular healthCitation20 and provided an appropriate control population. All patients had given prior written informed consent for use of de-identified data for research in accordance with the Declaration of Helsinki and approved by the Biomedical Human Research Ethics Advisory Panel of the University of New South Wales.
Inclusion criteria for all eyes were: age ≥50 years, absence of self-reported diabetes mellitus, hypertension, or any other systemic vascular-related disease. Single (randomly selected) eyes from each participant were included if the spherical equivalent refraction was under 5.00D (as a proxy for axial length).Citation21
All diseased eyes had intermediate AMD (large drusen and/or pigmentary abnormalities with at least medium drusen) diagnosed by at least two non-blind clinicians using colour fundus photography according to the Ferris et al. classification,Citation22 confirmed using OCT, with no evidence of other macular or optic nerve diseases or abnormalities. All normal eyes were similarly selected from a convenient, retrospective sample of age- and sex-matched participants, whereby normal diagnosis of no macular or optic nerve diseases or abnormalities was confirmed by at least two non-blind clinicians.
Image acquisition
A central retinal 45° colour fundus photograph (Kowa NonmydWx 3D 2D/3D Nonmydriatic Retinal Camera, Kowa Company Ltd.) and en face (6 × 6 mm or 788 × 788 pixels) OCT image (Zeiss HD-OCT 5000 AngioPlex, Carl Zeiss Meditec, Jena, Germany) were extracted for a single eye of participants.
En face OCT images across the total retinal slab were generated from OCT angiography volume scans due to a greater density of B-scans (350× B-scans per angiographic cube relative to 128× B-scans per non-angiographic cube),Citation23 by default software without any image processing. Images with signal strength (SSI) less than seven out of 10 or with significant and uncorrectable segmentation errors were excluded.
Vessel selection and measurement
Vessel selection was performed by random allocation to one of three independent graders (LNS, TR, or AF). Complete blinding of disease status was not possible as graders had to view the en face OCT image for grading. The largest arteriole and venule closest to the optic nerve head (i.e., first-order branches) were identified from colour fundus photography and en face OCT (). A base point was marked on the en face OCT image () between 14 and 75 pixels (107–571 µm) from the image edge to avoid edge artefacts.
Figure 1. Method for vessel selection and measurement. (A) Fundus photography and (B) corresponding en face OCT images were used to identify the largest arteriole and venule closest to the optic nerve head. Scale bar indicates 75 pixels (1 pixel = 7.61 µm). (C) For grader measurements, a position on the vessel within 75 pixels from the image edge was selected (base point, red) and the outer and inner diameters of each vessel measured perpendicular to the tangent of each base point.
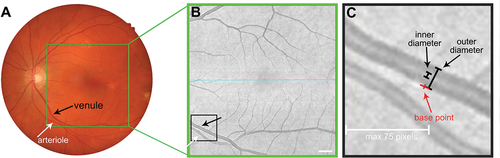
The average distance of base points from the optic nerve head was not significantly different between arterioles (1837 ± 544 µm) and venules (1751 ± 376 µm; p = 0.68), nor was it significantly different between AMD and normal eyes (AMD: 1820 ± 487 µm, normal: 1691 ± 350 µm; p = 0.57). Outer and inner diameters were then measured perpendicular to the tangent of each base point using the angle and line tool from ImageJ v1.52a (National Institutes of Health, Bethesda, MD, USA). Outer diameter end points were judged as the outside darker pixels of the vessel, while inner diameter end points were judged as the inside lighter pixels of the vessel ().
All three graders independently measured outer and inner diameters twice per eye. Pixels were converted to µm based on the approximation of 1 pixel = 7.61 µm (i.e., [6000 µm total scan width and height]/[788 × 788 pixels]).Citation24
Statistical analysis
Statistical analysis was performed using GraphPad Prism (Version 9.0.0 GraphPad Software San Diego, CA, USA) and Microsoft Excel (Version 16.42 Microsoft Inc Redmond, WAS, USA). Comparability of groups for demographic and ocular characteristics were conducted using unpaired Student’s t-tests or Fisher’s exact tests where appropriate.
Agreement of measurements within graders was assessed using Student’s t-test, and agreement between graders was assessed using intraclass-correlation co-efficient using a two-way random effects model for multiple graders. Agreement was considered poor, moderate, good, or excellent if the intraclass correlation co-efficient was <0.5, 0.5–0.74, 0.75–0.89, or >0.9, respectively.Citation25
Differences in vessel diameter between study groups were assessed using multivariable linear regression that included age, sex, refraction, and grader as covariables. Statistical significance was considered as p < 0.05.
Results
Participant demographics
A total of 46 intermediate AMD and 43 normal eyes were included in this study. All eyes had a first-order branch arteriole identifiable for analysis, while 40 (/46) intermediate AMD and 39 (/43) normal eyes had a first-order branch venule identifiable for analysis. There were no significant differences in age, sex, corrected visual acuity, spherical equivalent refraction, or SSI between AMD and normal eyes, for either arteriole or venule analyses ().
Table 1. Participant demographics.
Arteriole diameters
There were no significant differences for within-grader measurements of outer and inner diameters in AMD and normal eyes (Supplementary Table S1). As such, within-grader repeated measurements were pooled for each group, and then between-grader measurements were compared. There was excellent intraclass correlation for outer diameter between graders in AMD (0.95) and normal (0.96) eyes, and good intraclass correlation for inner diameter between graders in AMD (0.89) and normal (0.87) eyes ().
Table 2. Inter-grader agreement for arteriole and venule outer and inner diameter.
Comparing arterioles of intermediate AMD versus normal eyes, outer diameter (82.27 ± 19.79 µm vs 73.77 ± 16.11 µm; p < 0.05) and inner diameters (35.14 ± 8.39 µm vs 31.46 ± 8.12 µm; p < 0.05) were significantly greater (). Multivariable linear regression found no evidence of significant confounding from other measured covariables.
Figure 2. Arteriole diameter of normal and AMD eyes. (A) Outer and (B) inner diameter and (C) outer versus inner diameter. Central line of (A) and (B) represents the mean of the group, error bars represent SD. Lines in (C) represent the linear regression slope.
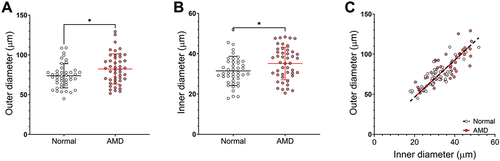
There was a significant correlation between outer and inner diameter for each group (Pearson’s co-efficient, rAMD = 0.80, rnormal = 0.85; p < 0.001), and no significant difference in the correlation between groups (p = 0.99; ). These results suggested that arteriole dilation may be present in AMD eyes with no significant effects on the ratio of outer to inner diameter.
Venule diameters
Similar to arterioles, there were no significant differences for within-grader measurements of outer and inner diameters in AMD and normal eyes (Supplementary Table S1) and as such, repeated measurements were pooled. The intraclass correlation coefficient for outer and inner diameters between graders was good to excellent in AMD and normal eyes (0.87–0.99; ).
In intermediate AMD compared to normal eyes, venules showed no significant difference in outer diameter (p = 0.17) but a significant increase in inner diameter (43.07 ± 9.49 µm vs 39.18 ± 10.08 µm; p < 0.05; ). Similar to arterioles, multivariable linear regression suggested no significant confounding from other measured covariables.
Figure 3. Venule diameter of normal and AMD eyes. (A) Outer and (B) inner diameter and (C) outer versus inner diameter. Central line of (A) and (B) represents the mean of the group, error bars represent SD. Lines in (C) represent the linear regression slope.
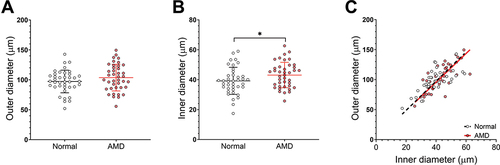
Outer and inner diameters were significantly correlated for each group (rAMD = 0.76, rnormal = 0.70; p < 0.001), and there was no difference in the correlation between groups (p = 0.63; ). Together, these results suggested that there may be inner vessel remodelling in AMD eyes, without significant changes in the outer diameter.
Discussion
This study found arterioles in intermediate AMD eyes exhibited dilation of outer and inner vessel diameters relative to normal eyes. Meanwhile, venules only exhibited an increase in inner retinal diameter, without significant changes in the outer diameter. Within- and between-grader measurements of vessel diameters were good to excellent for AMD and normal eyes, validating the novel use of en face OCT imaging for the measurement of retinal vessel calibres.
Overall, these results indicate that significant differences in vessel structure exist in intermediate AMD, reinforcing pathophysiological understanding of vascular changes in the early stages of AMD.
Arteriole dilation in intermediate AMD
In this study, outer and inner arteriole diameters were greater in eyes with AMD compared to normal eyes, suggesting arteriole dilation in the early stages of disease. This was supported by a previous report on arteriole dilation in association with early/intermediate AMD in a rural Chinese population.Citation8 Conversely, large epidemiology studies in Caucasian,Citation10 Indian,Citation11 and ChineseCitation26 populations have reported no associations between vessel calibre and AMD, albeit using colour fundus photography mostly without validation of within- and between-grader reliability. Dissimilarities between findings may also be associated with environmental and genetic variations between study populations, which are known to affect retinal vessel diameter.Citation27
Beyond the static measurements of retinal arteriole structure, dynamic (light stimulation) vessel analyses also support the findings in this study, whereby abnormal retinal arteriole dilation has been observed in eyes with drusen and reticular pseudodrusen, and AMD eyes with specific ‘age-related maculopathy susceptibility 2’ genotype.Citation28 These studies have suggested that the differences in arteriole vessel structure in AMD may be associated with endothelial dysfunction within the vessel wall.Citation28
Relatedly, circulating endothelial cells and markers of endothelial dysfunction are increased in the serum of patients with late AMDCitation29 and thought to contribute to choroidal neovascularisation. These findings are corroborated by observations of both outer and inner diameters being altered in intermediate AMD.
Venule remodelling in intermediate AMD
Many previous studies have reported no association between venular calibre and AMD in various populations.Citation8,Citation10,Citation11,Citation28 However, by employing en face OCT image analysis, this study revealed that while venule outer diameter remains unchanged in AMD eyes compared to normal eyes, venule inner diameter is significantly greater. This inner diameter change may reflect outward remodelling of the venule lumen, which has been observed in other systemic vascular diseases,Citation30,Citation31 especially considering that AMD and cardiovascular disease share a number of risk factorsCitation32 and potentially a common pathogenesis.Citation33 Alternatively, a greater venule inner diameter may simply indicate a change in the vessel axial light reflex.Citation34
The axial light reflex is speculated to be a function of both vessel diameter and flow velocity of erythrocytes, whereby disease-induced changes such as sclerosis of vessel walls or pathology that slows flow velocity leads to more reflectivityCitation35,Citation36 and therefore a broader axial light reflex. As the flow velocity of venules has been reported to be unaltered in AMD,Citation37 alterations in the venule wall composition may be responsible for the altered inner diameter of venules in AMD eyes seen in this study. Indeed, Klein et al.Citation38 found increased central retinal vein equivalent measures were associated with higher serum levels of high-sensitivity C-reactive protein, interleukin 6, and amyloid A, suggesting an association between retinal venule widening and inflammation. However, as most studies in this area are associated with the arteriolar light reflex as assessed in fundus photographs, the translation of these observations to OCT en face imaging remains limited.
Study limitations
The measurement and calculation of vessel parameters using en face OCT images in this study were novel and have not been assessed elsewhere in the literature. Good to excellent intra- and inter-grader agreement was observed using three blinded, independent graders, bolstering the reliability of the clinically accessible method.
Comparison against other retinal vessel imaging modalities such as colour fundus photography, including adjustment for axial length rather than using spherical equivalent refraction as a proxy measure,Citation21 however, is needed for further validation of this technique.
This study was also limited by its cross-sectional, retrospective design, whereby data were limited to self-reporting by participants of systemic disease and drug use, which may have some confounding impacts on retinal vessel calibre.Citation39 This study, however, serves as a proof-of-concept for using en face OCT images for vessel quantification and therefore serves as evidence for further exploration into retinal vessel changes in the early stages of AMD using resources such as large-scale prospective epidemiological biobank data with more comprehensive medical records.Citation40
Finally, it is unclear whether the inner diameter of retinal vessels measured in this study represented the axial light reflex or lumen as values overlapped with both possibilities.Citation34 Regardless, the findings confirmed that assessment of the outer diameter of retinal blood vessels alone (commonly via colour fundus photography) may be inadequate for observing finer-detailed pathological changes. Future studies should consider more sensitive approaches to retinal vessel imaging such as en face OCT or adaptive-optics OCT.
Conclusion
Significant differences in arteriole and venule outer and inner diameters can be observed in intermediate AMD eyes using the modality of en face OCT imaging. These results support further investigation of vascular contributions to the early stages of AMD.
Supplemental Material
Download PDF (85.9 KB)Acknowledgements
This work was supported, in part, by research grants from the Rebecca Cooper Foundation and the National Health and Medical Research Council of Australia (NHMRC grant #1174385) awarded to LNS. MT is supported by the Australian Research Training Program scholarship. Guide Dogs NSW/ACT provides support for the Centre for Eye Health (the clinic of recruitment).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08164622.2024.2311703
Additional information
Funding
References
- Wong WL, Su X, Li X et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014; 2: e106–116. doi:10.1016/S2214-109X(13)70145-1.
- Mullins RF, Johnson MN, Faidley EA et al. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Visual Sci 2011; 52: 1606–1612. doi:10.1167/iovs.10-6476.
- Chatziralli I, Theodossiadis G, Panagiotidis D et al. Choriocapillaris vascular density changes in patients with drusen: cross-sectional study based on optical coherence tomography angiography findings. Ophthalmol Ther 2018; 7: 101–107. doi:10.1007/s40123-018-0119-9.
- Borrelli E, Shi Y, Uji A et al. Topographic analysis of the choriocapillaris in intermediate age-related macular degeneration. Am J Ophthalmol 2018; 196: 34–43. doi:10.1016/j.ajo.2018.08.014.
- Cicinelli MV, Rabiolo A, Sacconi R et al. Retinal vascular alterations in reticular pseudodrusen with and without outer retinal atrophy assessed by optical coherence tomography angiography. Br J Ophthalmol 2018; 102: 1192–1198. doi:10.1136/bjophthalmol-2017-311317.
- Ahn SM, Lee SY, Hwang S-Y et al. Retinal vascular flow and choroidal thickness in eyes with early age-related macular degeneration with reticular pseudodrusen. BMC Ophthalmol 2018; 18: 184. doi:10.1186/s12886-018-0866-3.
- Trinh M, Kalloniatis M, Nivison-Smith L. Radial peripapillary capillary plexus sparing and underlying retinal vascular impairment in intermediate age-related macular degeneration. Invest Ophthalmol Visual Sci 2021; 62: 2. doi:10.1167/iovs.62.4.2.
- Yang K, Zhan SY, Liang YB et al. Association of dilated retinal arteriolar caliber with early age-related macular degeneration: the Handan eye study. Graefes Arch Clin Exp Ophthalmol 2012; 250: 741–749. doi:10.1007/s00417-011-1824-4.
- Jeganathan VSE, Kawasaki R, Wang JJ et al. Retinal vascular caliber and age-related macular degeneration: the Singapore Malay eye study. Am J Ophthalmol 2008; 146: 954–959.e1. doi:10.1016/j.ajo.2008.07.006.
- Liew G, Kaushik S, Rochtchina E et al. Retinal vessel signs and 10-year incident age-related maculopathy: the blue mountains eye study. Ophthalmology 2006; 113: 1481–1487. doi:10.1016/j.ophtha.2006.03.051.
- Chin YC, Wong TY, Cheung CMG et al. Retinal vascular caliber and age-related macular degeneration in an Indian population from Singapore. Ophthalmic Epidemiol 2014; 21: 224–229. doi:10.3109/09286586.2014.926941.
- Klein R, Clegg L, Cooper LS et al. Prevalence of age-related maculopathy in the atherosclerosis risk in communities study. Arch Ophthalmol 1999; 117: 1203–1210. doi:10.1001/archopht.117.9.1203.
- Pappelis K, Jansonius NM. Retinal vessel caliber measurement bias in fundus images in the presence of the central light reflex. Transl Vis Sci Technol 2023; 12: 16. doi:10.1167/tvst.12.7.16.
- Guymer R, Wu Z. Age-related macular degeneration (AMD): more than meets the eye. The role of multimodal imaging in today’s management of AMD-a review. Clin Exp Ophthalmol 2020; 48: 983–995. doi:10.1111/ceo.13837.
- Guo S, Yin S, Tse G et al. Association between caliber of retinal vessels and cardiovascular disease: a systematic review and meta-analysis. Curr Atheroscler Rep 2020; 22: 16. doi:10.1007/s11883-020-0834-2.
- Moreira-Neto CA, Lima LH, Zett C et al. En-face OCT and OCT angiography analysis of macular choroidal macrovessel. Am J Ophthalmol Case Rep 2021; 21: 101012. doi:10.1016/j.ajoc.2021.101012.
- Jiang X, Shen M, Gregori G et al. Swept-source OCT en face imaging of paravascular inner retinal defects. Ophthalmic Surg Lasers Imaging Retina 2021; 52: 407–411. doi:10.3928/23258160-20210628-10.
- Lee MD, Kaidonis G, Kim AY et al. En face optical coherence tomography angiography imaging versus fundus photography in the measurement of choroidal nevi. Ophthalmic Surg Lasers Imaging Retina 2017; 48: 741–747. doi:10.3928/23258160-20170829-09.
- Jamous KF, Kalloniatis M, Hennessy MP et al. Clinical model assisting with the collaborative care of glaucoma patients and suspects. Clin Exp Ophthalmol 2015; 43: 308–319. doi:10.1111/ceo.12466.
- Wang H, Kalloniatis M. Clinical outcomes of the centre for eye health: an intra-professional optometry-led collaborative eye care clinic in Australia. Clin Exp Optom 2021; 104: 795–804. doi:10.1080/08164622.2021.1878821.
- Gaurisankar ZS, van Rijn GA, Lima JEE et al. Correlations between ocular biometrics and refractive error: a systematic review and meta-analysis. Acta Ophthalmol 2019; 97: 735–743. doi:10.1111/aos.14208.
- Ferris FL, Wilkinson CP, Bird A et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013; 120: 844–851. doi:10.1016/j.ophtha.2012.10.036.
- Choi W, Mohler KJ, Potsaid B et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One 2013; 8: e81499. doi:10.1371/journal.pone.0081499.
- Carl Zeiss Meditec, Inc. CIRRUS HD-OCT User Manual – Models 500, 500.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155–163. doi:10.1016/j.jcm.2016.02.012.
- Xu L, Wang S, Li Y et al. Retinal vascular abnormalities and prevalence of age-related macular degeneration in adult Chinese: the Beijing eye study. Am J Ophthalmol 2006; 142: 688–689. doi:10.1016/j.ajo.2006.05.028.
- Sun C, Wang JJ, Mackey DA et al. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol 2009; 54: 74–95. doi:10.1016/j.survophthal.2008.10.003.
- Rabiolo A, Benatti L, Tomasso L et al. Retinal arterial dilation is impaired in eyes with drusen and reticular pseudodrusen. Retina 2019; 39: 2205–2211. doi:10.1097/IAE.0000000000002283.
- Machalińska A, Safranow K, Dziedziejko V et al. Different populations of circulating endothelial cells in patients with age-related macular degeneration: a novel insight into pathogenesis. Invest Ophthalmol Visual Sci 2011; 52: 93–100. doi:10.1167/iovs.10-5756.
- Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens 2013; 2013: 808353. doi:10.1155/2013/808353.
- van den Akker J, Schoorl MJC, Bakker ENTP et al. Small artery remodeling: current concepts and questions. J Vasc Res 2010; 47: 183–202. doi:10.1159/000255962.
- Tan JSL, Mitchell P, Smith W et al. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the blue mountains eye study. Ophthalmology 2007; 114: 1143–1150. doi:10.1016/j.ophtha.2006.09.033.
- Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol 1999; 6: 125–143. doi:10.1076/opep.6.2.125.1558.
- Brinchmann-Hansen O, Sandvik L. The width of the light reflex on retinal arteries and veins. Acta Ophthalmol (Copenh) 1986; 64: 433–438. doi:10.1111/j.1755-3768.1986.tb06948.x.
- Brinchmann-Hansen O. The light reflex on retinal arteries and veins. A theoretical study and a new technique for measuring width and intensity profiles across retinal vessels. Acta Ophthalmol 1986; 179: 1–53.
- Brinchmann-Hansen O, Myhre K, Sandvik L. The light reflex in retinal vessels and its relation to age and systemic blood pressure. Acta Ophthalmol (Copenh) 1987; 65: 206–212. doi:10.1111/j.1755-3768.1987.tb07002.x.
- Burgansky-Eliash Z, Barash H, Nelson D et al. Retinal blood flow velocity in patients with age-related macular degeneration. Curr Eye Res 2014; 39: 304–311. doi:10.3109/02713683.2013.840384.
- Klein R, Klein BEK, Knudtson MD et al. Are inflammatory factors related to retinal vessel caliber? The Beaver dam eye study. Arch Ophthalmol 2006; 124: 87–94. doi:10.1001/archopht.124.1.87.
- Wagner SK, Fu DJ, Faes L et al. Insights into systemic disease through retinal imaging-based oculomics. Transl Vis Sci Technol 2020; 9: 6. doi:10.1167/tvst.9.2.6.
- Sudlow C, Gallacher J, Allen N et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12: e1001779. doi:10.1371/journal.pmed.1001779.
