ABSTRACT
Clinical relevance
Choroidal thickness measurement is gaining popularity in clinical practice and research as an early indicator of myopia progression. Understanding the influence of temperature on choroidal thickness changes will improve the reliability of the measures.
Background
It has been suggested that environmental temperature may affect choroidal thickness and blood flow, with potential implications for ocular disease and refractive development. This study investigates the effect of changes in eyelid/ocular adnexa temperature on choroidal thickness.
Methods
In a paired-eye study, 20 young, healthy subjects received a warm stimulus (heat pack) over one closed eye and simultaneously a cold stimulus (ice pack) over the other for 10 min. Eyelid temperatures were monitored with thermal probes, and optical coherence tomography scans of the retina and choroid were taken before and after heating and cooling, and then every 5 min during a 15-min recovery period. Retinal and choroidal thicknesses were measured across the macular region (6 mm), including the subfoveal (1 mm), parafoveal (1–3 mm), and perifoveal (3–5 mm) regions, and compared between the cooled and warmed eyes.
Results
When the thermal stimuli were applied, eyelid surface temperatures changed predictably and remained significantly different (by approximately 10–15°C) between the eyes after 2 min (p < .001). Relative to the warmed eye, macular choroidal thickness in the cooled eye increased significantly after 10 min of treatment (p = .004). This choroidal thickening response occurred in the subfoveal, parafoveal, and perifoveal regions (all p < .05). Upon removal of the thermal stimuli, choroidal thickness rapidly returned to the baseline and was no longer different between the cooled and warmed eye (p = .641).
Conclusion
Cooling the anterior eye by application of a cold stimulus directly onto the closed eyelid caused a small but significant increase in choroidal thickness relative to warming the anterior eye, demonstrating that the choroid can modulate its thickness rapidly and transiently in response to local temperature changes.
Introduction
In humans, the outer retina, including the retinal pigment epithelium, is avascular and receives its blood supply entirely from the choroid. Choroidal blood flow accounts for approximately 85% of ocular blood flowCitation1 and far exceeds the metabolic requirements of the retina,Citation2 as the oxygen content of venous blood remains at approximately 95% of arterial blood.Citation3 This exceedingly high blood flow suggests that the choroidal circulation may have additional roles other than basic metabolic exchange.
Maintaining a stable retinal temperature is one proposed physiological function of seemingly excessive choroidal blood flow.Citation4 Such a thermoregulatory action might be physiologically necessary since the retina and choroid continuously absorb a large proportion (~25–33%)Citation5 of the light energy incident on the cornea. Choroidal circulation could regulate temperature by acting both as a heat sink and a heat source.Citation6,Citation7 Evidence for the choroid’s thermoregulatory role comes from studies on experimentally induced short-term temperature changes on the surface of the human eyes. In one study, a warming lamp held over closed eyelids for 10 min caused the ocular temperature to rise significantly but choroidal blood flow, as measured with a laser Doppler flowmeter, decreased rapidly, presumably to maintain a stable retinal temperature.Citation8
Despite the potential role of the choroid in retinal thermoregulation, the underlying mechanism remains largely unknown. The choroid is a dynamic structure with the capacity to change its thickness in response to various factors,Citation9 including exercise,Citation10 accommodation,Citation11 and retinal image defocus.Citation12 In humans, exposing one eye to a light source increases choroidal blood flow in the contralateral eye with a corresponding increase in the contralateral tissue temperature,Citation13 which suggests a centrally mediated response to temperature change.
However, a local thermoregulatory mechanism may also be involved, as choroidal thickness varies according to the heat flux density on the surface of the cornea,Citation14 and thickness and blood flow are positively correlated in both health,Citation15–17 and diseases (e.g., diabetic retinopathy, high myopia).Citation18–20 To the extent that changes in choroidal thickness and blood flow mediate the thermoregulatory function of the choroid, this could have important implications in ischaemic retinal disease, as reduced blood flow could compromise the ability of the choroid to regulate retinal temperature.
However, little is known about choroidal thickness responses to locally induced changes in ocular surface temperature. While obtaining direct measures of choroidal blood flow is challenging,Citation21 choroidal thickness can be readily measured using optical coherence tomography (OCT).
This study tested the hypothesis that changing the surface temperature of the anterior eye and adnexa alters choroidal thickness. A paired-eye, within-subject study design was used to stress test the ocular thermoregulatory system by simultaneously cooling one eye, warming the other, and comparing the choroidal thickness response between the cooled and warmed eye.
Methods
Study design
This prospective paired-eye study included 20 healthy adult subjects (14 females, age ± SD: 23 ± 3 years, spherical equivalent refraction (SER): Right eye: −1.93 ± 1.97D; Left eye: −1.99 ± 1.93D). Subjects were recruited primarily from staff and students of the Faculty of Medical and Health Sciences at the University of Auckland through advertisements. The study adhered to the tenets of the Declaration of Helsinki and received ethics approval from the University of Auckland Human Participants Ethics Committee (ref: 019994). All subjects provided informed consent in writing, and their data were de-identified.
The primary outcome was the relative change in choroidal thickness between the eyes receiving the two treatments: warm vs cold. Since temperature changes were induced via conduction (i.e., thermal stimuli touching the closed eyelid), potential mechanical effects were controlled by having both eyes receive the same mechanical stimulus but different thermal stimulation. However, as a result, there was no untreated thermal control eye. Consequently, relative effects between warmed and cooled eyes are reported. The secondary outcomes were comparisons of changes in eyelid surface temperature, forehead surface temperature, retinal thickness (RT), and intraocular pressure (IOP).
Experimental protocol
Before enrolment in the study, all subjects underwent an initial eye examination to establish eligibility. This included testing of visual acuity and measurement of refractive error with an open-field auto-refractor (NVision-K 5001, Shin-Nippon, Japan; http://www.shin-nippon.jp/) with subjects fixating a target at 6 m. Subjects were excluded if they had a best-corrected visual acuity greater than 0.00 LogMAR, high refractive error (SER of ±6.00D or greater), anisometropia greater than 1.00D, any history of ocular or cardiovascular diseases and medications, ocular surgery or trauma.
Before baseline measurements were made, subjects viewed a video movie for 20 min at 6 m with their habitual optical correction while seated and as still as possible, to minimise the influence of previous tasks on choroidal thickness. In addition, all subjects were asked to avoid cigarette smoking and caffeine intake for 6 h before starting the experiment. The room temperature was between 19.8°C and 20.7°C, and the ambient lighting was 10 lux. All experimental sessions were conducted between 1 and 5 PM to control for diurnal variation in choroidal thickness across subjects.
Following the 20-min stabilisation period the video was paused, and baseline measurements of eyelid surface temperatures and IOPs were recorded, and two OCT scans were taken of each eye. Each eye was then subjected to either warm or cold treatment (decided by a coin toss) for 10 min, using a bespoke heating/cooling device. The device consisted of an elastic headband with pockets sewn on, which rested on the surface of the closed eyelids, with holes so that temperature probes could be attached.
A heat pack (a pocket hand warmer, 96 g, https://www.kathmandu.co.nz/) was inserted into one pocket and an ice pack (~120 g) into the other. During the 10-min treatment period, eyelid surface temperatures were recorded every 2 min. After the 10-min treatment period, the temperature sensors, headband, and heat/ice packs were removed, and OCT images and then IOP measurements (TA01i, https://www.icaretonometer.com) were taken of both eyes.
Subjects then watched the video for further 15 min (the recovery period, with habitual correction as for the stabilisation period), during which both the eyelid surface temperature and OCT images were taken in both eyes every 5 min.
Temperature measurements
Eyelid surface temperatures were measured using a digital thermometer (QM1601, DIGITECH, China) with an operating range of −50 to 1300°C and a resolution of 0.1°C, via two external K-type thermocouple probes. Thermocouples were placed in contact with the upper eyelids of the closed eyes, during the application of both heat and cold stimuli giving simultaneous measures of temperature from the two eyes.
Optical coherence tomography
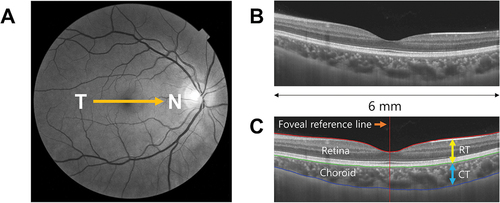
Cross-sectional chorioretinal images of both eyes were obtained using Topcon Deep Range Imaging Swept Source OCT (DRI SS-OCT Triton, Topcon Corp., Tokyo, Japan; http://www.topcon.co.jp/) in horizontal line scan mode (6 mm length, 128 averaged samples per line, 1024 intervals) centred on the fovea () while the subjects viewed the internal fixation light. The SS-OCT has a scan acquisition rate of 100,000 A-scans per second with a lateral resolution of 20 microns and depth resolution of 8 microns and uses a sweeping laser (tuneable range of approximately 100 nm) as a light source centred at a wavelength of 1050 nm.
Figure 1. Examples of (A) an OCT fundus image overlaid with a six mm horizontal line scan in a temporal (T) to nasal (N) direction, (B) a B-scan image from the OCT after averaging 128 individual scans from the same location, (C) a B-scan image after correction for tilt and layer segmentation. The software automatically segmented the internal limiting membrane (red), retinal pigment epithelium – choroid interface (green), and choroidoscleral interface (blue) for choroidal thickness (CT) and retinal thickness (RT) measurements.
For each subject, after the first scan, subsequent scans were performed under follow-up mode, in which the system automatically aligns the scan with the previous scan position using the infrared retinal image. In rare cases, where any of the 128 scan lines were missed (e.g., due to blink), or when the image quality score was poor (IQ score < 60), the scan was immediately repeated.
All B-scans, in their original resolution, were exported for further analysis using OCT image segmentation software developed at the Queensland University of Technology (OCT-Tool, ver. 4.0.4).Citation22 All the scans (n = 240) were de-identified and shuffled using a custom-written script in MATLAB (2017b; The MathWorks, USA) before horizontally aligning and processing through the OCT segmentation tool (). The software automatically placed a foveal reference line, but this line could be manually adjusted to ensure it passed through the centre of the foveal pit.
Each segmentation line (n = 128 per scan) was examined to ensure correct segmentation, and manually corrected with the ‘Correct Layer’ tool included in the software if required. Following segmentation, the image identifiers were restored, and the segmented data was imported into MATLAB. The macular choroidal thickness and retinal thickness (RT) were calculated as the mean of thickness measures along the 6 mm lateral width of the scan ().
To investigate whether the effect was focal or distributed throughout the choroid, regional choroidal thicknesses were calculated as the mean of the thickness measures in the subfoveal (1 mm), parafoveal (1–3 mm) and perifoveal (3–5 mm) regions along the line. No attempt was made to correct images for magnification effects because the primary parameter of interest was within-subject effects.
There was excellent repeatability of choroidal thickness between the two baseline measurements in both the cooled eye (e.g., macular choroidal thickness: r = .987, p < .001, mean [±95% CI] difference [second minus first] = −2.98 [−7.58, 1.61] µm) and the warmed eye (r = .998, p < .001, mean difference = 1.54 [−3.60, 0.51] µm).
Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS 24.0, SPSS Inc. Chicago, USA) and MATLAB (2017b; The MathWorks, USA). Separate analyses were conducted for the treatment and the recovery phase of the experiments. Some changes in eyelid surface temperature were not normally distributed and were compared using the non-parametric Friedman Test, with post-hoc pairwise comparisons using the Wilcoxon signed-rank test.
Normally distributed data are reported as mean (95% CI), while non-normally distributed data as median and interquartile ranges (25th percentile, 75th percentile). Changes (post-minus pre-treatment) in thickness and IOP following the treatment period were compared between the cooled and the warmed eye using two-sided paired t-tests. Similarly, changes in thickness during the recovery period were analysed with a two-way repeated-measures ANOVA using the type of eye (warmed vs cooled) and time points (pre-treatment baseline, 5, 10, and 15 min of the recovery period) as factors.
Post hoc multiple comparisons used Holm–Sidak corrections where appropriate. Correlation analysis was performed using Pearson’s R. Results were considered statistically significant at p < .05.
Results
Eyelid surface temperature
The surface temperature of the eyelid increased rapidly with the application of the warm stimulus (χ2 (5) = 71.56, p < 0.001) and decreased rapidly with the application of the cold stimulus (χ2 (5) = 94.90, p < .001) during the treatment period (). There was an increase in surface temperature in the warmed eye after just 2 min of treatment (difference = +5.25°C [4.65, 6.65], Z = −3.92, p < .001). Likewise, the surface temperature in the cooled eye decreased significantly after just 2 min of treatment (difference = −5.30°C [−8.45, −4.25], Z = −3.92, p < .001). Temperature changes continued throughout the 10-min treatment period, reaching a maximum increase of 7.25°C in the warmed eye and −8.60°C in the cooled eye.
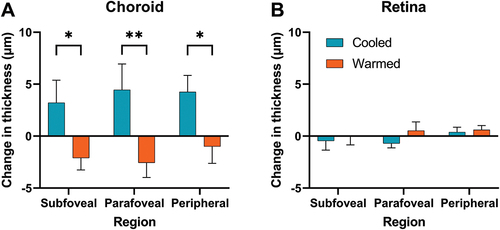
Figure 2. Time course of the changes in (a) eyelid surface temperature (b) choroidal thickness (CT) and (c) macular retinal thickness with a warm stimulus (orange) and a cold stimulus (blue). The yellow-shaded region indicates 10 min of the treatment period; the grey-shaded region indicates 15 min of the recovery period following the removal of the stimuli. *Indicates significantly different changes in choroidal thickness between the eyes. Data are presented as the median in (a) and mean in (b) and (c), and the error bars represent the interquartile range in (a) and ±1 standard errors of the mean in (b) and (c).
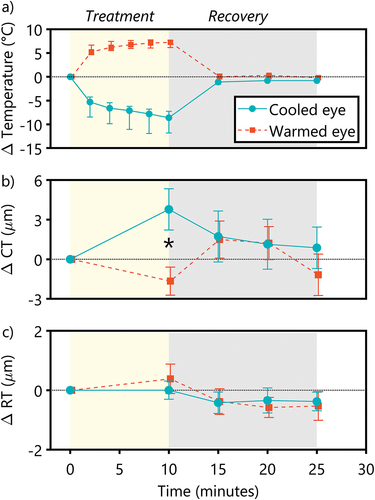
Following the removal of the warm stimulus, the surface temperature rapidly returned to baseline temperatures after just 5 min of recovery (difference from baseline = 0°C [−0.28, 0.60], Z = −1.09, p = .276), and remained stable throughout the recovery period (10 min: difference = 0.30 [−0.40, 0.58], Z = −1.11, p = .267; 15 min: difference = −0.20 [−0.45, 0.23], Z = −1.02, p = .306). The eyelid surface temperature of the eye with the cold stimulus also rapidly returned to the baseline after just 5 min of recovery (difference from baseline = 1.1°C [−1.60, −0.90], Z = −3.41, p = .001); however, there was a small but significant change from the baseline that persisted throughout the recovery period (10 min: difference = −0.80 [−1.10, −0.63], Z = −2.88, p = .004; 15 min: difference = −0.80 [−1.10, −0.45], Z = −3.18, p = .001).
During the treatment period, there was a significant difference in temperature changes between the cooled eye and the warmed eye at every time point (all p ≤ .001). During the recovery period, small but statistically significant differences in eyelid temperature between the eyes remained after 5 min (Z = −3.33, p = .001) and 10 min (Z = −3.24, p = .001) but not after 15 min of recovery (Z = −1.95, p = .051).
Choroidal thickness
Choroidal thickness in the cooled eye increased significantly after 10 min of treatment (difference = +3.78 µm [+1.07 to +6.89], t19 = 2.42, p = .026) but not in the warmed eye (difference = −1.65 µm [−3.63 to 0.49], t19 = −1.56, p = .135). After 10 min of thermal stimulation, there was a significant difference in the change in choroidal thickness between the eyes, with the change in choroidal thickness in the cooled eye higher compared to that in the warmed eye (difference = +5.43 µm [+1.93 to +8.92], t19 = 3.25, p = .004) (). Orthogonal regression analyses revealed that these changes in choroidal thickness were independent of baseline choroidal thickness in both the cooled eye (r = .03, p = .442) and the warmed eye (r = .001, p = .890).
After the removal of the thermal stimuli, choroidal thickness rapidly returned to baseline levels, and there was no longer any significant difference between the eyes (F1, 19 = 0.23, p = .641) across the recovery time (F3,57 = 1.14, p = .335). There was also no interaction between the eye and recovery time on changes in choroidal thickness during the recovery period (F3,57 = 0.69, p = .560), suggesting similar rates of return to thermoregulatory homoeostasis.
Changes in choroidal thickness in the subfoveal, parafoveal, and perifoveal regions between the cooled and the warmed eye followed a similar pattern (). The changes in choroidal thickness were significantly different between the eyes (F1, 57 = 23.2, p < .0001), and relative to the warmed eye, the choroid in the cooled eye was significantly thicker in the subfoveal (difference = +5.32 µm [1.21 to 9.43], p = .029), parafoveal (difference = +7.03 µm [1.65 to 12.41], p = .0046) and perifoveal (difference = +5.26 µm [1.69 to 8.83], p = .029) regions. This suggests that the observed effect was not localised but distributed throughout the choroid, consistent with overall changes in blood flow.
Retinal thickness
Compared to the baseline, the macular RT remained relatively stable, with no significant change even after 10 min of both the heat (difference = 0.38 µm [−0.65 to 1.42], t19 = 0.77, p = .450) and the cold treatment (difference = 0.00 µm [−0.638 to 0.639], t19 = 0.001, p = .999). Furthermore, there was no significant difference in the change of RT between the cooled and the warmed eye (difference = −0.38 µm [−1.71 to 0.95], t19 = −0.60, p = .555, ). Following the removal of the thermal stimuli, there were no changes in RT between the eyes (F1,19 = 0.06, p = .803) or across the recovery time (F3,57 = 1.50, p = .224). When stratified into regions (subfoveal, parafoveal, and perifoveal), again there were no significant differences between the cooled and the warmed eye (F1, 57 = 1.96, p = .17), nor across different regions of the retina (F2, 57 = 0.52, p = .60).
Intraocular pressure (IOP)
Following the application of the heat and the cold stimuli for 10 min, there was a significant decrease in IOP in both the cooled (difference = −2.35 mmHg [−3.43 to −1.27], t19 = −4.57, p < .001) and the warmed eye (difference = −1.40 mmHg [−2.49 to −0.31], t19 = −2.69, p = .014). However, the mean change in IOP between the two eyes was not different (difference = −0.95 mmHg [−2.34 to 0.44], t19 = −1.43, p = .168). The changes in IOP did not correlate with changes in macular choroidal thickness in either the cooled eye (r = .15, p = .528) or the warmed eye (r = −.03, p = .902).
Discussion
Using high-resolution OCT imaging, this study has shown the effect and time course of recovery of choroidal thickness upon cooling and warming of the anterior eye. Cooling the anterior eye caused a rapid increase in choroidal thickness, resulting in a relative difference in choroidal thickness change between the cooled and the warmed eye after 10 min. The choroidal response to local ocular cooling was rapid and transient, returning to the baseline within 5 min after the removal of the stimulus. This finding suggests that the choroid can rapidly modulate its thickness in response to changes in locally induced surface temperature. Interestingly, warming the anterior eye, in contrast, did not affect the choroidal thickness, at least in the short term, and the choroidal thickness remained relatively stable during both the treatment and recovery period. This may suggest a greater capacity for the normal choroidal blood flow to remove excess heat by acting as a heat sink than to add additional heat to the ocular tissues.
The present findings are consistent with previous speculations that anterior eye temperature changes could influence the thickness of the choroid,Citation7,Citation23 and perhaps expected as the choroid is thought to act as a temperature modulator to stabilise retinal temperature.Citation24 In monkeys, changes in choroidal blood flow (through changes in IOP) produce a reduction of retinochoroidal temperature under low ambient illumination and an increase in retinochoroidal temperature under high ambient illumination.Citation6 The choroidal action of retinal thermoregulation cannot be attributed to autoregulation of choroidal blood flow.Citation25
The relative increase in choroidal thickness with the cold stimulus could be due to choroidal blood flow compensating for the decreasing retinal temperature through a local mechanism. In cats with experimentally cooled conjunctiva, the retinal temperature remained relatively constant, presumably due to the temperature stabilising effect of choroidal blood flow, but the compensatory response was no longer present when vasoconstrictors were used.Citation26 It could also be argued that the opposite effect should then occur with anterior eye warming. However, no changes were observed in choroidal thickness with warming, contrary to that noted by others.Citation8 The neural mechanism by which the choroid senses changes in temperature is uncertain, and different mechanisms could underlie choroidal responses to increases and decreases in temperature. Moreover, anterior components of the eye could be better at dissipating excess heat, and so prevent conduction to the posterior retina.Citation24
Another possibility is that the application of a cold stimulus to the periorbital region could have induced a diving response, due to stimulation of the ophthalmic division of the trigeminal nerve,Citation27 as used in the cold-face test for assessing trigeminal-brainstem-vagal reflex integrity.Citation28–30 If this was the case, the observed increment in choroidal thickness could be due to the increased supply of blood to the central nervous system (including the eyes) while limiting the supply to peripheral organs. A centrally mediated response is plausible since light exposure in humans has been shown to change tissue temperature and choroidal blood flow in the contralateral eye.Citation13 However, no changes were observed in choroidal thickness with the warmed eye despite applying a thermal stress of similar magnitude. Since the choroid showed relative thickening in response to the cold stimulus, a systemic diving response induced by the application of cold stimuli could have negated the choroidal thinning response of the contralateral warmed eye. Nevertheless, the relative thickening of the choroid in the cooled eye compared to the warmed eye likely suggests a local mechanism underlying choroidal thickness modulation in response to changes in temperature.
The magnitude of choroidal thickness changes in this study was small but similar to the experimentally induced changes that have been observed in several previous studies.Citation11,Citation31–35 It is possible that changes in choroidal thickness were confined to sub-levels of the choroid, particularly the vascular luminal area, instead of the stromal area, resulting in only subtle changes in overall choroidal thickness. This is further supported by the present observation that the thickness changes were independent of the baseline choroidal thickness, and previous findings that changes in the luminal area were primarily responsible for diurnal changes in choroidal thickness.Citation36
Changes in choroidal thickness between the warmed and the cooled eye were consistent from the centre to the periphery, increasing with the cold treatment and decreasing with the warm treatment. This suggests that the choroidal responses to temperature changes are not localised but rather distributed throughout the choroid, and are presumably occurring via a global mechanism, such as changes in blood flow. This study is, however, unable to rule out a local mechanism since the thermal stimuli likely affected the entire anterior eye. It remains to be seen whether locally applied thermal stimuli would trigger localised choroidal responses to changes in temperature.
Interestingly, a reduction in IOP was observed in both the warmed and the cooled eyes. Alterations in IOP could cause changes in choroidal thickness, as previous reports have shown both a negativeCitation37 and a positive correlationCitation38 between IOP and choroidal thickness changes. However, changes in IOP would not explain the selective increase in choroidal thickness with the cold stimulus, but not with the warm stimulus, as IOP was reduced in both cases. Rather, the removal of mechanical stimuli likely resulted in decreased IOP in both the cooled and the warmed eye. A reduction in IOP after ocular cooling has been previously noted, but unlike the present results, the study found no change in IOP after ocular warming.Citation39
This study lacks responses from control subjects in the absence of any thermal stimuli, which limits the absolute quantification of effects. Previous studies by the authors and other groups have found that the choroidal thickness remains relatively stable in control groups over similar periods,Citation40–43 although the observed change in choroidal thickness in response to thermal stimuli in this study appeared smaller than that exhibited by the control group in a previous study.Citation12 Nonetheless, the relative change in choroidal thickness observed between two contrasting thermal stimuli demonstrates the dynamic potential of the choroid to respond to temperature changes.
In this study, thermal stimuli were applied to the anterior eye, and measurement of retinal temperature was not possible. Therefore, the transference of temperature changes from the anterior eye to the retina is unknown. However, the anterior segment is also known to regulate the temperature of the posterior eye (although to a lesser extent).Citation24 Changes in surface temperature on the anterior eye observed in this study presumably influenced the temperature at the back of the eye. As the eyelids of participants were closed during temperature application, this would have prevented changes in tear evaporation, which also regulates eye temperature.Citation44 Although changes in tissue temperature may have influenced the reliability of OCT measures, no changes were observed in OCT measures of RT, suggesting that this effect, if present, was minimal and the observed changes were not artefacts induced by errors to the time-of-flight assumptions made by the OCT machine.
Another limitation is that the magnitude of the observed effect (average choroidal thickness change between the cooled and the warmed eye) was smaller than the axial resolution of the OCT instrument. Whether application of thermal stimuli for a longer duration produces a stronger choroidal response remains unknown. A higher resolution instrument, such as a low-coherence interferometer, may allow more precise quantification of axial measures as a surrogate for choroidal response to thermal stimuli.
Lastly, the lack of correction for lateral magnification of the OCT images might have affected inter-eye comparisons of parafoveal and peripheral choroidal thickness measurements. However, this is unlikely to influence the results since subjects with greater than 1.00D anisometropia were excluded, and there was a strong correlation in SER between the cooled and warmed eyes (r = 0.91, p < 0.0001).
In conclusion, changes in ocular surface temperature can produce rapid, local, and transient changes in choroidal thickness that are asymmetrical around heating and cooling. The potential clinical impact of these choroidal responses induced by relatively large magnitude changes in temperature remains unresolved. Nevertheless, consideration of changes in ambient or ocular surface temperature seems necessary when conducting studies on choroidal thickness.
Acknowledgements
The authors thank Joy Qin from the University of Auckland for assistance with data collection and Professor Scott Read from the Queensland University of Technology for providing the OCT segmentation software.
Disclosure statement
Safal Khanal: None
Philip RK Turnbull: None
Lucia Kim: None
John R. Phillips: Dr. Phillips is an inventor on patents related to contact lenses for myopia control
Additional information
Funding
References
- Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res 1973; 15: 15–29. doi: 10.1016/0014-4835(73)90185-1.
- Country MW. Retinal metabolism: a comparative look at energetics in the retina. Brain Res 2017; 1672: 50–57. doi: 10.1016/j.brainres.2017.07.025.
- Alm A, Bill A. The oxygen supply to the retina. I. Effects of changes in intraocular and arterial blood pressures, and in arterial PO2 and PCO2 on the oxygen tension in the vitreous body of the cat. Acta Physiol Scand 1972; 84: 261–274. doi: 10.1111/j.1748-1716.1972.tb05177.x.
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res 2010; 29: 144–168. doi: 10.1016/j.preteyeres.2009.12.002.
- Geeraets WJ, Williams RC, Chan G et al. The loss of light energy in retina and choroid. Arch Ophthal 1960; 64: 606–615. doi: 10.1001/archopht.1960.01840010608020.
- Parver LM, Auker CR, Carpenter DO. The stabilizing effect of the choroidal circulation on the temperature environment of the macula. Retina 1982; 2: 117–120. doi: 10.1097/00006982-198200220-00008.
- Turnbull PRK, Phillips JR. Ocular effects of virtual reality headset wear in young adults. Sci Rep 2017; 7: 16172. doi: 10.1038/s41598-017-16320-6.
- Nagaoka T, Yoshida A. The effect of ocular warming on ocular circulation in healthy humans. Arch Ophthal 2004; 122: 1477–1481. doi: 10.1001/archopht.122.10.1477.
- Ostrin LA, Harb E, Nickla DL et al. IMI-the dynamic choroid: new insights, challenges, and potential significance for human myopia. Invest Ophthalmol Vis Sci 2023; 64: 4. doi: 10.1167/iovs.64.6.4.
- Sayin N, Kara N, Pekel G et al. Choroidal thickness changes after dynamic exercise as measured by spectral-domain optical coherence tomography. Indian J Ophthalmol 2015; 63: 445–450. doi: 10.4103/0301-4738.159884.
- Woodman-Pieterse EC, Read SA, Collins MJ et al. Regional changes in choroidal thickness associated with accommodation. Invest Ophthalmol Vis Sci 2015; 56: 6414–6422. doi: 10.1167/iovs.15-17102.
- Chiang S-H, Phillips JR, Backhouse S. Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic Physiol Opt 2015; 35: 405–413. doi: 10.1111/opo.12218.
- Parver LM, Auker CR, Carpenter DO. Choroidal blood flow. III. Reflexive control in human eyes. Arch Ophthal 1983; 101: 1604–1606. doi: 10.1001/archopht.1983.01040020606021.
- Anatychuk L, Pasyechnikova N, Naumenko V et al. Temperature and heat flux density of the eye surface in healthy individuals with different subfoveal thickness of the choroid. Acta Ophthalmol 2022; 100. doi: 10.1111/j.1755-3768.2022.035.
- Kim M, Kim SS, Kwon HJ et al. Association between choroidal thickness and ocular perfusion pressure in young, healthy subjects: enhanced depth imaging optical coherence tomography study. Invest Ophthalmol Vis Sci 2012; 53: 7710–7717. doi: 10.1167/iovs.12-10464.
- Novais EA, Badaró E, Allemann N et al. Correlation between choroidal thickness and ciliary artery blood flow velocity in normal subjects. Ophthalmic Surg Lasers Imag Retina 2015; 46: 920–924. doi: 10.3928/23258160-20151008-04.
- Kim DY, Silverman RH, Chan RVP et al. Measurement of choroidal perfusion and thickness following systemic sildenafil (Viagra®). Acta Ophthalmol 2013; 91: 183–188. doi: 10.1111/j.1755-3768.2011.02305.x.
- Querques G, Lattanzio R, Querques L et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci 2012; 53: 6017–6024. doi: 10.1167/iovs.12-9692.
- Zhang Z, Qi Y, Wei W et al. Investigation of macular choroidal thickness and blood flow change by optical coherence tomography angiography after posterior scleral reinforcement. Front Med (Lausanne) 2021; 8: 658259. doi: 10.3389/fmed.2021.658259.
- Biberoglu E, Eraslan M, Midi I et al. Ocular blood flow and choroidal thickness changes after carotid artery stenting. Arq Bras Oftalmol 2020; 83: 417–423. doi: 10.5935/0004-2749.20200081.
- Khanal S, Turnbull PRK, Vaghefi E et al. Repeatability of arterial spin labeling MRI in measuring blood perfusion in the human eye. J Magn Reson Imag 2019; 49: 966–974. doi: 10.1002/jmri.26323.
- Alonso-Caneiro D, Read SA, Collins MJ. Automatic segmentation of choroidal thickness in optical coherence tomography. Biomed Opt Express 2013; 4: 2795. doi: 10.1364/BOE.4.002795.
- Yildirim Y, Kaya A, Kar T. Temperature control role of the choroid may affect choroidal thickness after dynamic exercise. Indian J Ophthalmol 2015; 63: 930. doi: 10.4103/0301-4738.176033.
- Parver LM. Temperature modulating action of choroidal blood flow. Eye 1991; 5: 181–185. doi: 10.1038/eye.1991.32.
- Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res 2000; 32: 249–256. doi: 10.1159/000055622.
- Stiehl WL, Gonzalez-Lima F, Carrera A et al. Active defence of retinal temperature during hypothermia of the eye in cats. J Physiol 1986; 81: 26–33.
- Gooden BA. Mechanism of the human diving response. Integr Physiol Behav Sci 1994; 29: 6–16. doi: 10.1007/BF02691277.
- Brown CM, Sanya EO, Hilz MJ. Effect of cold face stimulation on cerebral blood flow in humans. Brain Res Bull 2003; 61: 81–86. doi: 10.1016/S0361-9230(03)00065-0.
- Khurana RK. Cold face test: adrenergic phase. Clin Auton Res 2007; 17: 211–216. doi: 10.1007/s10286-007-0422-3.
- Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res 2006; 16: 202–207. doi: 10.1007/s10286-006-0332-9.
- Sander BP, Collins MJ, Read SA. Short-term effect of low-dose atropine and hyperopic defocus on choroidal thickness and axial length in young myopic adults. J Ophthalmol 2019; 2019: 1–8. doi: 10.1155/2019/4782536.
- Hoseini-Yazdi SH, Vincent SJ, Collins MJ et al. Regional alterations in human choroidal thickness in response to short-term monocular hemifield myopic defocus. Ophthalmic Physiol Opt 2019; 39: 172–182. doi: 10.1111/opo.12609.
- Delshad S, Collins MJ, Read SA et al. The human axial length and choroidal thickness responses to continuous and alternating episodes of myopic and hyperopic blur. PLOS ONE 2020; 15: e0243076. doi: 10.1371/journal.pone.0243076.
- Lou L, Ostrin LA. Effects of narrowband light on choroidal thickness and the pupil. Invest Ophthalmol Vis Sci 2020; 61: 40. doi: 10.1167/iovs.61.10.40.
- Hoseini-Yazdi H, Vincent SJ, Read SA et al. Astigmatic defocus leads to short-term changes in human choroidal thickness. Invest Ophthalmol Vis Sci 2020; 61: 48. doi: 10.1167/iovs.61.8.48.
- Kinoshita T, Mitamura Y, Shinomiya K et al. Diurnal variations in luminal and stromal areas of choroid in normal eyes. Br J Ophthalmol 2016: bjophthalmol-2016–308594. doi: 10.1136/bjophthalmol-2016-308594.
- Hata M, Hirose F, Oishi A et al. Changes in choroidal thickness and optical axial length accompanying intraocular pressure increase. Jpn J Ophthalmol 2012; 56: 564–568. doi: 10.1007/s10384-012-0173-0.
- Maul EA, Friedman DS, Chang D-T et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology 2011; 118: 1571–1579. doi: 10.1016/j.ophtha.2011.01.016.
- Shamshad MA, Amitava AK, Ahmad I et al. Changes in central retinal artery blood flow after ocular warming and cooling in healthy subjects. Indian J Ophthalmol 2010; 58: 189–194. doi: 10.4103/0301-4738.62641.
- Chiang STH, Phillips JR. Effect of atropine eye drops on choroidal thinning induced by hyperopic retinal defocus. J Ophthalmol 2018; 2018: 1–6. doi: 10.1155/2018/8528315.
- Ostrin LA, Sah RP, Queener HM et al. Short-term myopic defocus and choroidal thickness in children and adults. Invest Ophthalmol Vis Sci 2024; 65: 22. doi: 10.1167/iovs.65.4.22.
- Chiang S-H, Turnbull PRK, Phillips JR. Additive effect of atropine eye drops and short-term retinal defocus on choroidal thickness in children with myopia. Sci Rep 2020; 10: 18310. doi: 10.1038/s41598-020-75342-9.
- Chiang STH, Chen TL, Phillips JR. Effect of optical defocus on choroidal thickness in healthy adults with presbyopia. Invest Ophthalmol Vis Sci 2018; 59: 5188–5193. doi: 10.1167/iovs.18-24815.
- Craig JP, Singh I, Tomlinson A et al. The role of tear physiology in ocular surface temperature. Eye 2000; 14: 635–641. doi: 10.1038/eye.2000.156.