ABSTRACT
Background
NKT cell population is a relatively well-characterized immune cell subset. Numerous publications have characterized the phenotypical features of its subpopulations even in human pregnancy. Nevertheless, there have not been studies investigating the distribution of the NKT cells based on the surface presence of the CD8 receptor.
Methods
Thirty-four pregnant women from the first trimester, 30 from the second, and 36 from the third trimester of pregnancy in addition to 35 healthy non-pregnant women have been involved in the study. PBMCs were isolated from peripheral blood, CD8+ and CD8- NKT cells were then studied by flow cytometry using monoclonal antibodies. Immune checkpoint molecules and intracellular markers were also measured.
Results
Substantial differences were revealed in the proportions of the NKT cell subpopulations in the healthy control cohort and the pregnant groups. By comparing the investigated groups, significant changes were detected in the expression levels of PD-L1, TIGIT, CD155, and NKG2D. Further associations were observed through examination of the relative expressions of TIGIT and CD226 in the CD8+ NKT subset.
Conclusion
Data suggest that the CD8+ NKT cells are under fine regulation during healthy human pregnancy.
Introduction
Pregnancy is a natural model of active immunotolerance, in which the maternal immune system simultaneously faces two challenges; besides accepting the semiallogeneic fetus, the maternal immune system has to be prepared for immune defense, mostly against infections. Pregnancy is a physiological condition that includes the altered ratio of different lymphocyte subpopulations in comparison with the healthy non-pregnant status.
CD56 can be found on most natural killer (NK) cells and a subset on CD3+ T cells making up 1 -11-% of human peripheral blood lymphocytes (Romero-Olmedo et al. Citation2021). NK cells and their receptor expression were profoundly investigated in different malignancies and viral infections but there is limited data regarding NKT cell activity under the same circumstances (Konjevic et al. Citation2012; Konjević et al. Citation2016; Vuletić et al. Citation2013). However, the nomenclature of NKT cells is very controversial. They are often referred to as NK-like T cells or CD3+CD56+ NKT-like cells (Meggyes et al. Citation2019; Romero-Olmedo et al. Citation2021). NKT cells recognize lipid-based antigens and are separated into two broad classes: type 1 and type 2 NKT cells (Pellicci et al. Citation2020). Type 1 cells recognize the α-galactosylceramide (α-GalCer) and are less frequent, representing fewer than 1% of T cells in the human blood and liver, but they are still one of the most abundant TCR specificities (Godfrey et al. Citation2015; Miko et al. Citation2008). Humans appear to have greater numbers of type 2 NKT cells: those that express diverse TCRs with broader lipid antigen specificities. Nevertheless, these cells are difficult to identify, and little is known about how they develop (Pellicci et al. Citation2020).
Romero-Olmedo A.J. et al. recently performed a deep phenotyping of human peripheral blood CD56+ T cells by 41-parameter mass cytometry, distinguishing five major phenotypically distinct subsets, including multiple innate-like and classical T-cell populations (Romero-Olmedo et al. Citation2021). CD56+ T cells may also express other NK cell markers and comprise γδTCR+ T cells as well as CD8+, CD4+, CD4-CD8- double-negative (DN) T cells expressing diverse/invariant (iNKT) αβ chains of the TCR and TCRVα7.2+CD161+ mucosal associated invariant (MAIT) cells (Meggyes et al. Citation2018; Montoya et al. Citation2007). Their result shows that CD3+CD56+ T cells comprised mainly CD8+ and γδTCR+ T cells (36.1% and 27.1%) compared to less abundant CD4+, DN, and MAIT cells, making up 6.4%, 9% and 4.9%, respectively (Romero-Olmedo et al. Citation2021). These data indicate that NKT cells are more heterogeneous than previously appreciated.
Several immune checkpoint molecules have been suggested to function as inhibitory signaling mediators for maintaining immune tolerance and homeostasis. There also exist the membrane-bound (surface receptor) and cell-free soluble molecules. The most studied membrane-bound molecules are CTLA-4, TIM-3, LAG-3, PD-1 and TIGIT.
An increasing number of studies report that the PD-1 receptor, also known as CD279, is expressed by various leukocytes, including T and B lymphocytes, monocytes, NK cells, and dendritic cells (McBride et al. Citation2020; Miko et al. Citation2019). Signaling through PD-1 is executed through interaction with its cognate ligands, including PD-L1 and PD-L2. PD-L1 is expressed by many immune cell types (resting T cells, B cells, dendritic cells, macrophages) and various tissues, including the placenta, heart, and spleen (Miko et al. Citation2019).
LAG-3 was discovered 25 years ago as a molecule that is up-regulated on activated CD4+ T and CD8+ T cells and a subset of NK cells (Triebel et al. Citation1990). LAG-3 impacts the function of CD8+ T cells and NK cells, neither of which interacts with MHC Class II. This led for the speculation about the existence of alternate ligands for LAG-3. One of its ligands, Gal-3, having been identified on tumor cells, can promote the progression of malignancy (Cocks and Mills Citation2021; Friedman et al. Citation2020). Fibrinogen-like protein 1 (FGL1), a liver-secreted protein, is another ligand for LAG-3 that is independent from MHC-II and, thus, blocking the LAG-3/FGL1 pathway can stimulate tumor immunity and inhibit tumor growth (Guo et al. Citation2020; Wang et al. Citation2019).
CD226, a member of the immunoglobulin superfamily, is a functional protein initially expressed on T cells, NK cells, NKT cells, B cells, monocyte/macrophage, dendritic cells (DC), megakaryocyte/platelet lineage, and hematopoietic precursor cells (Huang et al. Citation2020). In recent years, the function of CD226 has increasingly been researched and understood. More evidence increasingly shows that CD226 is closely related to the occurrence of autoimmune diseases, infectious diseases, and tumors. In 2003, Bottino et al. confirmed that the ligands of human CD226 were CD155 and CD112 (Bottino et al. Citation2003). CD226, as an adhesion molecule, promotes the migration, activation, proliferation, differentiation and function of CD8 + T cells (Huang et al. Citation2020). More and more evidence shows that CD226 is involved in the biological function of NK cells (Huang et al. Citation2020; Wagner et al. Citation2017). CD226, together with T cell immunoreceptor with Ig and ITIM domains (TIGIT), is also involved in regulating NK cell function.
TIGIT, a co-inhibitory molecule on T cells, exerts immunosuppressive effects by competing with CD226 for the same CD155 ligand (Lozano et al. Citation2012). Indeed, CD226, as a costimulatory factor, plays an important role in the development of various diseases. Thus, manipulating CD226 expression and function may be a feasible therapeutic strategy for many immune-related diseases and tumors (Yeo et al. Citation2021). Despite the available publications regarding the investigated immune checkpoint molecules (PD-1, TIGIT, CD226 and LAG-3), their exact role in pathophysiological functions is not fully understood. However, based on our current investigations, these molecules could have a clinical relevance in reproductive immunology.
The systemic tolerance and maternal-fetal tolerance protect the fetus from rejection and lead to a successful pregnancy. Therefore, the relationship between immune checkpoint molecules and pregnancy could be particularly interesting. Immune checkpoint molecules, such as PD-1, LAG-3, and TIGIT, have been extensively investigated, but their impact on NKT cells is less described throughout healthy pregnancy. Furthermore, the analysis of NKT cells regarding CD8 positivity or CD8 negativity aiming at a better understanding of their phenotypic and functional characterization has not yet been performed during human healthy pregnancy.
Materials and methods
Ethical approval
Informed, written consent was obtained from all participants in accordance with a protocol approved by the Regional and Local Research Ethics Committee at the Medical School, University of Pecs (Reference number: 6149). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Participants, sample collection
34 healthy pregnant volunteers were enrolled from the first, 30 from the second and 36 from the third trimesters of pregnancy in cooperation with the Department of Obstetrics and Gynecology at the University of Pecs (). Each group had different patients. For a control group 35 healthy age-matched, non-pregnant women were also recruited. Under the EU-GDPR and due to the Privacy, Informational and Healthy Data Act Regulations, all personal and health-related data obtained about the donors during blood donation were processed anonymously, confidentially and securely, and were not provided to the research team for further demographic analysis. The health status of each of the participating women was identified; none of them had a significant medical history, were taking medications including hormonal contraceptives, nor had any recent illnesses. All women affected by pregnancy-related complications and/or infection, pre-pregnancy disease, in vitro fertilization pregnancies, immune-associated disease, diabetes mellitus or AIDS were excluded. None of the participants was a tobacco consumer/smoker.
Table 1. Gynecological and demographic data of the participated women.
10 ml of venous blood was drawn into heparinized tubes and immediately transported to the laboratory for further investigation.
Sample collection, PBMC separation, and cryopreservation
After peripheral blood mononuclear cells (PBMCs) had been separated from heparinized venous blood on Ficoll-Paque density (GE Healthcare, USA) gradient. Isolated cells were washed in RPMI 1640 medium (Lonza, Switzerland), counted and centrifuged. PBMCs were then resuspended in human serum containing 10% DMSO (Sigma-Aldrich, USA) for cryoprotection. Next, the cells were aliquoted in cryovials and stored in a −80°C mechanical freezer. On the day of fluorescent cell labeling, the cryovials were warmed up as quickly as possible in a 37°C water bath and DMSO was washed out twice in RPMI 1640 medium.
Fluorochrome-Conjugated monoclonal antibodies
Thawed PBMCs were used for surface and intracellular staining and analysis. The following monoclonal antibodies were used: Brilliant Violet (BV421)-conjugated anti-human CD226 (clone: D×11, BD Biosciences, USA), BV421-conjugated anti-human PD-L1 (Clone: MIH1, BD Biosciences, USA), BV510-conjugated anti-human CD3 (Clone: UCHT1, BD Biosciences, USA), fluorescein isothiocyanate (FITC)-conjugated anti-human CD4 (Clone: RPA-T4, BD Biosciences, USA), FITC-conjugated anti-human granzyme B (Clone: GB11, BD Biosciences, USA), phycoerythrin (PE)-conjugated anti-human CD112 (Clone: R2.525, BD Biosciences, USA), PE-conjugated anti-human galectin-3 (Clone: B2C10, BD Biosciences, USA), PE-conjugated anti-human PD-1 (Clone: PD1.3, Beckmann-Coulter, USA), PE-conjugated anti-human TIGIT (Clone: A1553 G, Biolegend, USA), Peridinin-Chlorophyll-Protein (PerCP)-conjugated anti-human LAG-3 (Clone: 11C3C65, Biolegend, USA), PE/Cy7-conjugated anti-human NKG2D (Clone: 1D11 BD Biosciences, USA), PE/Cy7-conjugated anti-human Perforin (Clone: dG9, Biolegend, USA), allophycocyanin (APC)-conjugated anti-human CD56 (Clone: B159, BD Biosciences, USA), APC-conjugated anti-human CD155 (Clone: R2.525, Biolegend, USA), APC/H7-conjugated anti-human CD8 (Clone: SK1, BD Biosciences, USA).
Flow cytometric staining and measuring
106 thawed PBMCs were labeled by monoclonal antibodies for 30 min at room temperature in darkness. Then the cells were washed with PBS (BioSera, France) and resuspended in 300 µl of PBS containing 1% paraformaldehyde (PFA, Sigma-Aldrich, USA) and stored at 4°C in complete darkness until FACS analysis. Various flow cytometric analyses were performed with a BD FACS Canto II flow cytometer (BD Immunocytometry Systems, Belgium) with the BD FACS Diva V6. software (BD Biosciences, USA) for data acquisition. Flow cytometric data analysis was performed by FCS Express V4 (De Novo Software, USA).
Intracellular staining
Following surface labeling, the samples were washed with PBS and fixed in 4% PFA solution for 10 min at room temperature in complete darkness. After that, the cells were washed with 2 ml of PBS and incubated with 1:10 diluted FACS Permeabilizing Solution 2 (BD Biosciences, USA) for 10 min at room temperature in darkness. The cells were then washed with 2 ml of PBS and stained with FITC-conjugated anti-human granzyme B, PE-Cy7-conjugated anti-human perforin and PE-conjugated anti-human Galectin-3 antibodies for 30 min at room temperature in complete darkness. Finally, the samples were washed again with 2 ml of PBS, fixed with 1% PFA and stored at 4°C in darkness until FACS analysis was performed.
Statistical analysis
To investigate the effect of the surface receptor CD8 and the impact of different trimesters on the expression pattern of immune checkpoint molecules in NKT cells, Two-Way-ANOVA was applied for statistical significance, testing in R, version 4.0.5 (R: a language and environmentfor statistical computing). Explanatory variables were loge transformed before analysis. Decisions on the transformation of variables depended on visual inspection of “model-checking plots” in R for the models with transformed vs. untransformed variables. These plots allow checking assumptions about the normality of residuals and variance homogeneity. For pair-wise comparisons, Tukey post hoc tests were conducted to compare each cell type/trimester combination to another.
To test the relationship between the relative TIGIT and CD226 expression of CD8+ and CD8- cells across trimesters (from healthy controls to the third trimester) linear regression analyses were performed. P values and coefficients of determination (r2) were calculated in R.
Results
Phenotype analysis of peripheral blood NKT cells throughout pregnancy and in healthy controls
Based on the gating strategy (.), two different NKT cell subpopulations were determined based on the presence of CD8 surface marker in the peripheral blood of healthy pregnant women in each trimester of pregnancy and in healthy non-pregnant women. The percentage of CD8+ NKT cells in the lymphocyte gate was significantly higher in healthy controls, and during the first and third trimesters than that of CD8- NKT cells; nevertheless, these results did not reach the level of significance during the second trimester (). There is no difference in the percentages each of CD8+ and CD8- NKT cells among the investigated groups (). When the proportion of the CD8+ and CD8- NKT cell populations was analyzed in the NKT cell population, the CD8+ NKT cells showed a significant increase in healthy controls and in women inall trimesters than the CD8- NKT cells (). However, the percentage of the CD8- NKT cell population significantly decreased in the healthy control group than in women in the second trimester ().
Figure 1. Gating strategy for flow cytometry analysis. Selection method of the investigated peripheral CD8+ and CD8- NKT immune cell subpopulations.
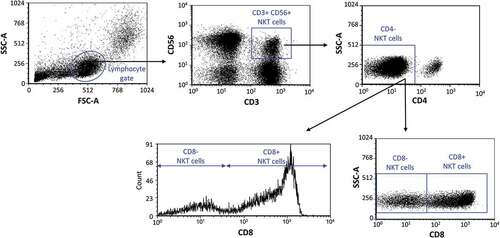
Figure 2. Percentage of the CD8 receptor positive and CD8 receptor negative NKT cells in all lymphocytes (a) and in the NKT subpopulation (b) in the three trimester of healthy pregnancy and in healthy controls. The solid bars represent medians of 35, 34, 30 and 36 determinations respectively, the boxes indicate the interquartile ranges, and the whiskers show the most extreme observations. The middle square within the box represents the mean value. Statistically significant differences with p-values <0.01*** and <0.03** are indicated.
PD-1 and PD-L1 expression by CD8+ and CD8- NKT cells throughout pregnancy
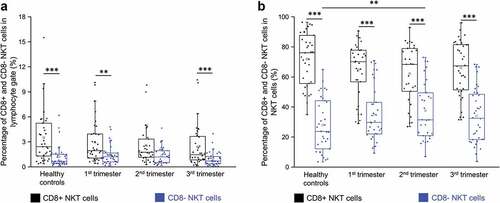
We measured the surface expression of PD-1 and PD-L1 on NKT cells by flow cytometry. PD-1 expression by neither CD8+ nor CD8- NKT cells exhibited any significant difference among the investigated groups (). CD8+ NKT cells from women in the third trimester showed a significantly decreased PD-L1 expression than those from the first- and second trimesters and the healthy control group (). CD8- NKT cells from women in the third trimester showed a significant decrease in PD-L1 expression only in comparison to the healthy control group ().
Figure 3. PD-1 receptor expression (a) and PD-L1 ligand expression (b) by CD8 receptor positive and CD8 receptor negative NKT cells in the three trimester of healthy pregnancy and in healthy controls. The solid bars represent medians of 15, 13, 10 and 18 determinations respectively, the boxes indicate the interquartile ranges, and the whiskers show the most extreme observations. The middle square within the box represents the mean value. Statistically significant differences with p-values <0.01***are indicated. Representative FACS plots and histograms show the expression of PD-1 surface marker (c) and PD-L1 surface molecule (d) by cells in the lymphocyte gate. To determine the positivity of PD-1 and PD-L1 fluorescent minus one (FMO) control was used.
TIGIT and CD226 receptors expression by CD8+ and CD8- NKT cells throughout pregnancy
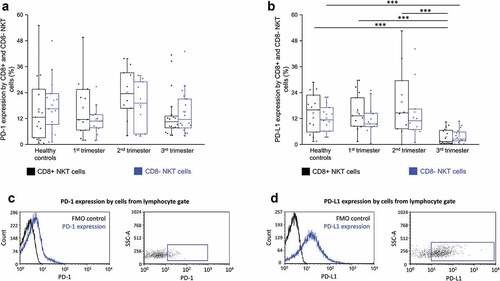
Among the inhibitory immune checkpoint receptors, the levels of TIGIT were the highest on NKT cells in pregnant women. The expression of TIGIT by CD8+ NKT cells showed a significant increase than that of their CD8- counterparts in healthy control women as well as women in the second and third trimesters during pregnancy (). CD226 expression showed no significant difference by CD8+ NKT cells and their CD8- counterparts in any of the investigated groups ().
Figure 4. TIGIT receptor expression (a) and CD226 receptor expression (b) by CD8 receptor positive and CD8 receptor negative NKT cells in the three trimester of healthy pregnancy and in healthy controls. The solid bars represent medians of 18, 20, 19 and 16 determinations respectively, the boxes indicate the interquartile ranges, and the whiskers show the most extreme observations. The middle square within the box represents the mean value. Statistically significant differences with p-values and <0.03**, <0.05* are indicated. Representative FACS plots and histograms show the expression of TIGIT surface marker (c) and CD226 surface molecule (d) expression by cells in the lymphocyte gate. To determine the positivity of TIGIT and CD226 FMO control was used.
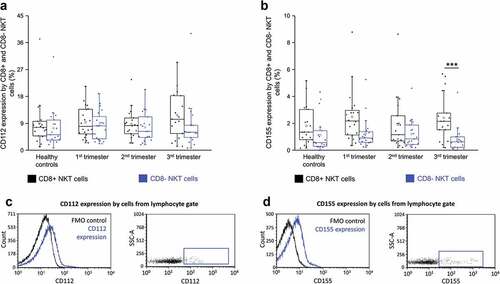
CD112 and CD155 ligands expression by CD8+ and CD8- NKT cells throughout pregnancy
CD112 expression by CD8+ NKT cells and their CD8- counterparts showed no significant difference in any of the investigated groups (). The expression of CD155 was significantly increased by CD8+ NKT cells than their CD8- counterparts in the third trimester only ().
Figure 5. CD112 expression (a) and CD155 receptor expression (b) by CD8 receptor positive and CD8 receptor negative NKT cells in the three trimester of healthy pregnancy and healthy controls. The solid bars represent medians of 19, 20, 19 and 17 determinations respectively, the boxes indicate the interquartile ranges, and the whiskers show the most extreme observations. The middle square within the box represents the mean value. Statistically significant differences with p-values <0.01*** are indicated. Representative FACS plots and histograms show the expression of CD112 surface marker (c) and CD155 surface molecule (d) expression by cells in the lymphocyte gate. To determine the positivity of CD112 and CD155 FMO control was used.
Relative expression of TIGIT and CD226 on CD8+ and CD8- NKT cells throughout pregnancy
We also investigated the ratio of the relative CD226 and relative TIGIT expressions (by mean fluorescent intensity) on NKT cells based on CD8 positivity in the peripheral blood of healthy pregnant women during each trimester of pregnancy and in healthy control women.
The value of the relative CD226/TIGIT expression ratio on CD8+ NKT cells was lower in all investigated trimesters than that of CD8- NKT cells, but it reached a level of significance only in the second and third trimesters of healthy pregnancy (). There is no difference in the values of the relative CD226/TIGIT expression ratio in healthy control individuals ().
Figure 6. The ratio of the CD226/TIGIT mean fluorescent intensity value on CD8 receptor positive and CD8 receptor negative NKT cells in the three trimester of healthy pregnancy and in healthy controls (a). The solid bars represent medians of 20, 19, 16 and 15 determinations respectively, the boxes indicate the interquartile ranges, and the whiskers show the most extreme observations. The middle square within the box represents the mean value. Statistically significant differences with p-values <0.03** and <0.05* are indicated. Linear regression results presenting the relationship between the relative TIGIT and CD226 expression of CD8 cells among the three trimester of pregnancy and healthy control group (b). Coefficients of determination (r2) are presented on each figure.
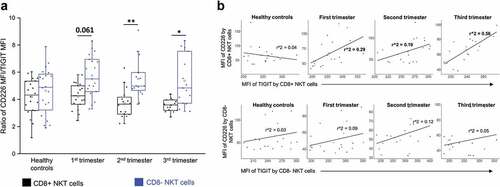
Through investigating the linear regression between the relative expression of CD226 and TIGIT surface receptors by NKT cell subpopulations based on the presence of CD8 receptors, a positive relationship was observed in the case of CD8+ NKT cells during pregnancy in all investigated trimesters. However, the regression was moderate only in the third trimester (). No relationship was found regarding CD8- NKT cells.
LAG-3 and Gal-3 expressions by CD8+ and CD8- NKT cells throughout
LAG-3 expression by CD8+ NKT cells and their CD8- counterparts showed no significant difference in any of the investigated groups (Figure S1). Gal-3 expression by CD8+ NKT and their CD8- counterparts ells showed no significant difference in any of the investigated groups (Figure S2).
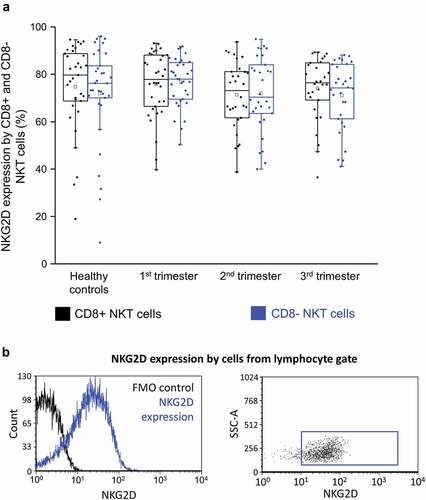
NKG2D expression by CD8+ and CD8- NKT cells throughout pregnancy
There was no significant difference in the expression of activating NKG2D receptor between CD8+ NKT cells and CD8- cells in any of the investigated groups ().
Figure 7. NKG2D expression (a) by CD8 receptor positive and CD8 receptor negative NKT cells in the three trimester of healthy pregnancy and in healthy controls. The solid bars represent medians of 33, 34, 30 and 36 determinations respectively, the boxes indicate the interquartile ranges, and the whiskers show the most extreme observations. The middle square within the box represents the mean value. Representative FACS plots and histograms show the expression of NKG2D surface marker (b) expression by cells in the lymphocyte gate. To determine the positivity of NKG2D FMO control was used.
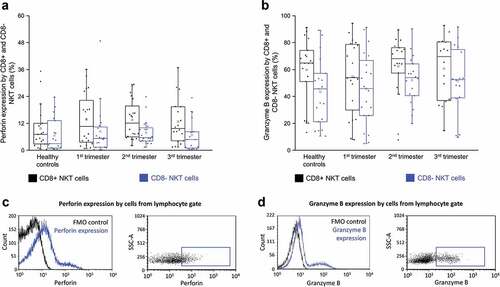
Perforin and granzyme B expressions by CD8+ and CD8- NKT cells throughout pregnancy
Besides the expression of inhibitory and stimulatory immune checkpoint receptors and their ligands, the effector functions of these cells were also examined. These experiments were performed to determine which subset expressed the highest cytotoxic potential. As shown in , there was no difference in the expression of perforin between CD8+ NKT cells and their CD8- counterparts in any of the investigated groups. Granzyme B expression by CD8+ NKT cells and their CD8- counterparts in any of the investigated groups (). At the same time, granzyme B content was measured and found to be significantly less in TIGIT+ CD8- subpopulation than in their TIGIT+ CD8+ counterpart in the second trimester of pregnancy (Figure S2b).
Figure 8. Intracellular perforin expression (a) and granzyme B receptor expression (b) by CD8 receptor positive and CD8 receptor negative NKT cells in the three trimester of healthy pregnancy and in healthy controls. The solid bars represent medians of 20, 21, 21 and 18 determinations respectively, the boxes indicate the interquartile ranges, and the whiskers show the most extreme observations. The middle square within the box represents the mean value. Representative FACS plots and histograms show the expression of perforin surface marker (c) and granzyme B intracellular molecule (d) expression by cells in the lymphocyte gate. To determine the positivity of perforin and granzyme B FMO control was used.
Discussion
NKT cells comprise a small subset of lymphocytes being able to release Th1 or Th2 cytokines after TCR ligation and induce perforin-, granzyme-, Fas- and TNF-related cytotoxicity (McCarthy et al. Citation2007). Many studies have investigated the alterations in the numbers of NKT cells during pregnancy (Abu-Raya et al. Citation2020; Boyson et al. Citation2008; Hosseini et al. Citation2019; Miko et al. Citation2021; Yuan et al. Citation2015), even though NKT cells have never been thoroughly investigated in terms of CD8 positivity or CD8 negativity when particularly focusing on their immune checkpoint molecules expression (PD-1, TIGIT, CD226, and LAG-3) or functional differences (perforin and granzyme B expression). The possible role of NKT cells regarding CD8 positivity or CD8 negativity throughout pregnancy and in pathological conditions is still to be elucidated, although elevated levels of NKT cells in the blood and their activation have been reported to be accompanied with poor pregnancy outcomes (Meggyes et al. Citation2019; Miko et al. Citation2008; Yuan et al. Citation2015).
In this study, we first sought to determine the distribution of NKT cell subsets during the three trimesters of gestation and in the healthy non-pregnant state.
When we compared the percentages of CD8+ and CD8- NKT cells in the lymphocyte gate among the investigated groups (healthy control, first, second and third trimesters) we found a significant increase in CD8+ NKT cells, in healthy control women and in women during the first and third trimester than in CD8- NKT cells. de Andres et al. found no significant difference comparing the proportion of CD3+CD56+CD8+ cells between healthy pregnant and healthy non-pregnant control women (de Andrés et al. Citation2017).
We also analyzed the proportion of these NKT subpopulations by investigating the NKT cell group instead of the lymphocyte gate. The most remarkable finding was the significant increase in the percentage of CD8+ NKT cell subpopulation than that of the CD8- NKT cells in the NKT cell group in not only healthy control women, but also women in all trimesters of healthy pregnancy. Most of the NKT cells were CD8+. This difference in the percentage of the NKT subpopulations was not seen in neither the second nor the third trimester.
An increased tendency in the expression of the co-inhibitory molecule TIGIT on CD8+ NKT cells was observed, in comparison to that of their CD8- counterparts, in the peripheral blood of women in the first trimester. However, the expression of other inhibitory immune checkpoint molecules (PD-1, CD226 and LAG-3), together with the stimulatory receptors (CD226 and NKG2D) by CD8+ and CD8- NKT cells, showed no difference in the first trimester. Upon investigating the expression of the ligands of these receptors, we observed that none of these ligands, i.e. PD-L1, CD155, CD112 and Gal-3, showed any significant difference between the two subpopulations of NKT cells in this early trimester.
The regulatory function of CD8+ NKT cells has been previously reported in several mouse models to be characterized by Th1 responses through the secretion of a Th2 cytokine profile (Marrero et al. Citation2015; Mars et al. Citation2002; Singh et al. Citation2018). Neither perforin nor granzyme B used in the functional characterization of NKT cells showed a prominent difference during the first trimester. Nonetheless, the subpopulation changes observed here, especially the increase in the level of CD8+ NKT cells in the peripheral blood during the first trimester, lead us to speculate that these cells could play a possible role in modulating immune responses in the early stages of human pregnancy.
The second trimester of pregnancy is, from an immunological point of view, the calmest time for the mother and her fetus. This is a period of rapid fetal growth and development. The mother, placenta, and fetus are symbiotic, and the predominant immunological feature is induction of an anti-inflammatory state (Mor et al. Citation2011).
When the percentages of the CD8+ and CD8- NKT cell population were analyzed in the NKT cell group, the proportion of CD8+ NKT cells showed a significant increase in women in the second trimester than that of CD8- NKT cells. The other notable finding regarding immune cell distribution is the increased in the percentage of the circulating CD8- NKT cells in women in the second trimester compared to that in healthy controls. By investigating the inhibitory and stimulatory immune checkpoint molecules and their ligands, we observed that only TIGIT showed a significant alteration; specifically, the expression of TIGIT by CD8+ NKT cells was significantly higher than that of CD8- NKT cells in peripheral blood of women in the second trimesters. It is well known that TIGIT shifts the balance away from Th1 and Th17 immunity and towards Th2 immunity and IL-10, targeting the immune response at multiple levels, mainly through its action on antigen presenting cells, effector T cells, and Treg cells (Anderson et al. Citation2016). The increased expression of TIGIT by CD8+ NKT cells contributes to the impaired function of these potentially cytotoxic phenotypes and changes along the course of pregnancy.
After analyzing the proportion of the NKT cell subpopulations in either the lymphocyte gate or the NKT cell group during the third trimester, we observed a significant increase, in both cases, in the percentages of the CD8+ NKT cells than those of their CD8- counterparts.
We found that the circulating CD8+ NKT cells are characterized by a more pronouncedup-regulation of the co-inhibitory TIGIT receptors, together with an upregulated expression of CD155, which can serve as a ligand for two immune checkpoint receptors (TIGIT and CD226) with opposite functions simultaneously, than that of their CD8- NKT counterparts. TIGIT not only binds with much higher affinity to CD155 than to CD112, but this binding is much stronger compared to the one found regarding CD226 (Anderson et al. Citation2016). These data allow us to speculate that the overall inhibitory signals delivered through TIGIT are significantly more pronounced than the stimulatory ones through CD226 and have a beneficial outcome to healthy pregnancy. However, the degree of co-expression of TIGIT and CD226 receptors by NKT cells gave interesting results. The relative CD226/TIGIT expression ratio was lower on CD8+ NKT than of their CD8- counterparts in all investigated trimesters but significant only in the second and third trimesters of healthy pregnancy. Furthermore, we found a notable relationship between CD226 and TIGIT expression by CD8+ NKT cells in all investigated trimesters during healthy pregnancy. Among the inhibitory receptors, the levels of TIGIT were the highest, whereas among the activating receptors, the levels of CD226 were the highest. Therefore, we can hypothesize that these CD8+ NKT cells are under a fine regulation during healthy human pregnancy. Careful analysis is needed to determine whether this mechanism is operative in different disease setting. Regarding PD-L1 expression, there is no significant difference between CD8+ and CD8- NKT cells in the third trimester, and only the CD8+ NKT third trimester subsets downregulated their PD-L1 expression compared to their CD8- counterparts in each of the first and second trimesters and in the healthy control group. The decreased expression of PD-L1 the ligand for the inhibitory immune checkpoint molecule, PD-1, indicates that this receptor might contribute to the possible activation of NKT cell function regardless of CD8 positivity in the third trimester.
Increased peripheral type II NKT cells at the late stages of gestation may be partially hormonally induced (Orlova and Shirshev Citation2009), although de Andres et al. found no correlation between NKT cell subsets and sex hormones during healthy pregnancy (de Andrés et al. Citation2017). However, effects of hormones on NKT cells, and more specifically on cell activity, have not been clearly elucidated.
We must note that the present study has limitations. We did not have the opportunity to perform a follow-up study investigating the same pregnant women in each trimester. We had different women in each of the groups instead. Next, the tested sample size and the available reagents we used for phenotypic and functional characterization were quite limited.
Several research groups have pointed out that the non-pathologic form of inflammation and participating immune cells with inflammatory or Th1 properties during pregnancy are critical not only for proper implantation and decidualization but also labor initiation to end the third trimester (Mor et al. Citation2011; Murrieta-Coxca et al. Citation2019). Our results regarding the relative CD226/TIGIT, perforin and granzyme-B expression denote that the CD8+ NKT cells are potentially more active than their CD8- counterparts, presumably playing a role in the immune changes in the third trimester. However, more studies are needed to investigate the association between circulating NKT cells and immunological changes before labor.
Conclusions
The possible role of NKT cells regarding CD8 positivity or CD8 negativity throughout pregnancy is still to be elucidated. Our study is the first to investigate these subpopulations focusing on their immune checkpoint molecules expression (PD-1, TIGIT, CD226, and LAG-3). The most remarkable finding was the significant increase of the percentage of CD8+ NKT cells subpopulation compared that of CD8- NKT cells in the NKT cell group in not only healthy control women but also women in all trimesters of healthy pregnancy. The expression of TIGIT by CD8+ NKT cells was significantly higher, while the value of the relative CD226/TIGIT expression ratio on CD8+ NKT cells was significantly lower in peripheral blood of women in the second and third trimesters compared to those of CD8- NKT cells. These changes by CD8+ NKT cells contribute to the impaired function of these potentially cytotoxic phenotypes and changes along the course of pregnancy.
Author contributions
M.M. contributed to the planning and execution of the flow cytometric experiments, and to the writing of the paper. D.U.N. performed statistical analyses. B.P. performed the immunolabeling of the isolated leukocytes. I.S.A.D. performed the flow cytometric analyses. L.SZ. involved in the funding support of the project and in the writing and supervision of paper.
Supplemental Material
Download PDF (125.1 KB)Acknowledgements
We would like to thank the University of Pecs, Medical School for the institutional and technical support and the Flow Cytometry Core Facility at the Szentágothai Research Centre, University of Pecs for their collaboration. We also would like to thank Etelka Nemeth Meszarosne for her help in the sample collection and all the women who participated in the study.
Disclosure statement
The authors report there are no competing interests to declare.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08820139.2022.2119863
Additional information
Funding
References
- Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. 2020. Maternal immunological adaptation during normal pregnancy. Front Immunol. 11:2627.
- Anderson AC, Joller N, Kuchroo VK. 2016. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 44(5):989–1004.
- Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, et al. 2003. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 198(4):557–67.
- Boyson JE, Aktan I, Barkhuff DA, Chant A. 2008. NKT cells at the maternal-fetal interface. Immunol Invest. 37(5–6):565–82.
- Cocks MM, Mills AM. 2021. The immune checkpoint inhibitor LAG-3 and its ligand GAL-3 in vulvar squamous neoplasia. Int J Gynecol Pathol. 41(2):113–121.
- de Andrés C, Fernández-Paredes L, Tejera-Alhambra M, Alonso B, Ramos-Medina R, Sánchez-Ramón S. 2017. Activation of blood CD3 + CD56 + CD8 + T cells during pregnancy and multiple sclerosis. Front Immunol. 8:196.
- Friedman LA, Ring KL, Mills AM. 2020. LAG-3 and GAL-3 in endometrial carcinoma: emerging candidates for immunotherapy. Int J Gynecol Pathol. 39(3):203–12.
- Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. 2015. The burgeoning family of unconventional T cells. Nat Immunol. 16(11):1114–23.
- Guo M, Yuan F, Qi F, Sun J, Rao Q, Zhao Z, Huang P, Fang T, Yang B, Xia J. 2020. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+T cells in hepatocellular carcinoma using multiplex quantitative analysis. J Transl Med. 18(1). doi:10.1186/s12967-020-02469-8
- Hosseini S, Shokri F, Pour SA, Khoshnoodi J, Jeddi-Tehrani M, Zarnani AH. 2019. Diminished frequency of menstrual and peripheral blood NKT-Like cells in patients with unexplained recurrent spontaneous abortion and infertile women. Reprod Sci. 26(1):97–108.
- Huang Z, Qi G, Miller JS, Zheng SG. 2020. CD226: an emerging role in immunologic diseases. Front Cell Dev Biol. 0:564.
- Konjevic G, Jurisic V, Jovic V, Vuletic A, Mirjacic Martinovic K, Radenkovic S, Spuzic I. 2012. Investigation of NK cell function and their modulation in different malignancies. Immunol Res. 52(1–2):139–56.
- Konjević G, Vuletić A, Mirjačić Martinović K, Colović N, Čolović M, Jurišić V. 2016. Decreased CD161 activating and increased CD158a inhibitory receptor expression on NK cells underlies impaired NK cell cytotoxicity in patients with multiple myeloma. J Clin Pathol. 69(11):1009–16.
- Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. 2012. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 188(8):3869–75.
- Marrero I, Ware R, Kumar V. 2015. Type II NKT cells in inflammation, autoimmunity, microbial immunity, and cancer. Front Immunol. 6:6–11.
- Mars LT, Laloux V, Goude K, Desbois S, Saoudi A, Van Kaer L, Lassmann H, Herbelin A, Lehuen A, Liblau RS. 2002. Cutting edge: vα14-Jα281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J Immunol. 168(12):6007–11.
- McBride MA, Patil TK, Bohannon JK, Hernandez A, Sherwood ER, Patil NK. 2020. Immune checkpoints: novel therapeutic targets to attenuate sepsis-induced immunosuppression. Front Immunol. 11:624272.
- McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, et al. 2007. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 204(5):1131–44.
- Meggyes M, Miko E, Lajko A, Csiszar B, Sandor B, Matrai P, Tamas P, Szereday L. 2019. Involvement of the PD-1/PD-L1 co-inhibitory pathway in the pathogenesis of the inflammatory stage of early-onset preeclampsia. Int J Mol Sci. 20(3):583.
- Meggyes M, Miko E, Szigeti B, Farkas N, Szereday L. 2019. The importance of the PD-1/PD-L1 pathway at the maternal-fetal interface. BMC Pregnancy Childbirth. 19(1):74.
- Meggyes M, Szanto J, Lajko A, Farkas B, Varnagy A, Tamas P, Hantosi E, Miko E, Szereday L. 2018. The possible role of CD8+/Vα7.2+/CD161++ T (MAIT) and CD8+/Vα7.2+/CD161 lo T (MAIT-like) cells in the pathogenesis of early-onset pre-eclampsia. Am J Reprod Immunol. 79(2):e12805.
- Miko E, Barakonyi A, Meggyes M, Szereday L. 2021. The role of Type I and Type II NKT cells in materno-fetal immunity. Biomedicines. 9:1901.
- Miko E, Meggyes M, Doba K, Barakonyi A, Szereday L. 2019. Immune checkpoint molecules in reproductive immunology. Front Immunol. 10:846.
- Miko E, Szereday L, Barakonyi A, Jarkovich A, Varga P, Szekeres-Bartho J. 2008. The role of invariant NKT cells in pre-eclampsia. Am J Reprod Immunol. 60(2):118–26.
- Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, Rugeles MT, Atkinson MA, Landay AL, Wilson SB. 2007. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. 122:1–14.
- Mor G, Cardenas I, Abrahams V, Guller S. 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 1221(1):80–87.
- Murrieta-Coxca JM, Rodríguez-Martínez S, Cancino-Diaz ME, Markert UR, Favaro RR, Morales-Prieto DM. 2019. IL-36 cytokines: regulators of inflammatory responses and their emerging role in immunology of reproduction. Int J Mol Sci. 20(7):1649.
- Orlova EG, Shirshev SV. 2009. Leptin as an immunocorrecting agent during normal pregnancy. Bull Exp Biol Med. 148(1):75–78.
- Pellicci DG, Koay HF, Berzins SP. 2020. Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat Rev Immunol. 20(12):756–70.
- R: a language and environment for statistical computing. https://www.r-project.org/
- Romero-Olmedo AJ, Schulz AR, Huber M, Brehm CU, Chang HD, Chiarolla CM, Bopp T, Skevaki C, Berberich-Siebelt F, Radbruch A, et al. 2021. Deep phenotypical characterization of human CD3 + CD56 + T cells by mass cytometry. Eur J Immunol. 51(3):672–81.
- Singh AK, Tripathi P, Cardell SL. 2018. Type II NKT cells: an elusive population with immunoregulatory properties. Front Immunol. 9:1969.
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. 1990. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 171(5):1393–405.
- Vuletić A, Jurišić V, Jovanić I, Milovanović Z, Nikolić S, Konjević G. 2013. Distribution of several activating and inhibitory receptors on CD3−CD56+ NK cells in regional lymph nodes of melanoma patients. J Surg Res. 183(2):860–68.
- Wagner AK, Kadri N, Snäll J, Brodin P, Gilfillan S, Colonna M, Bernhardt G, Höglund P, Kärre K, Chambers BJ. 2017. Expression of CD226 is associated to but not required for NK cell education. Nat Commun. 8(1):1–14.
- Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, Chen L, Chen Y, Zhu G, Yin W, et al. 2019. Fibrinogen-Like Protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 176(1–2):334–47.e12.
- Yeo J, Ko M, Lee DH, Park Y, Jin HS. 2021. Tigit/cd226 axis regulates anti-tumor immunity. Pharmaceuticals. 14(3):1–20.
- Yuan J, Li J, Huang SY, Sun X. 2015. Characterization of the subsets of human NKT-like cells and the expression of Th1/Th2 cytokines in patients with unexplained recurrent spontaneous abortion. J Reprod Immunol. 110:81–88.
