?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
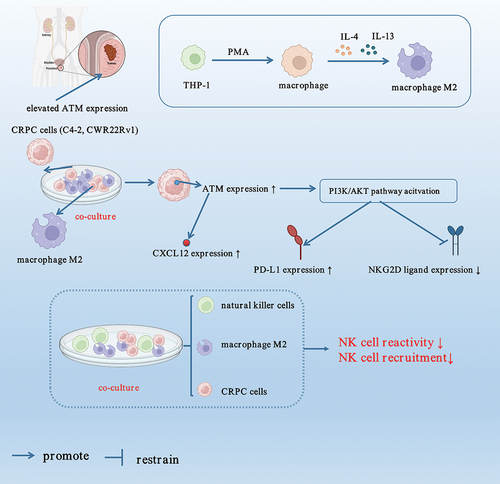
Castration-resistant prostate cancer (CRPC) in males is associated with a poor prognosis and a higher risk of treatment-related adverse effects, with high mortality among cancers globally. It is thus imperative to explore novel potential molecules with dual therapeutic and biomarker functions. Based on the recent research findings, the expression levels of ataxia telangiectasia mutant kinase (ATM) in prostate cancer (PC) tissues collected from CRPC patients were higher than hormone-dependent PC patients. Using CRPC cell lines (C4–2 and CWR22Rv1), the transwell chamber experiments revealed ATM promoted macrophage recruitment in CRPC cells in vitro via C-X-C motif chemokine ligand 12 (CXCL12). Further in vitro investigations demonstrated that polarized macrophages prevented NK cell recruitment and reduced the immunocidal activity of NK cells against CRPC cell lines. Moreover, ATM boosted programmed death receptor ligand 1 (PD-L1) expression while inhibiting NK group 2D (NKG2D) ligand expression in selected cell lines via PI3K/AKT signaling pathway. The in vivo investigations revealed ATM induced proliferation of CRPC and macrophage recruitment, while the NK cell recruitment was found to suppress ATM expression and CRPC proliferation. In conclusion, it could be demonstrated that inhibiting ATM increased the susceptibility of CRPC to NK cell inhibitors by dampening the CXCL12 and PI3K/AKT-PD-L1 pathways, thereby offering a novel and individualized treatment protocol for treating CRPC.
Introduction
Prostate cancer (PC) is currently the second most prevalent malignancy in men globally and ranks the fifth leading cause of cancer-related mortality (Liu et al., Citation2022). Recent studies have shown that approximately 10–20% of individuals diagnosed with PC metastases are susceptible to developing castration-resistant prostate cancer (CRPC) within five years of their initial diagnosis (Asay et al., Citation2020). Once castration resistance emerges, the median survival time is estimated to be around 14 months, significantly increasing mortality rates (Fu et al., Citation2022). Furthermore, 15–33% of patients will experience metastasis within the first two years following their initial diagnosis (Hirst et al., Citation2012). Given the rapid progression, aggressive nature, and unfavorable prognosis associated with CRPC, it is vital to investigate the underlying mechanisms and identify novel biomarkers to enhance CRPC treatment strategies.
Tumor cells have been shown to interact molecularly with M2 macrophages and natural killer (NK) cells (Krneta et al., Citation2017), thereby polarizing macrophages to M2 phenotype by secreting cytokines and chemokines (Jackute et al., Citation2018), thereby promoting proliferation and migration of tumor cells (Stefaniuk et al., Citation2020). Moreover, tumor cells upregulate immune checkpoint molecules to suppress the cytotoxicity of NK cells, which can eliminate tumor cells; thus, inhibition of NK activity by tumor cells results in their immune escape (Jain et al., Citation2021). Among different types of tumor cells, CRPC cells have been shown to interact strongly with M2 macrophages and NK cells (Xu et al., Citation2018), thereby facilitating macrophage polarization into M2 phenotype by secreting cytokines and chemokines such as IL-17 (Wu et al., Citation2022). The NK cell infiltration has been demonstrated in various studies to play an important role in PC advancement (Sakellariou et al., Citation2020), preferentially targeting PC stem-like cells through the TRAIL/DR5 signaling pathway (Seki et al., Citation2021). In addition, PC cells expressing Core2 O-glycans can better avoid NK cell immunity and survive in the host systemic circulation, which ultimately aids in progressing PC metastasis (Okamoto et al., Citation2013). Therefore, understanding the molecular interactions between CRPC cells, M2 macrophages, and NK cells may reveal potential targets for CRPC treatment.
ATM, a member of the class-IV phosphoinositide 3-kinase (PI3K)-related kinase family, is a vital regulator of the DNA damage response and maintains genomic integrity in eukaryotic cells (Abraham, Citation2004; Ueno et al., Citation2022). Recent evidence suggested that ATM kinase plays a pivotal role in the pathophysiology of various malignancies, despite being best recognized for its involvement in directing the nuclear DNA damage response (Qi et al., Citation2016). Similarly, a study revealed a close correlation between increased ATM expression in breast cancer lymph node metastasis and tumor invasion (Sun et al., Citation2012), suggesting reduced ATM expression may inhibit ovarian cancer metastasis (Yin et al., Citation2016). Moreover, ATM has been identified as a breast and pancreatic cancer susceptibility gene (Dalmasso et al., Citation2021), and a study also discovered a correlation between pathogenic mutations in ATM and an increased incidence of gastric cancer, advocating a broad ATM tumor spectrum (Choi et al., Citation2016). Considering these results, the current investigation establishes whether ATM also plays a role in CRPC development.
Within the scope of this study, it was hypothesized that ATM might play a crucial part in CRPC progression. For this purpose, the function of ATM in recruiting macrophages and the proliferation of CRPC were investigated, in addition to exploring the effect of NK cell recruitment on CPRC and the tumor transplantation model. The findings are envisaged to pave a path for understanding the role of NK cells and macrophage recruitment in CRPC progression.
Materials and methods
Sample collection
The samples from PC patients were surgically collected from patients between May 2019 to July 2020 at our hospital to investigate the expression of ATM in CRPC tissues. Written informed patient consent was obtained before sample collection. All patients were male aged 59 to 74 years, with a mean age of 68.6. A total of 40 surgical specimens were collected from patients who underwent surgery for PC. Among all patients, 20 had hormone-dependent prostate cancer (HDPC), 20 had CRPC, and only those HDPC patients were included in the study who had HDPC without local or distant metastasis and received radical prostatectomy, as with the other participants. The remaining 20 patients diagnosed with CRPC had developed a variety of local or distant metastasis and had all undergone palliative surgery to alleviate the symptoms brought on by a urinary tract infection. The Second Affiliated Hospital of Soochow University Ethics Committee gave ethical approval before commencing the study.
Cell culture
The human PC cell lines C4–2 and CWR22Rv1 were obtained from Cell Bank at the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and incubated at 37°C in a humidified incubator with 5% CO2 supply, and the medium was changed once every 48 h.
When required, CWR22Rv1 and C4–2 cells were pre-treated with diluted inhibitors of various cell signaling pathways, including JAK inhibitor 1 (5 µM, CAS457081-03-7, EMD Millipore, Germany), Stattic (10 µM, CAS19983-44-9, EMD Millipore, Germany), LY294002 (10 µM, Abcam, UK), SB203580 (10 µM, Sigma-Aldrich, USA), and U0126 (10 µM, Cell Signaling Technology, USA), which inhibit JAK1/JAK2, STAT3, PI3K/AKT, MAPK, and ERK, respectively.
Isolation of primary NK cells
After overnight incubation with 100 units/mL IL-2, primary NK cells were isolated from the peripheral blood mononuclear cells (PBMCs) of healthy donors using an NK cell isolation kit (ThermoFisher, USA) following the methodology provided by the manufacturer.
THP-1 macrophage culture and polarization
The THP-1 human monocyte cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in T-75 flasks using RPMI-1640 media (HyClone, USA) supplemented with 10% FBS (Gibco, USA) and 1% penicillin/streptomycin (P/S, Gibco, USA). The flasks were placed in an incubator at 37°C with a 5% CO2 atmosphere, and the media was changed once every 48 h. Before the THP-1 monocytes became adherent, they were differentiated into type 0 macrophages (M0 macrophages) by treatment with 200 nM phorbol-12-myristate-13-acetate (PMA, Abcam) for three days, which was then proceeded macrophage polarization into M1-like macrophages by incubation with LPS (100 ng/mL, Sigma Aldrich, USA) and IFN-gamma (20 ng/mL, R&D SYSTEMS, Minneapolis, MN, USA) for 96 h, and M2-like macrophages by incubation with IL-4 (20 ng/mL, R&D Systems, USA) and IL-13 (20 ng/mL, R&D Systems, USA), which were added to M0 macrophages and incubated for five days. The macrophage polarization was then validated by flow cytometry.
Flow cytometry
The polarization efficiency and confirmation were elucidated using flow cytometry. The macrophages at a density of 1 × 105 were treated with fluorescent-specific antibodies in PBS containing 10% FBS for 20 min at 4°C. The cells were then gently washed in triplicates and subjected to analysis using a BD FACSCanto II flow cytometer equipped with FlowJo software (Tree Star, USA). The details of the antibodies used for flow cytometry in this study are given in .
Table 1. Antibodies table.
Co-culture system and transwell chemotaxis assay
The THP-1 and CRPC co-culture systems were generated using 24-well transwell plates (Nest, China). Briefly, CRPC cells in the logarithmic growth phase were collected and allowed to remain in serum-free media until reaching the density of 1 × 105 cells/mL. The cells in lower wells were seeded with a 1 mL CRPC cell suspension, while the upper chambers received either un-transfected or transfected THP-1 cells (1 × 106 cells in 200 µL medium) via 3 µm polycarbonate membrane inserts. There was no physical interaction between the co-cultured THP-1 macrophages and CRPC cells. While unco-cultured macrophages were used as control. The media in the upper chambers were collected after 48 h of incubation and for cytokine markers analysis. Polycarbonate membrane inserts (8 μm thickness) were employed for studying macrophage recruitment by tumor cells, where macrophages migrated through the membrane. In contrast, the non-migratory cells were carefully removed from the upper chamber by scraping the upper surface of the transwell membrane, we then fixed and stained the migrated macrophages with 0.1% crystal violet in formalin. Cells were counted by observing six randomly chosen fields in each well with a microscope.
NK cytotoxicity assay
The cytotoxicity of NK cells against CRPC cells was performed using a lactate dehydrogenase (LDH) release assay, employing a previously reported method (Shen et al., Citation2017; Yang et al., Citation2017). The following day, CRPC cells (2,500–5,000 cells) were plated, followed by adding NK cells in different ratios (1:1, 1:5, and 1:15, target cells/effector cells). After co-culturing for 4 h, an aliquot of 50 µL media was used for the LDH cytotoxic assay, performed using an LDH cytotoxic assay kit (ThermoFisher, USA). A correction was made to the experimental release by subtracting the spontaneous release of effector cells at the corresponding dilutions. The percentage cytotoxicity was then calculated using the following equation.
Quantitative polymerase chain reaction (qPCR)
For qPCR analysis, the mRNA was isolated using an RNA Extract Kit (ThermoFisher, USA), which was then reverse transcribed into cDNA using a reverse-transcription kit (Yeasen, China) on a C1000 Touch Thermal Cycler (Bio-Rad, USA), and subjected to qPCR analysis using a ViiA7 system (Life Technologies, USA). The results were normalized to GAPDH, the gene that serves as the housekeeping gene. The details of primers used for each gene are listed in .
Table 2. Primers used for PCR.
ATM siRNA-mediated knockdown and over-expression
ATM siRNA and control vector were designed and obtained from GenePharma (Shanghai, China) to knock down ATM expression. Briefly, CRPC cells were transfected with 6 µg siRNA with Lipofectamine™ 2000 reagent (Invitrogen, USA) per manufacturer’s protocols. The targeting sequences used were si-ATM#1: sense: 5’-AGAAGUAAGUGAUGUUAUAAA-3,’ si-ATM#1: antisense: 5’-UAUAACAUCACUUACUUCUGU-3;’ si-ATM#2: sense: 5’-CCAUGAGUCUAGUACUUAAUG-3,’ si-ATM#2: antisense: 5’-UUAAGUACUAGACUCAUGGUU-3;’ si-ATM#3: sense: 5’-GGUCAAAUACUUCAUCAAAUG-3,’ si-ATM#3: antisense: 5’-UUUGAUGAAGUAUUUGACCAA-3;’ si-NC: 5’-UUCUCCGAACGUGUCACGU. Cells were treated with 50 nM DTX for 6 h and analyzed 24 h post-transfection. A total of 2.5 μg pcDNA3.1-His and pcDNA3.1-Flag-His-ATM (Addgene, USA) were transfected into tumor cells using LipofectamineTM 2000 reagent to overexpress ATM (Invitrogen, USA), and analyzed following 24 h of transfection.
Animal experiments
The in vivo experimentation was conducted using BALB/c nude mice (male, age = eight weeks, weight = 18–20 g) purchased from the Shanghai Model Organisms Center (China). All animals were acclimatized for seven days in a room maintained at a constant temperature of 22 ± 1°C, relative humidity of 50 ± 1%, and a light/dark cycle of 12 h per day, with free access to food and water. All animal care procedures were carried out per Chinese national guidelines.
Orthotopic xenografts were established by orthotopically injecting CRPC cells into animals. During the castration process, a small surgical incision was made with a sterile surgical scalpel, followed by testicle removal after being excised with the scalpel. The incision was then closed using a sterile 3–0 nonabsorbable suture. Before anesthesia termination, all animals were administered 0.05 to 0.1 mg·kg−1 buprenorphine subcutaneously. In preparation for the orthotopic injection, animals were shaved, swabbed with betadine and alcohol, inflicting a transverse incision in the lower abdomen, and anesthesia induction using isoflurane. The bladder, seminal vesicles, and prostate were partially removed from the abdominal cavity to expose the anterior prostate. Using a 22-gauge sterile needle, 1 × 106 CRPC cells were injected into the anterior prostate in a media/Matrigel mixture (1:1 [v/v], total injection volume 20 μL). Surgical clips were used to close the incision after the urogenital organs had been reinserted into the body cavity.
Some animals received an injection of primary NK cells (5 × 106 cells per mouse) into the tail vein 7 and 9 d after the CRPC cell injection. The size of the tumor was measured once every three days for a total of three weeks. All animals were then euthanized using asphyxiation with CO2 after the experiments, which was in line with the recommendations made by the AVMA Panel on Euthanasia.
Tumors were collected and weighed at the time of sacrifice to compare across groups. Tumor specimens were preserved in 4% paraformaldehyde for histological analysis like hematoxylin and eosin (H&E) staining, immunofluorescence, and immunohistochemistry. All experiments on animals were carried out under the supervision of the ethics committee of the Second Affiliated Hospital of Soochow University and by the guidelines established by the National Institutes of Health (NIH) regarding the care of humans and the use of laboratory animals.
H&E staining
The transplantation tumor tissues were sliced into appropriate diameters and then embedded in paraffin. The tissue sections of 4 µm thickness were prepared for H&E staining (Servicebio, China) as per directions provided by the manufacturer using a microtome. Briefly, the sections were first treated with 3% hydrogen peroxide for 20 min, then blocked nonspecific binding sites for 1 h using QuickBlockTM immunostaining blocking reagent. The sections were incubated with each solution sequentially and washed, viewed under Axio Scope A1 optical microscope (Zeiss, Germany), and respective sections photographed.
Immunohistochemistry
Paraffin-embedded PC and mice tumor transplant specimens were sectioned into 4 μm thick slices and subjected to immunohistochemical examination. Following antigen retrieval of each segment, endogenous peroxidase was inhibited with 3% hydrogen peroxide for 30 min, and nonspecific binding sites were blocked with QuickBlockTM immunostaining blocking reagent (Beyotime, China) for 1 h. The slides were incubated with primary antibodies at 4°C for 12 h, followed by treatment with HRP-conjugated secondary antibodies against mouse and rabbit (Dako, Denmark) for 2 h at room temperature. To visualize the proteins, 3,3’-diaminobenzidine (DAB) (Dako, Denmark) was added, the mixture incubated for 10 min, and respective sections were photographed with Axio Scope A1 optical microscope (Zeiss, Germany). The principal antibodies used are presented in .
Immunofluorescence
CRPC cells and mouse transplantation tumor tissues were obtained for immunofluorescence. CRPC cells grown in six-well plates were fixed with 4% paraformaldehyde for 30 min at room temperature before being rinsed with PBS. After embedding in paraffin, serial sections of the tissues were made with a thickness of 4 μm for immunohistochemistry. Following that, non-specific binding sites were blocked using QuickBlockTM immunostaining blocking reagent (Beyotime, China), and the endogenous peroxidase was blocked with 3% hydrogen peroxide. Sections and wells were incubated with primary antibodies at 4°C for 12 h. The sections and wells were then gently washed of the primary antibodies and incubated again with secondary antibodies at room temperature for 1 h before being stained with 4,’6-diamidino-2-phenylindole (DAPI) (Keygen, China) for 5 min. The target proteins were then observed using a laser confocal microscope (Zeiss, Germany). The details of the primary and secondary antibodies are given in .
Western blotting
The protein was extracted from the cultivated cells and the mouse transplanting tumor tissues using RIPA buffer (Sigma Aldrich, USA). The proteins were separated using SDS-PAGE, followed by proteins deposition onto PVDF membranes (GE, USA). The membranes were then treated with specific antibodies at 4°C overnight. After being rinsed three times, membranes were incubated with secondary antibodies for 2 h at room temperature. Chemiluminescence reagents were used to detect the proteins (Absin, China). The band intensities were quantitatively analyzed using Image-Pro Plus 6.0 (Media Cybernetics, USA). GAPDH served as the reference standard. contains the western blotting primary and secondary antibodies.
Statistical analysis
All data are presented as mean ± SD. Statistical analysis was performed using SPSS (version 25.0, IBM Corp. Armonk, USA) and GraphPad Prism (Version 8.0; GraphPad, USA) software. Data were analyzed statistically using one-way and two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests among more than two groups. The inter-group differences were analyzed by employing the student’s t-test. The P < .05 indicated statistical significance.
Results
ATM was highly expressed in CRPC and positively associated with PD-L1 expression
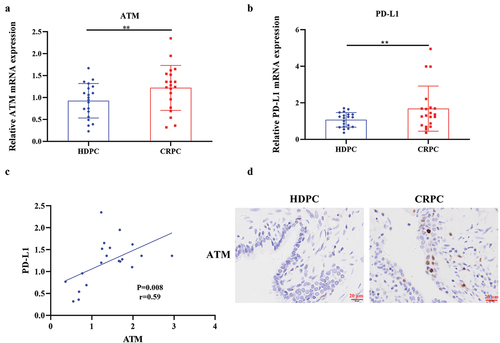
Understanding the ATM function in CPRC advancement, the levels of ATM-mRNA in PC patients with CRPC (n = 20) and HDPC (n = 20) were studied. Results indicated that the ATM-mRNA levels in CPRC tissues were significantly elevated, as shown in , which were further confirmed via immunohistochemical analysis (). It had been reported that ATM might control the expression of PD-L1 in a range of cancers (Shen et al., Citation2019; Zhang et al., Citation2018), therefore, the amount of PD-L1 present in the PC tissues of patients diagnosed with CRPC and HDPC was determined. By comparing the PD-L1-mRNA expression levels in CRPC and HDPC tissues, results showed that CRPC had much higher levels compared to HDPC tissues (). The linear regression and correlation analysis demonstrated that the levels of PD-L1-mRNA in CRPC tissues had a moderate direct link with the levels of ATM-mRNA (). All these results envisioned that ATM expression dramatically increased in CRPC tissues and was favorably related to PD-L1 expression.
Figure 1. ATM was highly expressed in CRPC and positively associated with PD-L1 expression. a, b. Expression of ATM and PD-L1 mRNA was measured by qPCR (n = 20) in CRPC and HDPC tissues. GAPDH was used to normalize the level of mRNA expression. c. the link between ATM and PD-L1 mRNA expression was calculated using a linear correlation analysis. The Spearman correlation coefficient (r) and the accompanying p (probability) value were shown. d. ATM expression in PC tissues from patients with CRPC was evaluated by IHC analysis. Scale bar = 20 μm. Data are presented as the mean ± SD. **P < .01. Data in a and B were normally distributed and analyzed using Student’s t-tests.
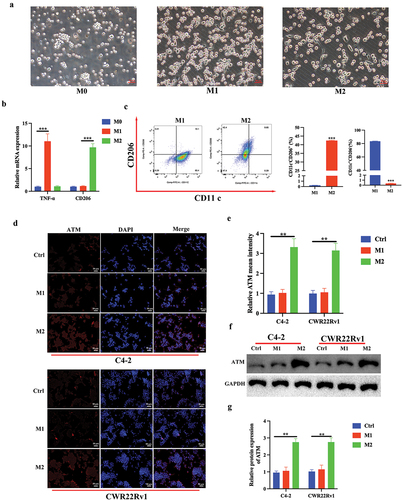
M2 macrophage polarization promotes ATM expression in CRPC cells in vitro
It has been demonstrated that increased macrophages in tumor tissues and polarization of localized macrophages play an essential role in PC progression (Han et al., Citation2020). Therefore, the effect of elevated ATM expression on macrophage polarization during the course of CRPC was investigated. The THP-1 monocyte cell line was first induced to develop into M0 type macrophages by PMA, and further differentiated into M1 or M2 macrophages by using LPS/IFN-gamma or IL-4/IL-13, respectively (). In contrast to M0 THP-1 cells, M1 macrophages preferentially expressed pro-inflammatory genes (TNF-α), whereas M2 macrophages mainly expressed anti-inflammatory genes (CD206) (). The polarization efficiency of macrophages was further studied using flow cytometry, quantification of M2 macrophages showed an increased level of CD206, which is crucial for the activation of M2 macrophages, and a decreased level of CD11c as compared with M1 macrophages (). Similarly, the relative abundance of ATM in CRPC cell lines (C4–2 and CWR22Rv1) following co-culture with THP-1 polarized macrophages (M1 and M2) was further investigated, which revealed significantly higher ATM expression in CRPC cell lines when co-cultured with M2 macrophages (), which were further validated by western blotting for ATM protein expression as shown in (). These findings suggest that the presence of M2 macrophages upregulates ATM expression in CRPC cells beyond basal levels through a positive feedback loop involving the initial recruitment of macrophages by baseline ATM. This study elucidates the interaction between CRPC cells and M2 macrophages through ATM signaling, and reveals an amplifying mechanism by which macrophages heighten ATM expression in CRPC.
Figure 2. M2 macrophage polarization promoted ATM expression in CRPC cells in vitro. a. Visualization of morphology of M0 THP-1 (left), M1 THP-1 (middle) and M2 THP-1 (right) by light microscopy, scale bar = 50 μm. b. mRNA expression levels of TNF-α and CD206. c. Flow cytometry analysis of treated THP-1 cells was performed for CD11c (M1, macrophage marker) and CD206 (M2, macrophage marker) to evaluate M1 and M2 macrophages polarization, respectively. d, e. immunofluorescent staining of ATM in CRPC cell lines (C4–2 and CWR22Rv1) after co-culturing with THP-1 polarized macrophages (M1 and M2). Scale bar = 50 μm. f, g. the level of ATM protein expression in CRPC cell lines (C4–2 and CWR22Rv1) after co-culturing with THP-1 polarized macrophages (M1 and M2). GAPDH was used to normalize protein expression. Data were presented as the mean ± SD, n = 3; **P < .01, ***P < .001. Data were analyzed by using two-way ANOVA with Tukey’s multiple comparisons.
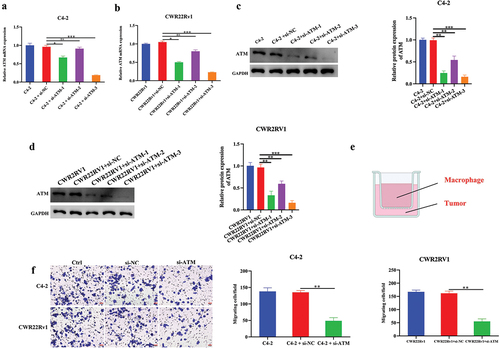
ATM promotes macrophage recruitment in CRPC cells
The involvement of ATM in macrophage recruitment in vitro was studied. As measured by qPCR and western blotting, the ATM was efficiently knocked down in CRPC cell lines (), and si-ATM-3 was selected for future investigations owing to its highest knockdown efficiency. Further, C4–2 or CWR22Rv1 cells with THP-1 polarized macrophages were co-cultured using transwell chambers (). The capacity of macrophage recruitment in CRPC cells was further evaluated using a transwell invasion experiment. The results indicated that knocking down ATMs in CRPC cell lines dramatically reduced their ability to attract macrophages in vitro (), suggesting that ATM could regulate macrophage recruitment in CRPC cells. Collectively, ATM promoted macrophage recruitment in CRPC cells in vitro.
Figure 3. ATM promotes the ability of macrophage recruitment in CRPC cells in vitro. a, b. q-PCR analysis of ATM knockdown efficiency in CRPC cell lines. GAPDH was used for normalization. c, d. Western blotting analysis of a knockdown of ATM in CRPC cell lines. GAPDH was used for normalization. e. co-cultured CRPC cell lines with M2 macrophages using transwell chambers. f. transwell assays for CRPC cell lines after co-culturing with or without M2 macrophages. Scale bar = 50 μm. Data are presented as the mean ± SD; n = 3; **P < .01; ***P < .001. Data were analyzed by using one-way ANOVA with Tukey’s multiple comparisons.
The role of ATM-CXCL12 in macrophage recruitment to CRPC cells
Since ATM was shown to stimulate the recruitment of macrophages in CRPC cells, the stimulating effect of ATM on particular cytokine was determined in vitro. Following co-culturing with M2 macrophages, qPCR was used to analyze the expression of several macrophage recruitment-related cytokines (CCL2, CCL3, CCL4, CCL5, CXCL1, CXCL12, CXCL13, and TNF-α) in ATM knockdown CRPC cell lines. The results showed that only the level of CXCL12 mRNA expression was significantly decreased in ATM knockdown CRPC cells (). Moreover, ATM knockdown in CRPC cell lines also reduced CXCL12 protein expression, where the relative decline was more pronounced following co-culture with M2 macrophages (). Similarly, the capacity of macrophage recruitment in CXCL12 knockdown and CXCL12 over-expressed CRPC cells were studied in CRPC cells co-cultured with M2 macrophages, and results demonstrated that CXCL12 could promote macrophage recruitment in CRPC cell lines (), demonstrating ATM could improve the ability of CRPC cells to recruit macrophages in vitro via CXCL12.
Figure 4. The role of ATM-CXCL12 in macrophage recruitment to CRPC cells. a, b. mRNA expression of macrophage recruitment-related cytokines (CCL2, CCL3, CCL4, CCL5, CXCL1, CXCL12, CXCL13, and TNF-α) in ATM knockdown CRPC cell lines following a co-culture with THP-1 polarized macrophages. GAPDH was used for normalization. c, d. CXCL12 protein expression in ATM knockdown CRPC cell lines following a co-culture with M2 macrophages. GAPDH was used for normalization. e-h. transwell assays for CRPC cell lines following a knockdown of CXCL12 or over-expressed CXCL12 and co-culturing with or without M2 macrophages. Scale bar = 50 μm. Data are presented as the mean ± SD; n = 3; **P < .01; ***P < .001. Data were analyzed using two-way ANOVA with Tukey’s multiple comparisons test.
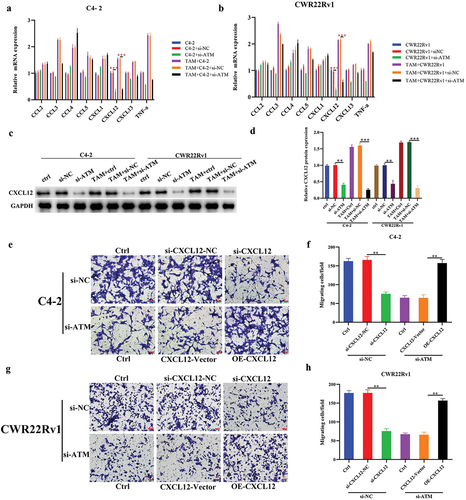
ATM promoted PD-L1 while concurrently suppressing NKG2D ligand expression in CRPC cells
As the expression of PD-L1 in CRPC tissues was found to have a positive link with ATM, it was determined whether ATM could also influence the expression of PD-L1 in CRPC cells after being cultured in vitro. Following a co-culturing with M2 macrophages, the ATM knockdown CRPC cell lines revealed lower PD-L1 expression (), further confirmed by immunofluorescence analysis as shown in . Natural killer group 2D (NKG2D) ligand was identified as a stimulatory axis responsible for increasing immune function; in contrast, the PD-L1 axis represents a crucial inhibitory immunological checkpoint (Zhao et al., Citation2019). Subsequently, the relative expression of NKG2D-activated ligands (MICA, MICB, ULBP-1, ULBP-2, and ULBP-3) was tested in CRPC cells after ATM knockdown and co-cultured with M2 macrophages in vitro. Results showed that ATM knockdown could significantly promote NKG2D-activated ligand expression in CRPC cells (). These findings suggested that ATM could promote PD-L1 expression but inhibit NKG2D ligand expression in CRPC cells.
Figure 5. ATM promotes PD-L1 while concurrently suppressing NKG2D ligand expression in CRPC cells. a, b. the level of PD-L1 expression in ATM knockdown CRPC cell lines after co-culture with M2 macrophages. GAPDH was used for normalization. c. immunofluorescence stained with PD-L1 (red) in ATM knockdown CRPC cell lines after co-culture with M2 macrophages. Scale bar = 50 μm. d-h. NKG2D-activated ligands (MICA, MICB, ULBP-1, ULBP-2, and ULBP-3) were expressed in ATM knockdown CRPC cell lines after co-culture with M2 macrophages. GAPDH was used for normalization. Data are presented as the mean ± SD; n = 3; **P < .01. P-values were calculated by one-way ANOVA with post hoc Tukey’s multiple comparison analysis.
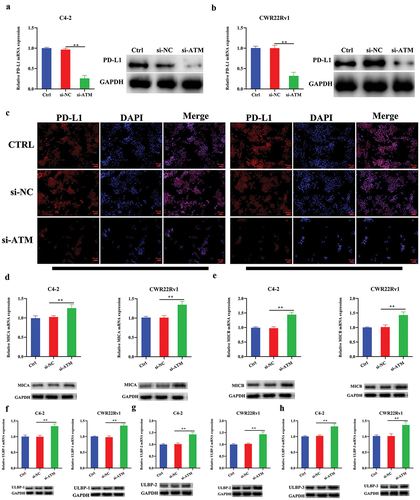
M2 macrophages operate in a paracrine manner on CRPC cells and NK cells, impairing NK cell immunocidal activity against CRPC cells
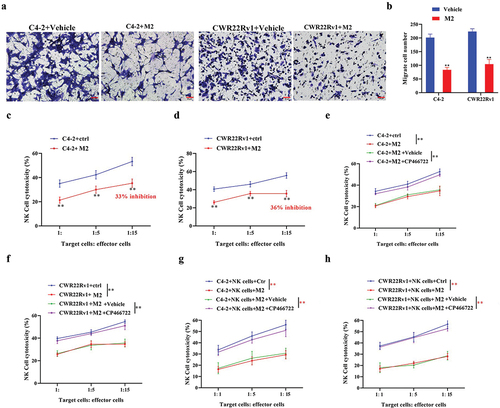
Recent research has shown that NK cell infiltration is essential to PC progression (Sakellariou et al., Citation2020). The co-cultured M2 macrophages and CRPC cells were further cultured with NK cells. The transwell analysis results suggested CRPC cell lines co-cultured with M2 macrophages showed a diminished capacity for the recruitment of NK cells (), in addition to a significant reduction in NK cell-mediated cytotoxicity (), which was significantly recovered after treatment with ATM inhibitor (CP466722) (). These data indicated that M2 macrophages hindered NK cells’ recruitment and reduced their immunocidal activity against CRPC cells.
Figure 6. M2 macrophages operate in a paracrine manner on CRPC cells and NK cells, impairing NK cell immunocidal activity against CRPC cells. a, b. The recruitment ability of CRPC cells to NK cells was detected using a transwell assay. c, d. co-culture of M2 macrophages with CRPC cell lines significantly reduced NK cell-mediated cytotoxic effects. e-h. THP-1 polarized macrophages were co-cultured with CRPC cell lines and NK cells. Treatment with an ATM inhibitor (CP466722) recovered NK cell-mediated cytotoxic effects. Data are presented as the mean ± SD; n = 3; **P < .01. Data were analyzed by using two-way ANOVA with Tukey’s multiple comparisons.
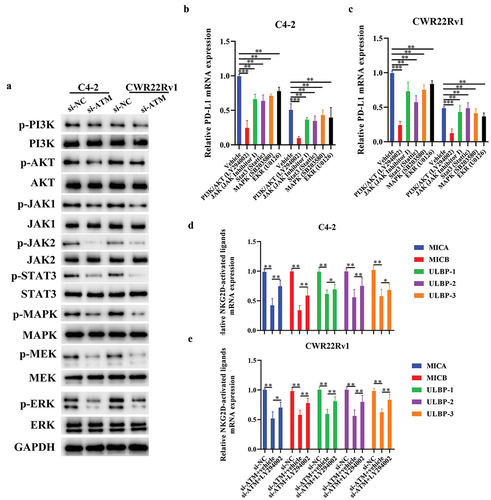
ATM promotes PD-L1 and suppresses NKG2D ligand expression via the PI3K/AKT signaling pathway in CRPC cells
The mechanism of ATM to downregulate NKG2D ligand expression while simultaneously elevating PD-L1 expression in vitro was evaluated by studying the activation of some ATM-related signaling molecules (PI3K/AKT, JAK1/JAK2, STAT3, MAPK MEK, and ERK) in CRPC cells lines. Western blotting results revealed dramatic deactivation of multiple ATM-related signaling pathways in ATM knockdown CRPC cells (). It was then preceded by investigating ATM-associated signaling pathways’ ability to control the expression of PD-L1/NKG2D ligands in CRPC cells. Interestingly, LY294002 (a PI3K/AKT inhibitor) significantly decreased the PD-L1 mRNA expression in CRPC cells to a greater extent than other signaling pathway inhibitors (). In addition, CRPC cells treated with LY294002 exhibited higher levels of NKG2D-activated ligand mRNA expression following an in vitro ATM knockdown (). Together, these results demonstrated that ATM in CRPC cells may increase the expression of PD-L1 and reduce the expression of NKG2D ligand via the PI3K/AKT signaling pathway.
Figure 7. ATM promotes PD-L1 and suppresses NKG2D ligand expression via the PI3K/AKT signaling pathway in CRPC cells. a. Activation of various ATM-related signaling molecules (PI3K/AKT, JAK1/JAK2, STAT3, MAPK, MEK, and ERK) in ATM knockdown CRPC cell lines. GAPDH was used for normalization. b, c. the level of PD-L1 mRNA expression in CRPC cell lines (C4–2 or CWR22Rv1) with inhibitors of potential signaling pathways, including PI3K/Akt (LY294002), JAK (JAK inhibitor 1), Stat3 (stattic), MAPK (SB203580), and MEK/ERK (U0126). GAPDH was used for normalization. d, e. the level of NKG2D ligand mRNA in ATM knockdown CRPC cell lines. GAPDH was used for normalization. Data are presented as the mean ± SD; n = 3; *P < .05; **P < .01. Data were analyzed using two-way ANOVA with Tukey’s multiple comparisons test.
ATM promotes CRPC and macrophage recruitment in vivo
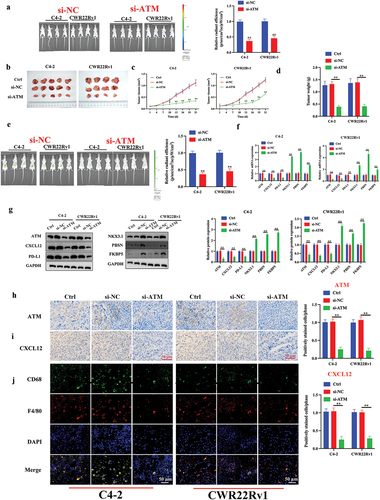
The in vitro results were further validated in animal models in vivo. A castrated CRPC in situ transplantation tumor model was created using an in situ injection, and tumor formation was detected using luminescence. shows satisfactory tumor formation after three weeks. The tumor weight and volume were recorded. Compared to normal CRPC cells, ATM knockdown CRPC cells could reduce the weight and volume of transplanted tumors (). A total of 5 × 106 fluorescently tagged THP-1 polarized macrophages from each mouse were delivered by tail vein injection. After 48 h, Epi-fluorescence was employed to assess the macrophage recruitment of CRPC cells in vivo (). Results showed that compared to the control group, mice injected with si-ATM CRPC cell lines attracted significantly fewer macrophages, indicating ATM’s ability to promote in vivo macrophage recruitment (). In addition, the levels of mRNA and protein expression in PC-related markers (NKX3.1, PBSN, and FKBP5) in transplanted tumors were also investigated. These markers exhibited enhanced expression in ATM knockdown CRPC cell-induced transplantation tumors (). Furthermore, qPCR, western blotting, and immunohistochemical analysis of transplantation tumors revealed that ATM might improve the ability of macrophage recruitment in CRPC cells via controlling CXCL12 in vivo (). To evaluate the level of macrophage infiltration, immunofluorescence was used to detect the macrophage-specific markers CD68 (a diagnostic of activated macrophages) (Bartuzi et al., Citation2014), and F4/80 (a marker of mature macrophages) (Fresno & Gironès, Citation2018), and results indicated that the ATM knockdown in the CRPC cell lines suppressed in vivo macrophage recruitment (), suggesting ATM can promote CRPC and macrophage recruitment in vivo.
Figure 8. ATM promotes CRPC and macrophage recruitment in vivo. a. luminescence of tumor formation after three weeks. b. photograph of CRPC cell lines induced transplantation tumors. c, d. the volume and weight of the transplantation tumors. e. epi-fluorescence was employed to assess the ability of CRPC cells to attract macrophages. f, g. the level of mRNA and protein expression of ATM, CXCL12, PD-L1, NKX3.1, PBSN, and FKBP5 in transplantation tumor tissues. GAPDH was used for normalization. h, i. the expression of ATM and CXCL12 in the transplantation tumor tissues was evaluated by an immunohistochemical analysis. Scale bar = 50 μm. j. immunofluorescent staining of CD68 (green) and F4/80 (red) in transplantation tumor tissues. Scale bar = 50 μm. Data are presented as the mean ± SD, n =5. *P < .05; **P < .01; ***P < .001. Data were analyzed by using two-way ANOVA with Tukey’s multiple comparisons.
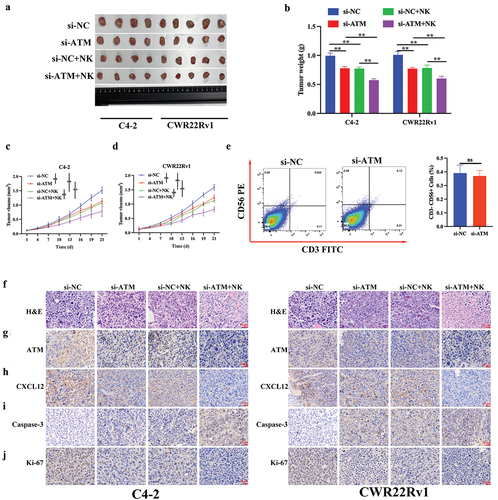
NK cell recruitment inhibits ATM expression and CRPC proliferation in vivo
A castrated CRPC in situ transplantation tumor model was created as described above. NK cells were injected into the tail vein on days 4 and 7, and tumor tissues were collected on day 25 (). The results revealed that the effect of an NK cell injection was consistent with the tumor growth-inhibiting effect played by blocking ATM, where both effects could be overlaid on each other (). To verify the relationship between ATM and CD56+ NK cells in CRPC, we found knockdown ATM in CRPC cells did not reduces tumor infiltrating CD56+ NK cell recruitment in xenograft tumors (). Moreover, an NK cell injection could also decrease the expression of ATM, CXCL12, and Ki-67 (a proliferation marker) and increase the level of caspase-3 (an apoptosis marker) expression in vivo (). Collectively, NK cell recruitment may inhibit ATM expression and CRPC proliferation in vivo.
Figure 9. NK cell recruitment inhibited ATM expression and CRPC proliferation in vivo. a. photograph of CRPC cell lines induced transplantation tumors (with or without) NK cell injection. b-d. the volume and weight of the transplantation tumors. e. fluorescence-activated cell sorting (FACS) study of CD56+CD3- NK cells in xenograft tumors with either si-NC or si-ATM group. f. The visualization of transplantation tumor tissues was evaluated by H&E staining. g-j. The expression of ATM, CXCL12, caspase-3, and ki-67 in transplantation tumor tissues was assessed by immunohistochemical analysis. Scale bar = 20 μm. Data are presented as the mean ± SD; n =5; **P < .01. Data were analyzed by using two-way ANOVA with Tukey’s multiple comparisons.
Discussion
PC is the most commonly diagnosed malignant tumor in men (Zhang et al., Citation2022; Zheng et al., Citation2019). Although androgen deprivation therapy (ADT) is an effective treatment option during the primary stage, patients with PC inevitably relapse into CRPC following ADT treatment for 18–24 months (Ge et al., Citation2022; Wu et al., Citation2019). CRPC, previously termed hormone-refractory PC, is a progressive disease despite medical or surgical castration. It has also been observed that patients diagnosed with CRPC often have an average survival period of fewer than 20 months (Grasso et al., Citation2012; Heidenreich et al., Citation2014); nevertheless, no treatment strategy has yet proven successful for CRPC, thereby necessitating exploring novel biomarkers for early diagnosis and treatment.
NK cells are essential to activating antigen-independent innate immune responses, which serve as the first line of defense against cancer development in its earliest stages (Marcus et al., Citation2014). It has been observed that NK cells have a role in the progression of CRPC. It has been reported that NK cells, T cells, and dendritic cells are among the primary types of immune cells invading tumors in PC (Yuan et al., Citation2013). Moreover, CD56+ NK cells increased after ADT treatment and were associated with a favorable CRPC prognosis (Tsukuda et al., Citation1993). Depletion of NK cells, according to the findings of several in vivo trials, may also contribute to enhanced tumor growth (Marcus et al., Citation2014). According to the results of our previous studies, M2 macrophages can obstruct the recruitment of NK cells and reduce the immunocidal activity of NK cells in vitro against CRPC cells. Thus, the implications of our findings suggest that NK cells play an important part in CRPC advancement.
As a member of the PI3K-like family, ATM kinase contains several downstream targets that govern multiple essential physiological processes, including DNA damage repair, apoptosis, proliferation, and other cellular stress responses (Boohaker & Xu, Citation2014; Khanna et al., Citation2001). Despite evidence suggesting that ATM may play a role in the development of many malignancies (Cao et al., Citation2021; Khanna et al., Citation2001), the association between ATM kinase and CRPC progression has not been reported. This study identified ATM kinase as an upstream modulator of CXCL12 and the PD-L1/NKG2D ligand. Evidence is mounting in favor of the hypothesis that ATM expression was high in CRPC tissues and correlated positively with PD-L1 expression. In addition, ATM promoted macrophage recruitment ability in CRPC cells via CXCL12 both in vitro and in vivo. Taken together, ATM is a putative kinase promoting CRPC through various mechanisms.
The positive connection between tumor cells and NK cells was found with the PD-L1 axis, which may govern the immunological activity of NK cells following their activation against tumor cells (Bae et al., Citation2012; Benson et al., Citation2010; González et al., Citation2008). A report suggested that high PD-L1 expression levels were associated with a poor prognosis and resistance to anti-cancer treatments (Mu et al., Citation2011). In addition, our recent work has shown that inhibition of IL-6-JAK/Stat3 signaling in CRPC cells could boost NK cell-mediated cytotoxicity by modifying PD-L1/NKG2D ligand levels (Xu et al., Citation2018). Since the PD-L1 axis has a negative anti-cancer impact, we investigated whether PD-L1 also participates in the progression of CRPC via inhibiting NK cell immune escape. The PI3K/AKT pathway is an essential intracellular signal transduction system advocating the initiation and advancement of several different forms of cancer (Chen, Citation2010; Zhang et al., Citation2016). In addition, the PI3K/AKT signaling pathway is highly activated in several other cellular biological activities, particularly cell proliferation and metastasis (Ma et al., Citation2013). For instance, the research by Shukla et al. found that the PI3K/AKT signaling pathway could control the invasion of PC cells (Shukla et al., Citation2007). Thus, our results demonstrated for the first time that ATM can serve a dual function, i.e., upregulate PD-L1 expression and downregulate NKG2D ligand expression in CRPC cells via PI3K/AKT signaling. Moreover, our findings also indicated that ATM might stimulate the production of PD-L1 while simultaneously suppressing the expression of NKG2D ligand in CRPC cells in vitro via PI3K/AKT signaling pathway. For these reasons, it is believed that PD-L1/NKG2D ligand and PI3K/AKT signaling pathways might be able to serve as potential therapeutic targets for CRPC patients.
In conclusion, this study showed potentially useful information depicting ATM contribution to CRPC development. Following co-culturing with M2 macrophage, ATM expression was increased in CRPC cells cultured in vitro, in addition to facilitating macrophage recruitment in CRPC cells through CXCL12. Moreover, polarized M2 macrophages inhibited NK cell recruitment and impaired NK cell immunocidal activity against CRPC cells in vitro (). Our findings revealed that NK cell recruitment inhibited ATM expression and CRPC proliferation in vivo. Thus, understanding the role of ATM and PD-L1/NKG2D in the evolution of CRPC could pave the path for exploring novel CRPC treatment strategies.
Author contributions
Conceptualization, HL J., J. Z., R. X.; Methodology, HL J., J. Z., R. X., YB. Z.; Software, BX. X.; Validation, HL J., J. Z., R. X., YC. Z., LJ. X.; Formal Analysis, DR. Y.; Investigation, HL J., J. Z., R. X.; Resources, J. G.; Data Curation, HL J., J. Z., R. X.; Writing-Original Draft Preparation, HL Jing., J. Z., R. X.; Writing-Review & Editing, YC. Z., LJ. X.; Visualization, Visualization.; Supervision, LJ. X.; Project Administration, HL J., J. Z., R. X.; Funding Acquisition, LJ. X., DR. Y., J. Z.
Supplemental Material
Download PDF (641.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All datasets generated for this study are included in the article.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08820139.2023.2258930.
Additional information
Funding
References
- Abraham, R. T. (2004). PI 3-kinase related kinases: ‘big’ players in stress-induced signaling pathways. DNA Repair, 3(8–9), 883–887. https://doi.org/10.1016/j.dnarep.2004.04.002
- Asay, S., Graham, A., Hollingsworth, S., Barnes, B., Oblad, R. V., Michaelis, D. J., & Kenealey, J. D. (2020). γ-tocotrienol and α-tocopherol ether acetate enhance docetaxel activity in drug-resistant prostate cancer cells. Molecules, 25(2), 398. https://doi.org/10.3390/molecules25020398
- Bae, D. S., Hwang, Y. K., & Lee, J. K. (2012). Importance of NKG2D-NKG2D ligands interaction for cytolytic activity of natural killer cell. Cellular Immunology, 276(1–2), 122–127. https://doi.org/10.1016/j.cellimm.2012.04.011
- Bartuzi, P., Wijshake, T., Dekker, D. C., Fedoseienko, A., Kloosterhuis, N. J., Youssef, S. A., Li, H., Shiri-Sverdlov, R., Kuivenhoven, J. A., de Bruin, A., Burstein, E., Hofker, M. H., & van de Sluis, B. (2014). A cell-type-specific role for murine Commd1 in liver inflammation. Biochimica et Biophysica Acta, 1842(11), 2257–2265. https://doi.org/10.1016/j.bbadis.2014.06.035
- Benson, D. M., Jr., Bakan, C. E., Mishra, A., Hofmeister, C. C., Efebera, Y., Becknell, B., Baiocchi, R. A., Zhang, J., Yu, J., Smith, M. K., Greenfield, C. N., Porcu, P., Devine, S. M., Rotem-Yehudar, R., Lozanski, G., Byrd, J. C., & Caligiuri, M. A. (2010). The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood, 116(13), 2286–2294. https://doi.org/10.1182/blood-2010-02-271874
- Boohaker, R. J., & Xu, B. (2014). The versatile functions of ATM kinase. Biomedical Journal, 37(1), 3–9. https://doi.org/10.4103/2319-4170.125655
- Cao, W., Shen, R., Richard, S., Liu, Y., Jalalirad, M., Cleary, M. P., D’Assoro, A. B., Gradilone, S. A., & Yang, D. Q. (2021). Inhibition of triple‑negative breast cancer proliferation and motility by reactivating p53 and inhibiting overactivated akt. Oncology Reports, 47(2). https://doi.org/10.3892/or.2021.8252
- Chen, J. (2010). The Src/PI3K/Akt signal pathway may play a key role in decreased drug efficacy in obesity-associated cancer. Journal of Cellular Biochemistry, 110(2), 279–280. https://doi.org/10.1002/jcb.22572
- Choi, M., Kipps, T., & Kurzrock, R. (2016). ATM mutations in cancer: Therapeutic implications. Molecular Cancer Therapeutics, 15(8), 1781–1791. https://doi.org/10.1158/1535-7163.Mct-15-0945
- Dalmasso, B., Pastorino, L., Nathan, V., Shah, N. N., Palmer, J. M., Howlie, M., Johansson, P. A., Freedman, N. D., Carter, B. D., Beane-Freeman, L., Hicks, B., Molven, A., Helgadottir, H., Sankar, A., Tsao, H., Stratigos, A. J., Helsing, P., Van Doorn, R. … Ghiorzo, P. (2021). Germline ATM variants predispose to melanoma: A joint analysis across the GenoMEL and MelaNostrum consortia. Genetics in Medicine, 23(11), 2087–2095. https://doi.org/10.1038/s41436-021-01240-8
- Fresno, M., & Gironès, N. (2018). Regulatory lymphoid and myeloid cells determine the cardiac immunopathogenesis of Trypanosoma cruzi infection. Frontiers in Microbiology, 9, 351. https://doi.org/10.3389/fmicb.2018.00351
- Fu, X., Zhao, J., Yu, G., Zhang, X., Sun, J., Li, L., Yin, J., Niu, Y., Ren, S., Zhu, Y., Xu, B., & Huang, L. (2022). OTUD6A promotes prostate tumorigenesis via deubiquitinating Brg1 and AR. Communications Biology, 5(1), 182. https://doi.org/10.1038/s42003-022-03133-1
- Ge, J., Wang, P., Ma, H., Zhang, J., & Muniyan, S. (2022). Solamargine inhibits prostate cancer cell growth and enhances the therapeutic efficacy of docetaxel via akt signaling. Journal of Oncology, 2022, 1–11. https://doi.org/10.1155/2022/9055954
- González, S., López-Soto, A., Suarez-Alvarez, B., López-Vázquez, A., & López-Larrea, C. (2008). NKG2D ligands: Key targets of the immune response. Trends in Immunology, 29(8), 397–403. https://doi.org/10.1016/j.it.2008.04.007
- Grasso, C. S., Wu, Y. M., Robinson, D. R., Cao, X., Dhanasekaran, S. M., Khan, A. P., Quist, M. J., Jing, X., Lonigro, R. J., Brenner, J. C., Asangani, I. A., Ateeq, B., Chun, S. Y., Siddiqui, J., Sam, L., Anstett, M., Mehra, R., Prensner, J. R. … Tomlins, S. A. (2012). The mutational landscape of lethal castration-resistant prostate cancer. Nature, 487(7406), 239–243. https://doi.org/10.1038/nature11125
- Han, I. H., Song, H. O., Ryu, J. S., & Hsieh, M. H. (2020). IL-6 produced by prostate epithelial cells stimulated with trichomonas vaginalis promotes proliferation of prostate cancer cells by inducing M2 polarization of THP-1-derived macrophages. PLoS Neglected Tropical Diseases, 14(3), e0008126. https://doi.org/10.1371/journal.pntd.0008126
- Heidenreich, A., Bastian, P. J., Bellmunt, J., Bolla, M., Joniau, S., van der Kwast, T., Mason, M., Matveev, V., Wiegel, T., Zattoni, F., & Mottet, N. (2014). EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. European Urology, 65(2), 467–479. https://doi.org/10.1016/j.eururo.2013.11.002
- Hirst, C. J., Cabrera, C., & Kirby, M. (2012). Epidemiology of castration resistant prostate cancer: A longitudinal analysis using a UK primary care database. Cancer Epidemiology, 36(6), e349–e353. https://doi.org/10.1016/j.canep.2012.07.012
- Jackute, J., Zemaitis, M., Pranys, D., Sitkauskiene, B., Miliauskas, S., Vaitkiene, S., & Sakalauskas, R. (2018). Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunology, 19(1), 3. https://doi.org/10.1186/s12865-018-0241-4
- Jain, A. S., Prasad, A., Pradeep, S., Dharmashekar, C., Achar, R. R., Ekaterina, S., Victor, S., Amachawadi, R. G., Prasad, S. K., Pruthvish, R., Syed, A., Shivamallu, C., & Kollur, S. P. (2021). Everything old is new again: Drug repurposing approach for non-small cell lung cancer targeting MAPK signaling pathway. Frontiers in Oncology, 11, 741326. https://doi.org/10.3389/fonc.2021.741326
- Khanna, K. K., Lavin, M. F., Jackson, S. P., & Mulhern, T. D. (2001). ATM, a central controller of cellular responses to DNA damage. Cell Death & Differentiation, 8(11), 1052–1065. https://doi.org/10.1038/sj.cdd.4400874
- Krneta, T., Gillgrass, A., Poznanski, S., Chew, M., Lee, A. J., Kolb, M., & Ashkar, A. A. (2017). M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. Journal of Leukocyte Biology, 101(1), 285–295. https://doi.org/10.1189/jlb.3A1215-552R
- Liu, J., Lin, F., Wang, X., Li, C., & Qi, Q. (2022). GATA binding protein 5-mediated transcriptional activation of transmembrane protein 100 suppresses cell proliferation, migration and epithelial-to-mesenchymal transition in prostate cancer DU145 cells. Bioengineered, 13(4), 7972–7983. https://doi.org/10.1080/21655979.2021.2018979
- Ma, Y., Qin, H., & Cui, Y. (2013). MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochemical & Biophysical Research Communications, 441(4), 958–963. https://doi.org/10.1016/j.bbrc.2013.11.010
- Marcus, A., Gowen, B. G., Thompson, T. W., Iannello, A., Ardolino, M., Deng, W., Wang, L., Shifrin, N., & Raulet, D. H. (2014). Recognition of tumors by the innate immune system and natural killer cells. Advances in Immunology, 122, 91–128. https://doi.org/10.1016/b978-0-12-800267-4.00003-1
- Mu, C. Y., Huang, J. A., Chen, Y., Chen, C., & Zhang, X. G. (2011). High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Medical Oncology, 28(3), 682–688. https://doi.org/10.1007/s12032-010-9515-2
- Okamoto, T., Yoneyama, M. S., Hatakeyama, S., Mori, K., Yamamoto, H., Koie, T., Saitoh, H., Yamaya, K., Funyu, T., Fukuda, M., Ohyama, C., & Tsuboi, S. (2013). Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity. Molecular Medicine Reports, 7(2), 359–364. https://doi.org/10.3892/mmr.2012.1189
- Qi, Y., Qiu, Q., Gu, X., Tian, Y., & Zhang, Y. (2016). ATM mediates spermidine-induced mitophagy via PINK1 and Parkin regulation in human fibroblasts. Scientific Reports, 6, 24700. https://doi.org/10.1038/srep24700
- Sakellariou, C., Elhage, O., Papaevangelou, E., Giustarini, G., Esteves, A. M., Smolarek, D., Smith, R. A., Dasgupta, P., & Galustian, C. (2020). Prostate cancer cells enhance interleukin-15-mediated expansion of NK cells. BJU International, 125(1), 89–102. https://doi.org/10.1111/bju.14893
- Seki, T., Shimizu, Y., Ishii, K., Takahama, Y., Kato, K., & Yano, T. (2021). NK cells can preferentially target prostate cancer stem-like cells via the TRAIL/DR5 signaling pathway. Biomolecules, 11(11), 1702. https://doi.org/10.3390/biom11111702
- Shen, M., Xu, Z., Xu, W., Jiang, K., Zhang, F., Ding, Q., Xu, Z., & Chen, Y. (2019). Inhibition of ATM reverses EMT and decreases metastatic potential of cisplatin-resistant lung cancer cells through JAK/STAT3/PD-L1 pathway. Journal of Experimental & Clinical Cancer Research, 38(1), 149. https://doi.org/10.1186/s13046-019-1161-8
- Shen, M. J., Xu, L. J., Yang, L., Tsai, Y., Keng, P. C., Chen, Y., Lee, S. O., & Chen, Y. (2017). Radiation alters PD-L1/NKG2D ligand levels in lung cancer cells and leads to immune escape from NK cell cytotoxicity via IL-6-MEK/Erk signaling pathway. Oncotarget, 8(46), 80506–80520. https://doi.org/10.18632/oncotarget.19193
- Shukla, S., Maclennan, G. T., Hartman, D. J., Fu, P., Resnick, M. I., & Gupta, S. (2007). Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. International Journal of Cancer. International Journal of Cancer, 121(7), 1424–1432. https://doi.org/10.1002/ijc.22862
- Stefaniuk, P., Szymczyk, A., & Podhorecka, M. (2020). The neutrophil to lymphocyte and lymphocyte to Monocyte Ratios as new prognostic factors in hematological malignancies – A narrative review. Cancer Management and Research, 12, 2961–2977. https://doi.org/10.2147/cmar.S245928
- Sun, M., Guo, X., Qian, X., Wang, H., Yang, C., Brinkman, K. L., Serrano-Gonzalez, M., Jope, R. S., Zhou, B., Engler, D. A., Zhan, M., Wong, S. T., Fu, L., & Xu, B. (2012). Activation of the ATM-Snail pathway promotes breast cancer metastasis. Journal of Molecular Cell Biology, 4(5), 304–315. https://doi.org/10.1093/jmcb/mjs048
- Tsukuda, M., Sawaki, S., & Yanoma, S. (1993). Suppressed cellular immunity in patients with nasopharyngeal carcinoma. Journal of Cancer Research and Clinical Oncology, 120(1–2), 115–118. https://doi.org/10.1007/bf01200735
- Ueno, S., Sudo, T., & Hirasawa, A. (2022). ATM: Functions of ATM kinase and its relevance to hereditary tumors. International Journal of Molecular Sciences, 23(1). https://doi.org/10.3390/ijms23010523
- Wu, G., Sun, Y., Xiang, Z., Wang, K., Liu, B., Xiao, G., Niu, Y., Wu, D., & Chang, C. (2019). Preclinical study using circular RNA 17 and microRNA 181c-5p to suppress the enzalutamide-resistant prostate cancer progression. Cell Death & Disease, 10(2), 37. https://doi.org/10.1038/s41419-018-1048-1
- Wu, N., Wang, Y., Wang, K., Zhong, B., Liao, Y., Liang, J., & Jiang, N. (2022). Cathepsin K regulates the tumor growth and metastasis by IL-17/CTSK/EMT axis and mediates M2 macrophage polarization in castration-resistant prostate cancer. Cell Death & Disease, 13(9), 813. https://doi.org/10.1038/s41419-022-05215-8
- Xu, L., Chen, X., Shen, M., Yang, D. R., Fang, L., Weng, G., Tsai, Y., Keng, P. C., Chen, Y., & Lee, S. O. (2018). Inhibition of IL-6-JAK/Stat3 signaling in castration-resistant prostate cancer cells enhances the NK cell-mediated cytotoxicity via alteration of PD-L1/NKG2D ligand levels. Molecular Oncology, 12(3), 269–286. https://doi.org/10.1002/1878-0261.12135
- Xu, L., Shen, M., Chen, X., Yang, D. R., Tsai, Y., Keng, P. C., Lee, S. O., & Chen, Y. (2018). In vitro-induced M2 type macrophages induces the resistance of prostate cancer cells to cytotoxic action of NK cells. Experimental Cell Research, 364(1), 113–123. https://doi.org/10.1016/j.yexcr.2018.01.041
- Yang, L., Shen, M., Xu, L. J., Yang, X., Tsai, Y., Keng, P. C., Chen, Y., & Lee, S. O. (2017). Enhancing NK cell-mediated cytotoxicity to cisplatin-resistant lung cancer cells via MEK/Erk signaling inhibition. Scientific Reports, 7(1), 7958. https://doi.org/10.1038/s41598-017-08483-z
- Yin, S., Wang, P., Yang, L., Liu, Y., Wang, Y., Liu, M., Qi, Z., Meng, J., Shi, T. Y., Yang, G., & Zang, R. (2016). Wip1 suppresses ovarian cancer metastasis through the ATM/AKT/Snail mediated signaling. Oncotarget, 7(20), 29359–29370. https://doi.org/10.18632/oncotarget.8833
- Yuan, H., Hsiao, Y. H., Zhang, Y., Wang, J., Yin, C., Shen, R., & Su, Y. (2013). Destructive impact of T-lymphocytes, NK and mast cells on basal cell layers: Implications for tumor invasion. BMC Cancer, 13(1), 258. https://doi.org/10.1186/1471-2407-13-258
- Zhang, H. J., Liu, Z., Kan, L., & Jeong, B. H. (2022). Prostate cancer susceptibility loci identified in GATA2 and ZMIZ1 in Chinese population. International Journal of Genomics, 2022, 1–6. https://doi.org/10.1155/2022/8553530
- Zhang, L., Xu, L. J., Zhu, J., Li, J., Xue, B. X., Gao, J., Sun, C. Y., Zang, Y. C., Zhou, Y. B., Yang, D. R., & Shan, Y. X. (2018). ATM‑JAK‑PD‑L1 signaling pathway inhibition decreases EMT and metastasis of androgen‑independent prostate cancer. Molecular Medicine Reports, 17(5), 7045–7054. https://doi.org/10.3892/mmr.2018.8781
- Zhang, Y., Bao, C., Mu, Q., Chen, J., Wang, J., Mi, Y., Sayari, A. J., Chen, Y., & Guo, M. (2016). Reversal of cisplatin resistance by inhibiting PI3K/Akt signal pathway in human lung cancer cells. Neoplasma, 63(3), 362–370. https://doi.org/10.4149/304_150806n433
- Zhao, R., Song, Y., Wang, Y., Huang, Y., Li, Z., Cui, Y., Yi, M., Xia, L., Zhuang, W., Wu, X., & Zhou, Y. (2019). PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Proliferation, 52(3), e12571. https://doi.org/10.1111/cpr.12571
- Zheng, X., Qiu, S., Liao, X., Han, X., Jin, K., Yang, L., & Wei, Q. (2019). The accumulation of metabolic syndrome components is associated with higher risk of positive surgical margin among patients with localized prostate cancer after radical prostatectomy. Onco Targets Ther, 12, 1613–1620. https://doi.org/10.2147/ott.S195148